Abstract
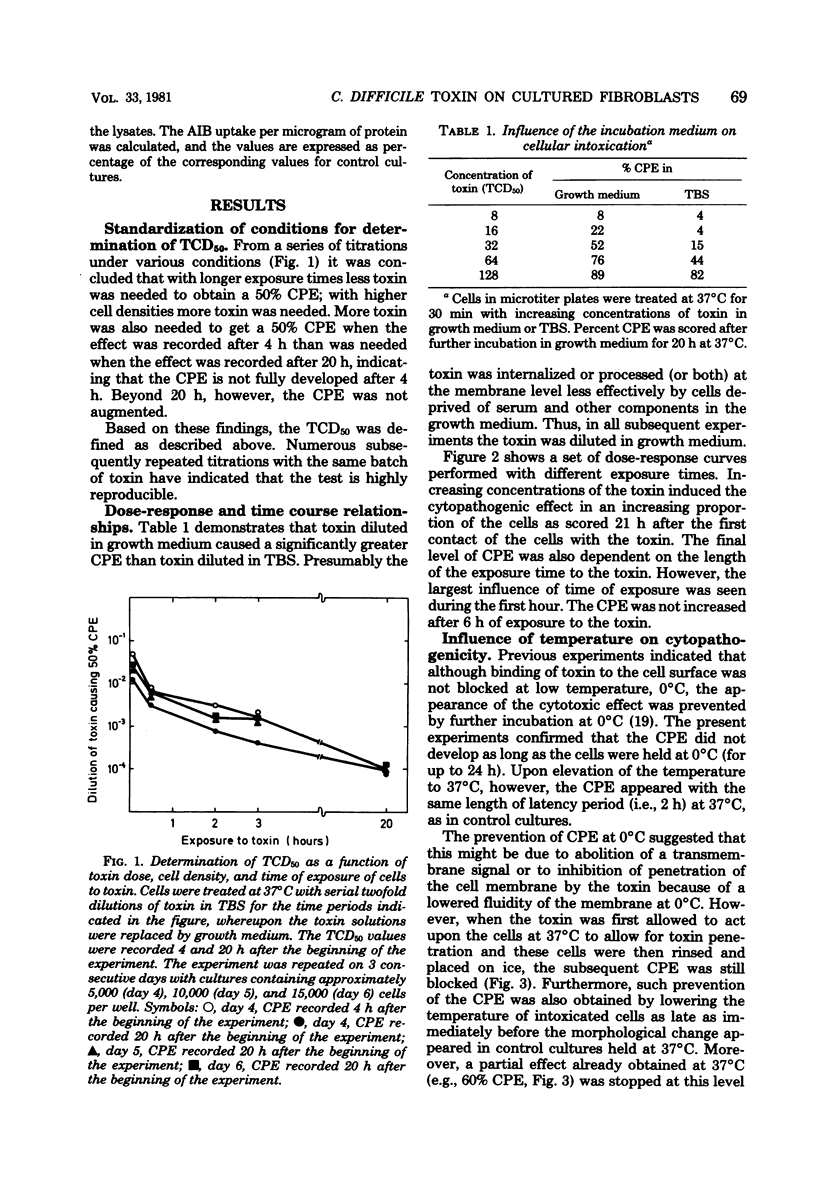
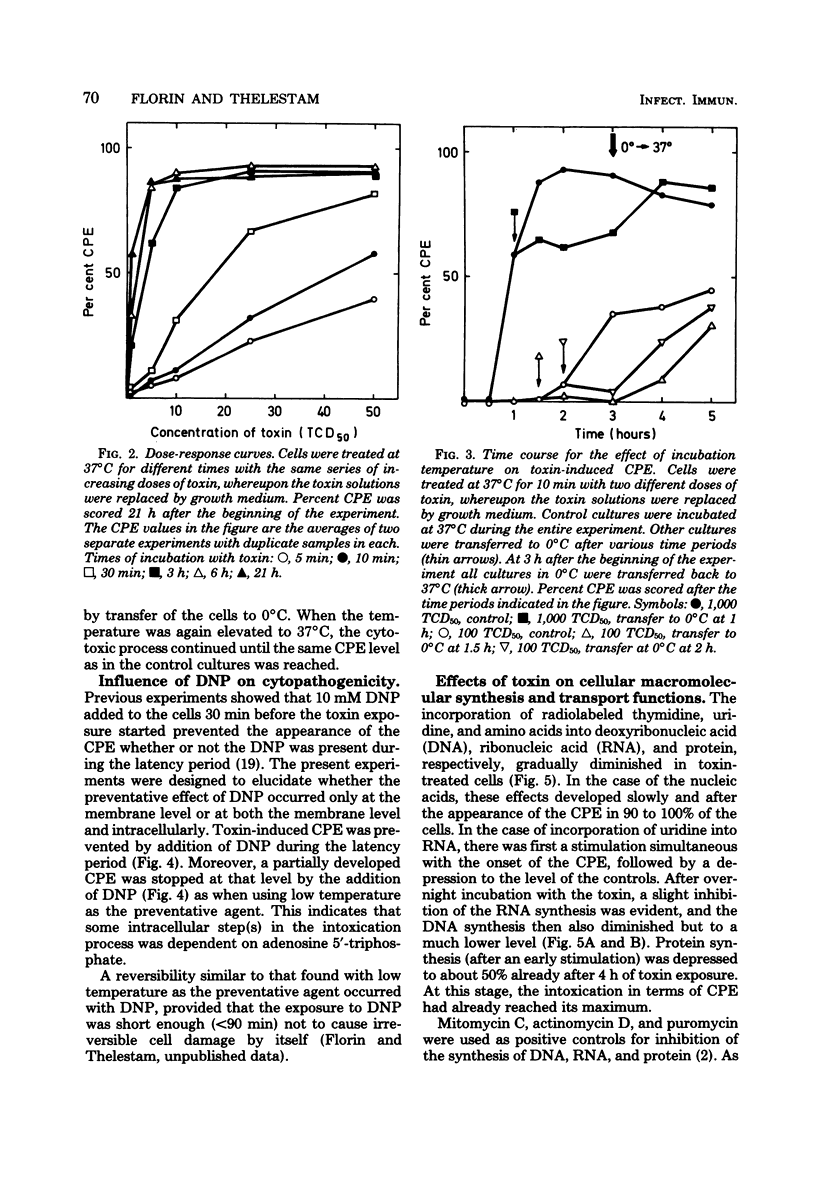
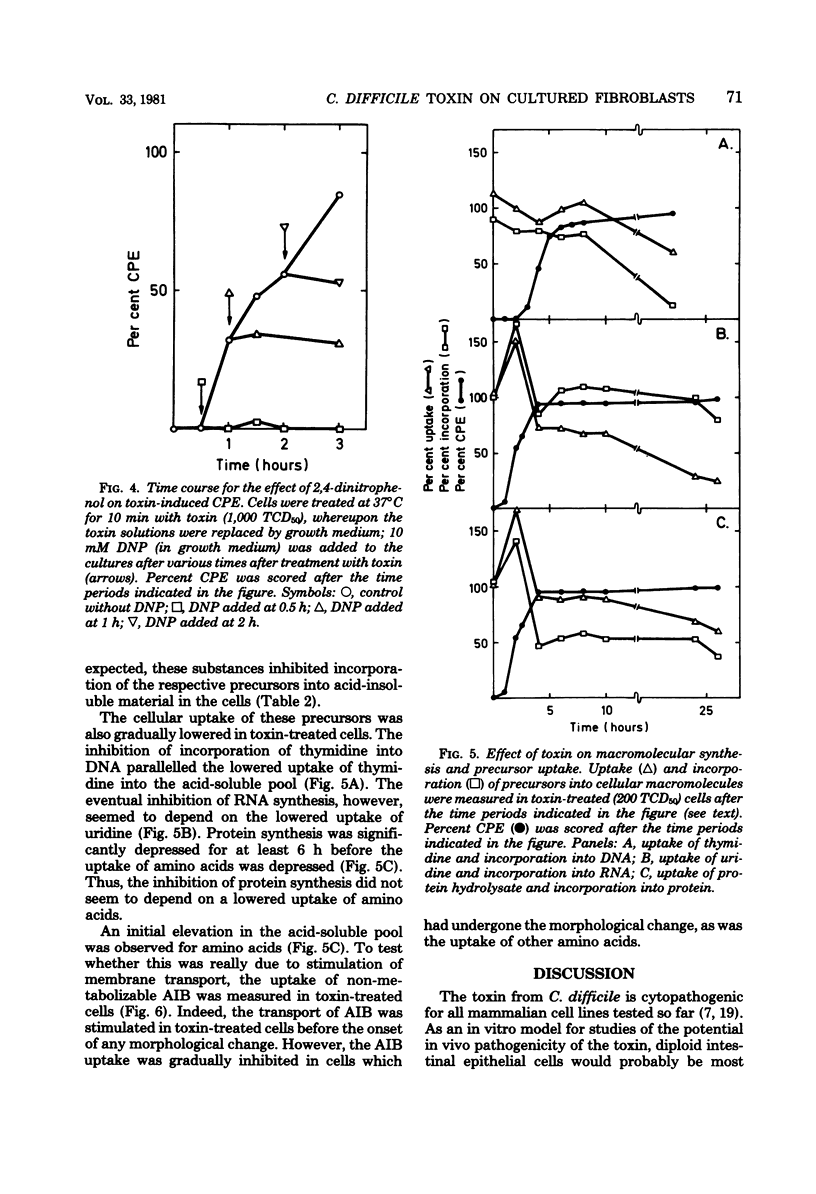
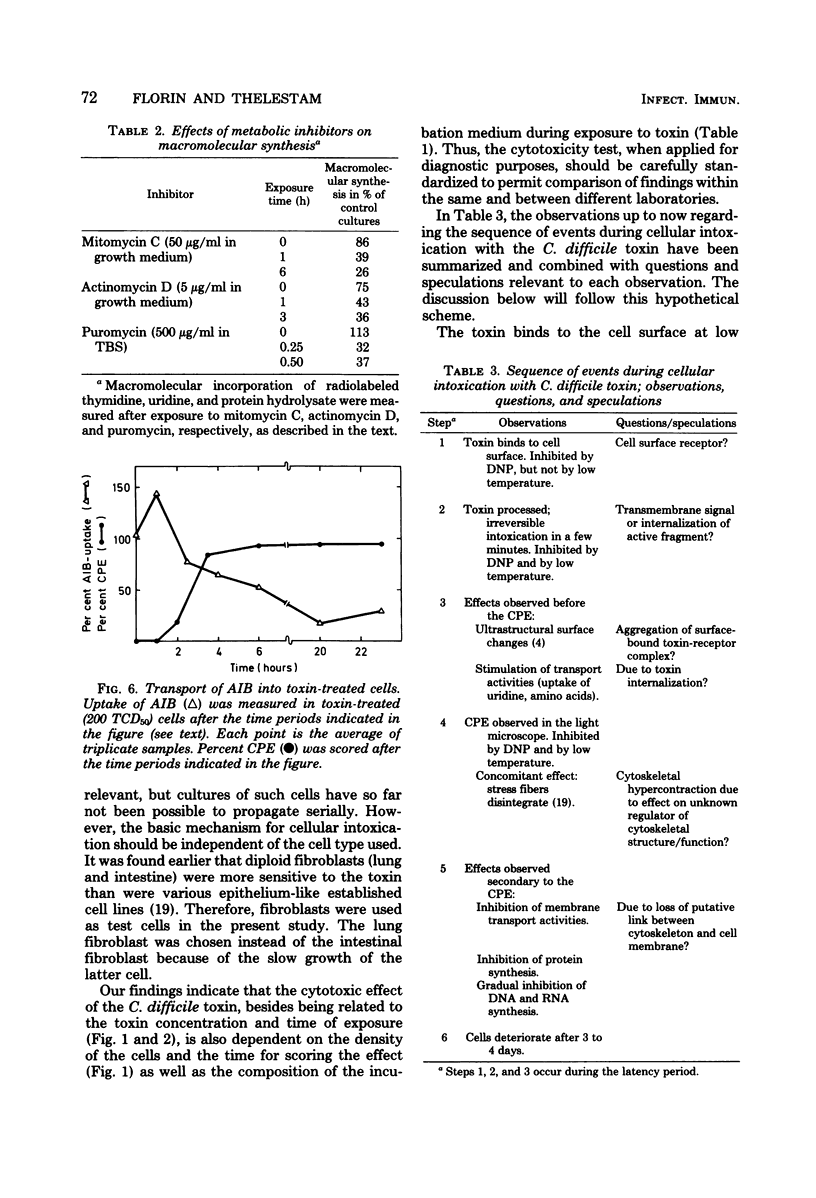
The cytopathogenic effect of partially purified toxin from Clostridium difficile on cultured human lung fibroblasts was studied. Conditions for determination of 50% tissue culture dose were standardized. The cytopathogenic effect of the toxin was dependent on toxin concentration, exposure time, and density of the cells. Transfer of the cells to 0 degrees C did not inhibit binding of toxin to the fibroblast surface, but prevented the development of the cytopathogenic effect. Both binding of toxin and some intracellular step(s) were prevented by 2,4-dinitrophenol. These preventative effects were reversible. Before and concomitantly with the appearance of the cytopathogenic effect, the cellular uptake of uridine and of amino acids was markedly stimulated. Protein synthesis was depressed when 100% of the cells showed the cytopathogenic effect, but the synthesis of nucleic acids was inhibited only several hours later. The primary cellular target for the toxin is still unknown.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersson T., Farber S., Foley G. E., Killander D., Zetterberg A. Cytochemical evaluation of metabolic inhibitors in cell culture. Exp Cell Res. 1965 Sep;39(2):365–385. doi: 10.1016/0014-4827(65)90041-8. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Bartlett J. G., Gorbach S. L., Onderdonk A. B. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect Immun. 1978 May;20(2):526–529. doi: 10.1128/iai.20.2.526-529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagraeus A., Tyrrell D. L., Norberg R., Norrby E. Actin filaments in paramyxovirus-infected human fibroblasts studied by indirect immunofluorescence. Arch Virol. 1978;57(4):291–296. doi: 10.1007/BF01320068. [DOI] [PubMed] [Google Scholar]
- George W. L., Rolfe R. D., Mulligan M. E., Finegold S. M. Infectious diseases 1979--antimicrobial agent-induced colitis: an update. J Infect Dis. 1979 Aug;140(2):266–268. doi: 10.1093/infdis/140.2.266. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B. Pseudomembranous colitis: Presence of clostridial toxin. Lancet. 1977 Dec 24;2(8052-8053):1312–1314. doi: 10.1016/s0140-6736(77)90363-4. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maness P. F., Edelman G. M. Assay for early cytoplasmic effects of the src gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2750–2754. doi: 10.1073/pnas.75.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. F., Godman G. C., Deitch A. D., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. I. Early events. J Cell Biol. 1974 May;61(2):481–500. doi: 10.1083/jcb.61.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. F., Godman G. C., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. II. Cortex and microfilaments. J Cell Biol. 1974 Aug;62(2):406–423. doi: 10.1083/jcb.62.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Purification and characterization of Clostridium difficile toxin. Infect Immun. 1979 Jul;25(1):191–201. doi: 10.1128/iai.25.1.191-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Bartlett J. G. Partial purification and characterization of a cytotoxin from Clostridium difficile. Rev Infect Dis. 1979 Mar-Apr;1(2):379–385. doi: 10.1093/clinids/1.2.379. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Brönnegård M. Interaction of cytopathogenic toxin from Clostridium difficile with cells in tissue culture. Scand J Infect Dis Suppl. 1980;(Suppl 22):16–29. [PubMed] [Google Scholar]


