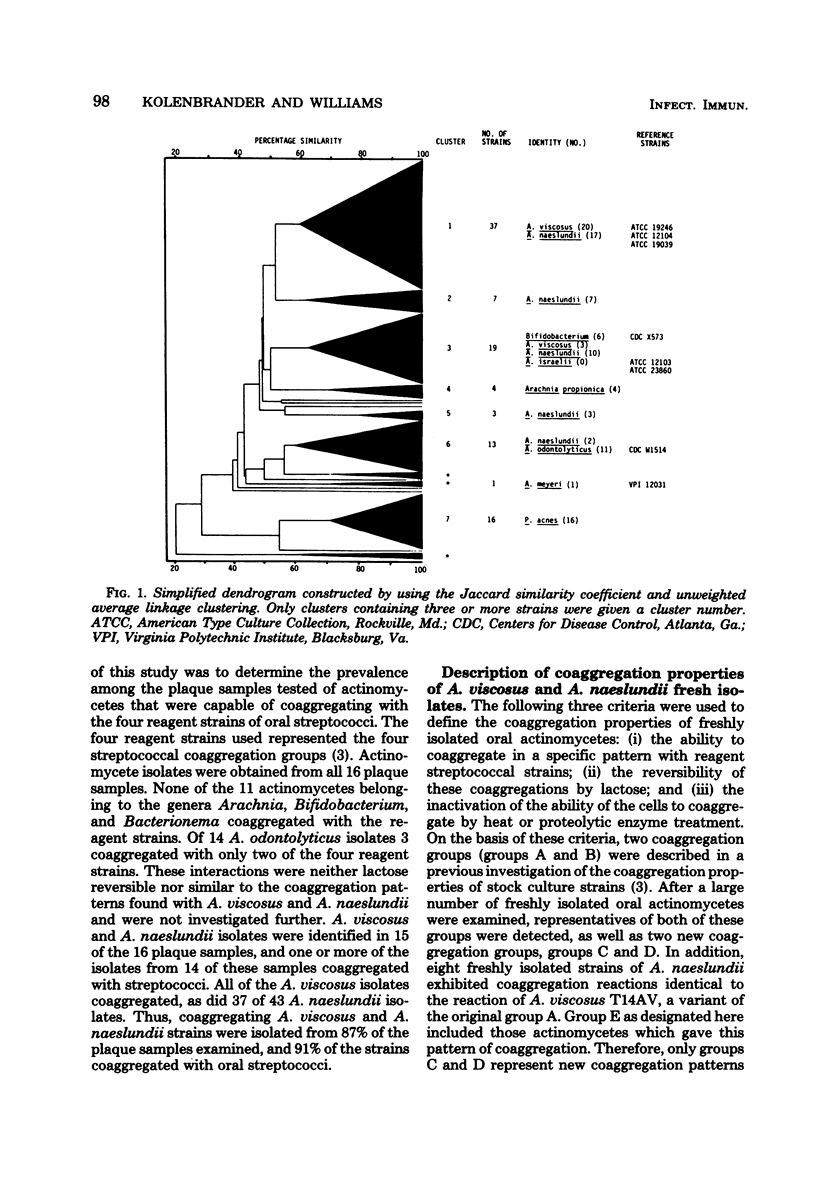
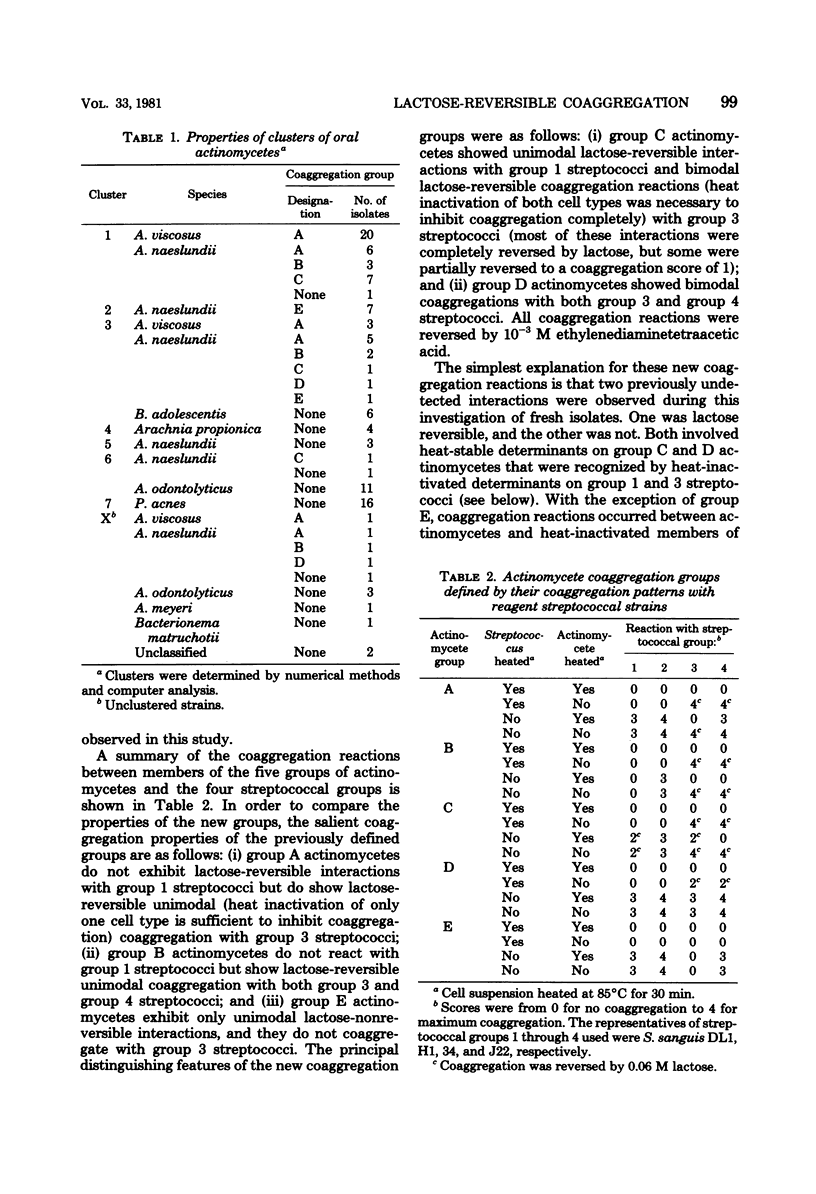
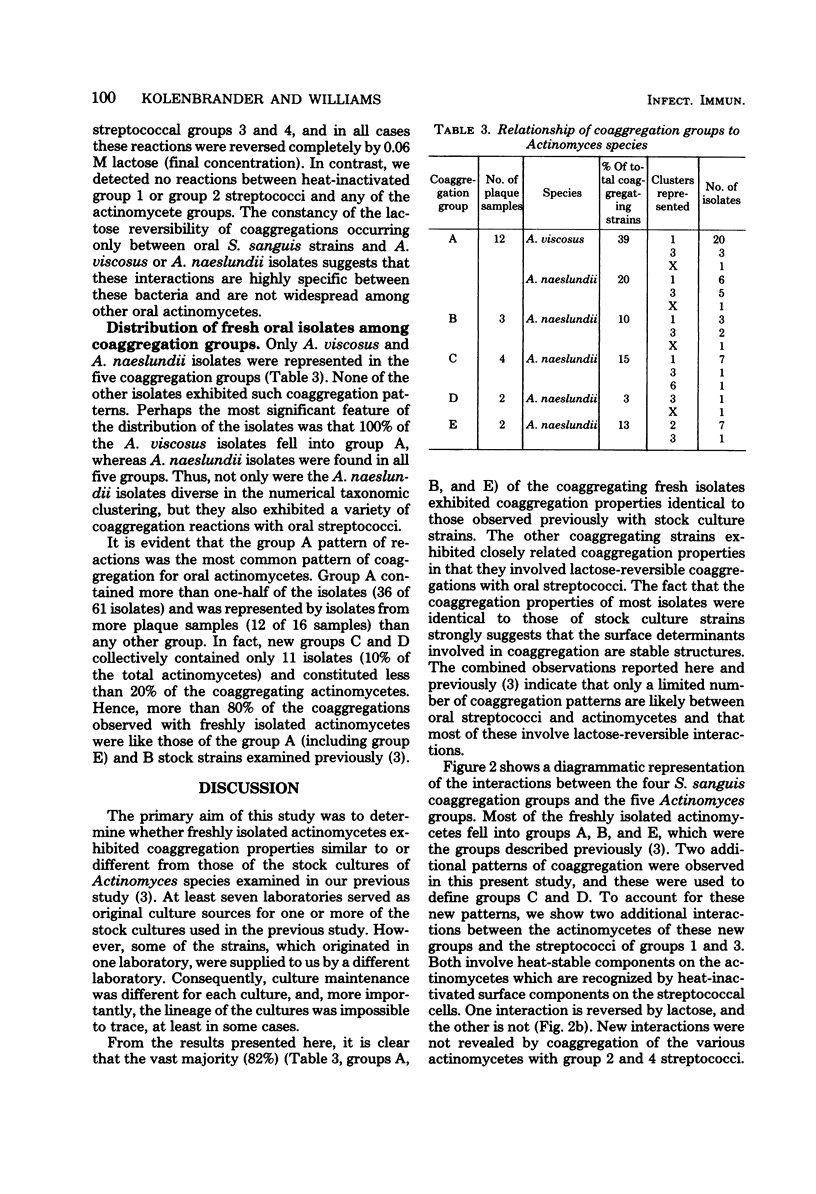
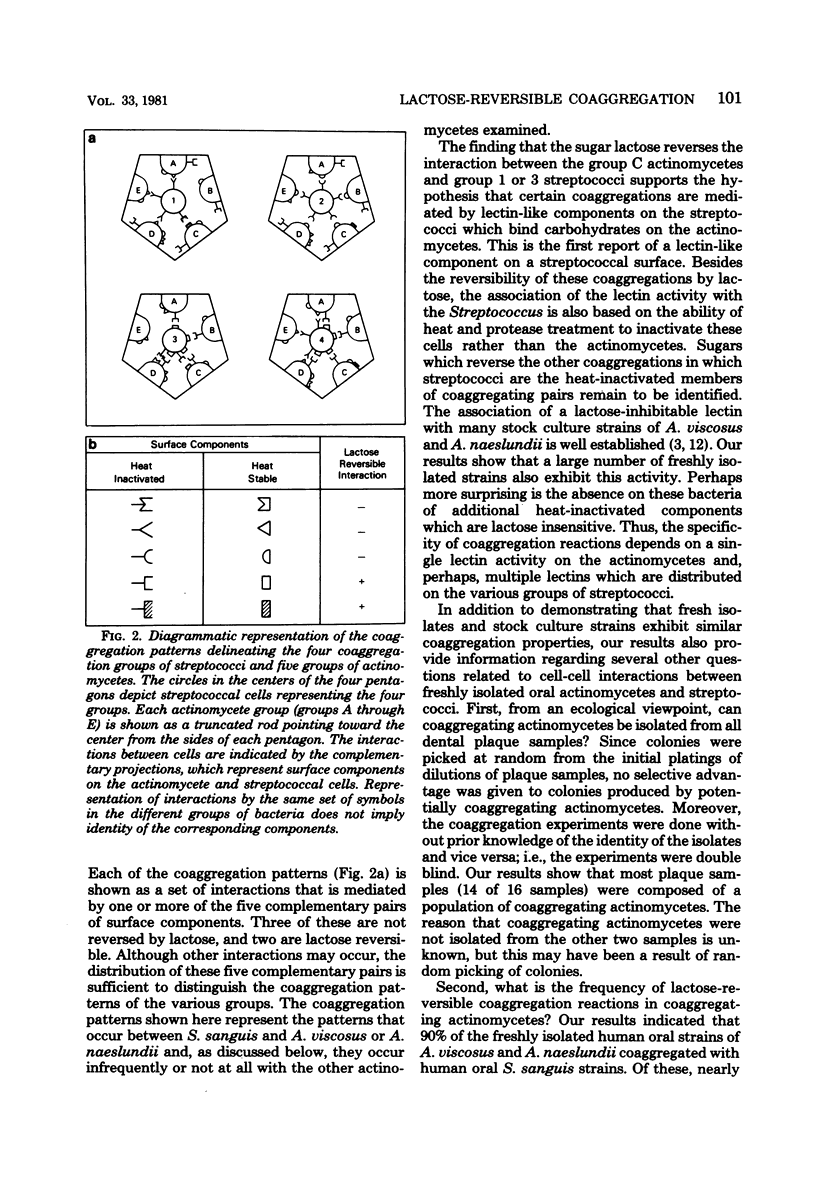
Abstract
Freshly isolated strains of oral actinomycetes were obtained from human dental plaque and were tested for the ability to coaggregate with common laboratory stock strains of Streptococcus sanguis. Strains belonging to the genera Actinomyces, Arachnia, Bifidobacterium, and Bacterionema were isolated. Only members of the genus Actinomyces coaggregated with the streptococci, and only Actinomyces viscosus and Actinomyces naeslundii exhibited lactose-reversible interactions. A total of 61 strains, consisting of all of the A. viscosus isolates and 86% of the A. naeslundii isolates, coaggregated; 87% inhibited lactose-reversible coaggregation. On the basis of this property and the altered ability of strains to coaggregate after heat treatment of the cells, we delineated four coaggregation groups. The other 13% of the strains constituted a fifth group, which was characterized by a pattern of closely related interactions that were not reversed by lactose. Compared with previously characterized coaggregation properties determined with stock culture strains of actinomycetes, more than 80% of these fresh isolates exhibited identical coaggregation properties. Thus, most of the coaggregation between freshly isolated oral actinomycetes and streptococci involves lactose-reversible cell-cell interactions, which suggests that such coaggregation is mediated by a network of lectin-carbohydrate interactions between complementary cell surface structures on the two cell types.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bladen H., Hageage G., Pollock F., Harr R. Plaque formation in vitro on wires by gram-negative oral microorganisms (Veillonella). Arch Oral Biol. 1970 Feb;15(2):127–133. doi: 10.1016/0003-9969(70)90048-8. [DOI] [PubMed] [Google Scholar]
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Fillery E. D., Bowden G. H., Hardie J. M. A comparison of strains of bacteria designated Actinomyces viscosus and Actinomyces naeslundii. Caries Res. 1978;12(6):299–312. doi: 10.1159/000260349. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K., Paul A. Role of bacterial interactions in the colonization of oral surfaces of Actinomyces viscosus. Infect Immun. 1980 Jul;29(1):83–90. doi: 10.1128/iai.29.1.83-90.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H. S., Genco R. J. Characterization of tufted streptococci isolated from the "corn cob" configuration of human dental plaque. Infect Immun. 1980 Jan;27(1):235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEATH P. H. The application of computers to taxonomy. J Gen Microbiol. 1957 Aug;17(1):201–226. doi: 10.1099/00221287-17-1-201. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]



