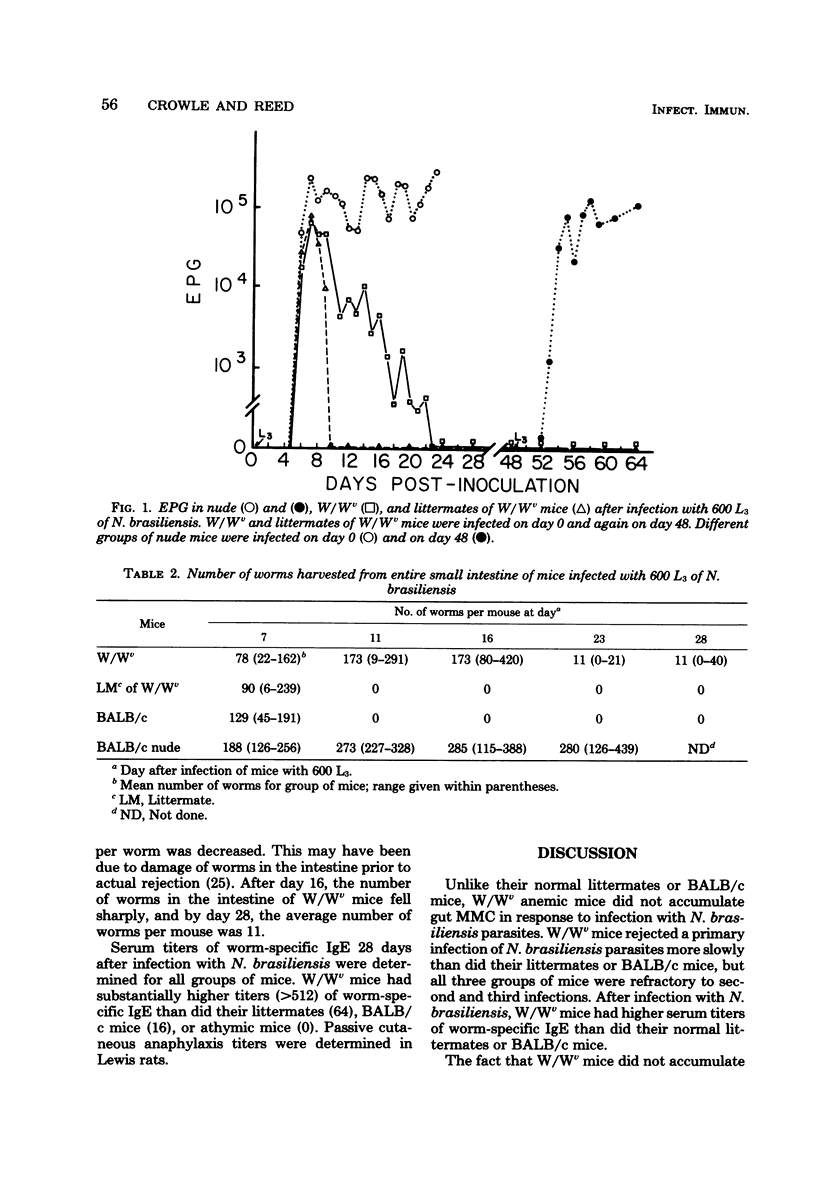
Abstract
The ability of W/Wv anemic mice to accumulate mucosal mast cells and to reject the intestinal parasite Nippostrongylus brasiliensis was examined. W/Wv mice did not accumulate mucosal mast cells in response to infections with N. brasiliensis. They eliminated a primary infection more slowly than did their normal littermate controls but were as refractory as controls to second and third infections. W/Wv mice had higher serum titers of worm-specific immunoglobulin E than did controls. These results indicate that mucosal mast cells are not an absolute requirement for N. brasiliensis rejection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreassen J., Hindsbo O., Ruitenberg E. J. Hymenolepis diminuta infections in congenitally athymic (nude) mice: worm kinetics and intestinal histopathology. Immunology. 1978 Jan;34(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W. Immune inflammatory responses to parasites: the role of basophils, mast cells and vasoactive amines. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):96–103. doi: 10.4269/ajtmh.1977.26.96. [DOI] [PubMed] [Google Scholar]
- BLOOM G., KELLY J. W. The copper phthalocyanin dye "Astrablau" and its staining properties, especially the staining of mast cells. Z Zellforch Microsk Anat Histochem. 1960;2:48–57. doi: 10.1007/BF00736491. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. The probable relationship of some or all mast cells to the T-cell system. Cell Immunol. 1977 May;30(2):358–360. doi: 10.1016/0008-8749(77)90079-x. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand. 1966;66(3):289–302. doi: 10.1111/apm.1966.66.3.289. [DOI] [PubMed] [Google Scholar]
- GINSBURG H., SACHS L. FORMATION OF PURE SUSPENSIONS OF MAST CELLS IN TISSUE CULTURE BY DIFFERENTIATION OF LYMPHOID CELLS FROM THE MOUSE THYMUS. J Natl Cancer Inst. 1963 Jul;31:1–39. [PubMed] [Google Scholar]
- Isaak D. D., Jacobson R. H., Reed N. D. The course of Hymenolepis nana infections in thymus-deficient mice. Int Arch Allergy Appl Immunol. 1977;55(1-6):504–513. doi: 10.1159/000231964. [DOI] [PubMed] [Google Scholar]
- Isaak D. D., Jacobson R. H., Reed N. D. Thymus dependence of tapeworm (Hymenolepis diminuta) elimination from mice. Infect Immun. 1975 Dec;12(6):1478–1479. doi: 10.1128/iai.12.6.1478-1479.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Okudaira H., Mauser L. E., Ishizaka K. Development of rat mast cells in vitro. I. Differentiation of mast cells from thymus cells. J Immunol. 1976 Mar;116(3):747–754. [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D., Manning D. D. Expulsion of Nippostrongylus brasiliensis from mice lacking antibody production potential. Immunology. 1977 Jun;32(6):867–874. [PMC free article] [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D. The immune response of congenitally athymic (nude) mice to the intestinal nematode Nippostrongylus brasiliensis. Proc Soc Exp Biol Med. 1974 Dec;147(3):667–670. doi: 10.3181/00379727-147-38412. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E. Reaginic antibodies and helminth infection. Vet Rec. 1973 Nov 3;93(18):480–483. doi: 10.1136/vr.93.18.480. [DOI] [PubMed] [Google Scholar]
- Kaliner M. A. The mast cell--a fascinating riddle. N Engl J Med. 1979 Aug 30;301(9):498–499. doi: 10.1056/NEJM197908303010910. [DOI] [PubMed] [Google Scholar]
- Keller R., Hess M. W., Riley J. F. Mast cells in the skin of normal, hairless and athymic mice. Experientia. 1976 Feb 15;32(2):171–172. doi: 10.1007/BF01937747. [DOI] [PubMed] [Google Scholar]
- Kind L. S., Macedo-Sobrinho B. Communications. Heterologous adoptive cutaneous anaphylaxis: a method for detecting reaginic antibody formation by cells of the mouse. J Immunol. 1973 Aug;111(2):638–640. [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Go S., Matsuda H., Hatanaka K., Seki M. Distribution of mast-cell precursors in hematopoeitic and lymphopoietic tissues of mice. J Exp Med. 1979 Sep 19;150(3):482–490. doi: 10.1084/jem.150.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Hatanaka K., Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977 Aug 4;268(5619):442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- Mekori T., Phillips R. A. The immune response in mice of genotypes W-Wv and Sl-Sld1. Proc Soc Exp Biol Med. 1969 Oct;132(1):115–119. [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Murray M., Jarrett W. F., Jennings F. W. Mast cells and macromolecular leak in intestinal immunological reactions. The influence of sex of rats infected with Nippostrongylus brasiliensis. Immunology. 1971 Jul;21(1):17–31. [PMC free article] [PubMed] [Google Scholar]
- Ogilvie B. M., Love R. J. Co-operation between antibodies and cells in immunity to a nematode parasite. Transplant Rev. 1974;19(0):147–169. doi: 10.1111/j.1600-065x.1974.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Ovary Z., Caiazza S. S., Kojima S. PCA reactions with mouse antibodies in mice and rats. Int Arch Allergy Appl Immunol. 1975;48(1):16–21. doi: 10.1159/000231289. [DOI] [PubMed] [Google Scholar]
- Potten C. S. Small intestinal cryptogenic cells in W/Wv mutant mice. Radiat Res. 1978 Apr;74(1):139–143. [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Leenstra F., Elgersma A. Thymus dependence and independence of intestinal pathology in a Trichinella spiralis infection: a study in congenitally athymic (nude) mice. Br J Exp Pathol. 1977 Jun;58(3):311–314. [PMC free article] [PubMed] [Google Scholar]
- Sharkis S. J., Wiktor-Jedrzejczak W., Ahmed A., Santos G. W., McKee A., Sell K. W. Antitheta-sensitive regulatory cell (TSRC) and hematopoiesis: regulation of differentiation of transplanted stem cells in W/Wv anemic and normal mice. Blood. 1978 Oct;52(4):802–817. [PubMed] [Google Scholar]
- Uber C. L., Roth R. L., Levy D. A. Expulsion of Nippostrongylus brasiliensis by mice deficient in mast cells. Nature. 1980 Sep 18;287(5779):226–228. doi: 10.1038/287226a0. [DOI] [PubMed] [Google Scholar]
- Whur P. Relationship of globule leucocytes to gastrointestinal nematodes in the sheep, and Nippostrongylus brasiliensis and Hymenolepis nana infections in rats. J Comp Pathol. 1966 Jan;76(1):57–65. doi: 10.1016/0021-9975(66)90048-x. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Sharkie S., Ahmed A., Sell K. W., Santos G. W. Theta-sensitive cell and erythropoiesis: identification of a defect in W/Wv anemic mice. Science. 1977 Apr 15;196(4287):313–315. doi: 10.1126/science.322288. [DOI] [PubMed] [Google Scholar]
- Wlodarski K. Mast cells in the pinna of Balb/c 'nude' (nu/nu) and heterozygotes (ny/+)mice. Experientia. 1976 Dec 15;32(12):1591–1592. doi: 10.1007/BF01924470. [DOI] [PubMed] [Google Scholar]



