Abstract
The goal of this research was to resolve the hypoxic and anoxic responses of maize (Zea mays) sucrose (Suc) synthases known to differ in their sugar regulation. The two maize Suc synthase genes, Sus1 and Sh1, both respond to sugar and O2, and recent work suggests commonalities between these signaling systems. Maize seedlings (NK508 hybrid, W22 inbred, and an isogenic sh1-null mutant) were exposed to anoxic, hypoxic, and aerobic conditions (0, 3, and 21% O2, respectively), when primary roots had reached approximately 5 cm. One-centimeter tips were excised for analysis during the 48-h treatments. At the mRNA level, Sus1 was rapidly up-regulated by hypoxia (approximately 5-fold in 6 h), whereas anoxia had less effect. In contrast, Sh1 mRNA abundance increased strongly under anoxia (approximately 5-fold in 24 h) and was much less affected by hypoxia. At the enzyme level, total Suc synthase activity rose rapidly under hypoxia but showed little significant change during anoxia. The contributions of SUS1 and SH1 activities to these responses were dissected over time by comparing the sh1-null mutant with the isogenic wild type (Sus+, Sh1+). Sh1-dependent activity contributed most markedly to a rapid protein-level response consistently observed in the first 3 h, and, subsequently, to a long-term change mediated at the level of mRNA accumulation at 48 h. A complementary midterm rise in SUS1 activity varied in duration with genetic background. These data highlight the involvement of distinctly different genes and probable signal mechanisms under hypoxia and anoxia, and together with earlier work, show parallel induction of “feast and famine” Suc synthase genes by hypoxia and anoxia, respectively. In addition, complementary modes of transcriptional and posttranscriptional regulation are implicated by these data, and provide a mechanism for sequential contributions from the Sus1 and Sh1 genes during progressive onset of naturally occurring low-O2 events.
Low O2 induces marked changes in physiology and gene expression in plants and poses an important environmental stress (for review from different perspectives, see Perata and Alpi, 1993; Sachs et al., 1996; Dolferus et al., 1997; Drew, 1997; Setter et al., 1997; Vartapetian and Jackson, 1997). Most genes are rapidly down-regulated under these conditions; however, there are notable exceptions. Some of these remain unidentified (Hake et al., 1985), whereas others that could facilitate ethanolic fermentation (Peschke and Sachs, 1993; Rivoal et al., 1997; Dennis et al., 1984, 1985; Paul and Ferl, 1991a) and glycolysis (Sachs et al., 1980; Bouny and Saglio, 1996) have been identified. Glycolytic genes that are markedly up-regulated under hypoxia and/or anoxia include Suc synthases (McCarty et al., 1986; Springer et al., 1986; Bailey-Serres et al., 1988; McElfresh and Chourey, 1988; Rowland et al., 1989; S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication), Glc-6-phosphate isomerase (Kelley and Freeling, 1984b), Fru- 1,6-bisphosphate (Kelley and Freeling, 1984a; Kelley and Tolan, 1986), enolase (Lal et al., 1991, 1994; S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication), aldolase (Hake et al., 1985; Dennis et al., 1988; S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication), and glyceraldehyde-3-phosphate dehydrogenase (Russell and Sachs, 1989).
Among these low-O2-induced proteins, Suc synthase occupies a prominent position because it typically catalyzes the essential first step in C use by Suc-importing cells. Although Suc synthase and invertase can both cleave Suc, invertase activity declines under anoxia (Guglielminetti et al., 1997; Y. Zeng and K.E. Koch, unpublished data). Suc synthase therefore has the major role in the utilization of Suc under low-O2 conditions (Guglielminetti et al., 1995; Perata et al., 1997). Previous studies in maize (Zea mays) indicated that the Sh1 gene for Suc synthase was strongly induced at the mRNA level under anaerobic conditions (McCarty et al., 1986; Springer et al., 1986; McElfresh and Chourey, 1988; Rowland et al., 1989; Taliercio and Chourey, 1989), however, debate has persisted regarding changes at the protein level. Although some studies have reported little (Rowland et al., 1989) or no change at the protein level (McElfresh and Chourey, 1988; Chourey et al., 1991), others have demonstrated closer associations between mRNA abundance and protein synthesis and enzyme activity in maize (Springer et al., 1986; Bailey-Serres et al., 1988; Guglielminetti et al., 1997), rice (Ricard et al., 1991), and Arabidopsis (Martin et al., 1993). Evidence presented in this study indicates that differences in the degree of O2-deprivation treatments, hypoxia versus anoxia, and the time course of experiments may be important factors contributing to these contradictory results.
In addition, the two Suc synthase genes in maize are differentially responsive to sugar availability (Koch et al., 1992; Koch, 1996). Sh1 is maximally expressed under conditions of limited carbohydrate supply, whereas Sus1 is up-regulated when sugars are abundant (Koch et al., 1992; Koch, 1996). Sus1 and Sh1 are typically “feast and famine” genes, respectively. Recent work in this area has indicated that metabolic C flux rather than sugar levels mediate signals to sugar-responsive genes (Koch, 1996; Jang et al., 1997). If so, then low O2 could alter input into these sugar-sensing systems. Research presented here was motivated in part by evidence that the sugar-responsive Sus1 and Sh1 genes also differed in the degree to which they responded to low O2 (McCarty et al., 1986), further supporting the possible link between sugar- and O2-signaling systems. In addition to long-term effects on carbohydrate depletion, both sugar and O2 levels can alter C flux through the first step in glycolysis, which is catalyzed by hexokinases, one or more of which can also mediate the first step in a sugar signal-transduction pathway (Koch, 1996; Jang et al., 1997). Work here tested Suc synthase responses for initial compatibility with such an interface between low-O2- and sugar-sensing systems.
Finally, increasing evidence points to distinct differences between hypoxic and anoxic stresses (Johnson et al., 1989, 1994; Andrews et al., 1994; Bouny and Saglio, 1996; He et al., 1996a, 1996b; Drew, 1997) that extend from gene expression to morphology and physiology. These differences may well include contrasting effects on Suc synthases. In past studies the Sus1 and Sh1 genes have exhibited varying degrees of low-O2 sensitivity (as noted above), however, the two types of low-O2 stress were not distinguished in these earlier experiments.
The purpose of this research was to further define the low-O2 responses of the maize Suc synthases, which are known to be differentially sugar modulated. Effects of hypoxia and anoxia were compared over time at the mRNA, protein, and enzyme activity levels, and results were examined in the context of a possible link between sugar- and O2-sensing systems. An additional, unexpected aspect of this work was the contrast in patterns of temporal regulation revealed for both Sus1 and Sh1 by time-course analyses of low-O2 responses. Both transcriptional and posttranscriptional modes of up-regulation are implicated.
MATERIALS AND METHODS
Maize (Zea mays L.) seeds of hybrid NK508, inbred W22, and an isogeneic sh1-deletion mutant (in a W22 background) were surface sterilized for 20 min in 0.525% (v/v) bleach, and rinsed with water for 20 min. Seeds were germinated in the dark at 18°C on two layers of moist 3MM paper (Whatman) in 27- × 39-cm glass pans. Each pan was sealed with plastic (except import and export tubes) and supplied with a continuous air flow of 1 L min−1 throughout the 5- to 7-d germination period. Entire seedlings were exposed to this environment and remained on moist filter paper throughout the experiment. During subsequent experimental treatments, terminal 1-cm tips were excised from primary roots at selected time points, weighed, frozen in liquid N2, and stored at −80°C. Approximately 90 root tips (approximately 0.65 g) were pooled for each sample, which was subdivided for analysis of enzyme activity and mRNA levels.
Hypoxic and Anaerobic Treatments
Experimental treatments were initiated when primary root length had reached approximately 5 cm (about 7 d for W22 and the sh1-null mutant, and 5 d for NK508). A positive pressure and gas flow of 1 L min−1was maintained for anoxic (N2 only), hypoxic (3% O2 in N2), and aerobic (ambient air) treatments. Each source was fully humidified prior to chamber entry. Entire seedlings were exposed and remained on moist filter paper. Despite vigorous gassing with N2, trace levels of O2 were possible. In addition, the onset of the low-O2 treatments was delayed somewhat for many root cells because of the initial short-term presence of internal O2 in these tissues. Root tips were sampled after 3, 6, 12, 24, and 48 h of treatment, and intact controls were monitored for the poststress regrowth that occurred to varying degrees in all instances (data not shown).
Enzyme Extraction and Assay
Frozen samples were ground to a fine powder in liquid N2 using a mortar and pestle. Frozen powder was transferred to a second, chilled mortar for continued extraction in medium containing 200 mm Hepes buffer, pH 7.5, 1 mm DTT, 5 mm MgCl2, 1 mm EGTA, 20 mm sodium ascorbate, 1 mm PMSF, and 10% (w/v) polyvinylpolypyrrolidone. The buffer-to-tissue ratio was 10:1. Buffered extract was centrifuged at 14,000g for 1 min, and the supernatant was dialyzed (10,000 Mr cutoff) at 4°C for 24 h against extraction buffer diluted 1:40. The buffer was changed several times during dialysis.
Suc synthase activity was assayed in the synthetic direction. Reaction buffer (70 μL) contained 50 mm Hepes-NaOH buffer, pH 7.5, 15 mm MgCl2, 10 mm Fru, 5 mm UDP-Glc, and 20 μL of enzyme extract. Assays were conducted at 30°C for 30 min and terminated by adding 70 μL of 30% KOH. Controls were terminated at 0 min. Unreacted Fru was removed during a subsequent 10-min incubation at 100°C. After cooling, each assay was incubated with 1 mL of 0.14% anthrone in H2SO4 at 40°C for 20 min, and A620 was measured. Protein was quantified according to the method of Bradford et al. (1976), with BSA as the standard.
RNA Extraction and Analysis
Frozen samples were ground to a fine powder in liquid N2. RNA was extracted using the method of McCarty (1986), and quantified by A260. Ten micrograms of total RNA was separated by electrophoresis in 1% agarose gels containing formaldehyde, transferred to a nylon membrane, and hybridized with maize cDNA probes for Sh1 and Sus1 (gift from L.C. Hannah, University of Florida, Gainesville), and Alcohol dehydrogenase 1 (Adh1, a gift from R. Ferl, University of Florida), as in Koch et al. (1992). Blots were visualized on radiographic film at −80°C, and the relative abundance of mRNA was quantified using a phosphor imager (Molecular Dynamics, Sunnyvale, CA).
Protein Gel Blots
Protein extracts were denatured and separated by SDS-PAGE in a vertical gel apparatus. Each lane was loaded with 5 μg of protein and electrophoresed at 4°C. Acrylamide was 7.5 and 3.0% (w/v) in the separating and stacking gels, respectively. Samples entered gels at a constant voltage of 200 V and moved through stacking and separating gels at 70 and 200 V, respectively. Proteins were electroblotted onto nitrocellulose membrane, blocked with BSA (3% [w/v] in PBS plus 0.05% Tween 20), and reacted with antibody against SH1 and SUS1 proteins (Koch et al., 1992) (a 1:2000 antibody dilution at 22°C for 1 h in PBS-Tween containing 1% [w/v] BSA). Following initial hybridization, membranes were rinsed with PBS-Tween for 3 × 20 min and reacted with secondary antibody (alkaline phosphatase-conjugated goat anti-rabbit IgG [Bio-Rad] diluted 1:2000 in PBS-Tween 20 containing 1% BSA). Antibody hybridization was visualized using nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate.
RESULTS
Sus1 mRNA Levels Respond Markedly to Hypoxia
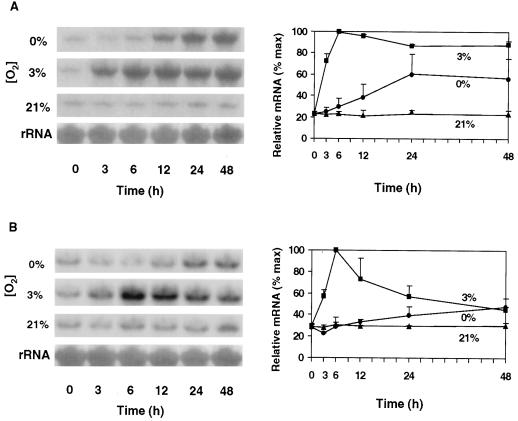
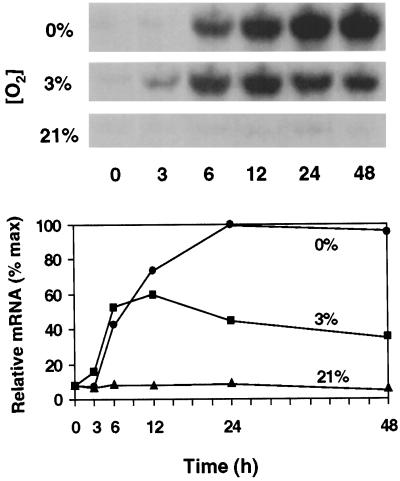
A pronounced feature of the low-O2 responses of root tips of maize seedlings shown in Figure 1 is the rapid increase of Sus1 mRNA abundance in response to hypoxia (3% O2). Within the first 6 h, Sus1 mRNA levels rose to levels 4- to 5-fold greater than in the aerobic controls of both the hybrid (NK508) and inbred (W22) maize genotypes tested. However, responses of the two genotypes differed in that the abundance of Sus1 mRNA in the NK508 (hybrid) plateaued at a high level for at least 48 h under hypoxia, whereas Sus1 message levels in the W22 (inbred) began to decrease after a peak at 6 h, declining to near noninduced levels by 48 h. This difference was initially unexpected because overt contrasts in growth or other low-O2 responses had not been evident between NK508 and W22. The latter was examined as a second, wild-type control for more precise comparisons with a sh1-null mutant in the same isogenic background.
Figure 1.
Time course and extent of change in Sus1 mRNA levels in root tips of intact hybrid (NK508) (A) and inbred (W22) (B) maize seedlings under 0% O2 (anoxic), 3% O2 (hypoxic), or 21% O2 (aerobic) conditions. Treatments were initiated after 5 to 7 d of germination, respectively, when roots had reached approximately 5 cm. The 1-cm tips of primary roots were excised at each time point (approximately 90 tips and 0.63 g). RNA gel blots were visualized by autoradiography, and abundance of 32P-mRNA was quantified with a phosphor imager. Ten micrograms of total RNA was loaded in each lane and uniformity was verified by visualization of rRNA bands. For each experiment, data from the three O2 treatments were obtained from the same blot. Error bars represent the means ± se of two to three experiments.
Under anoxic (0% O2) conditions Sus1 mRNA abundance increased much more slowly in both maize genotypes, having risen about 1- to 2-fold above aerobic controls within 24 h. Consistent with recent work (S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication), little or no Sus1 response was detected at the mRNA level prior to 12 h under anoxia. However, the slow increase was similar to that reported by McCarty et al. (1986) after 24 h of seedling submersion. The present work thus demonstrates a considerably stronger and more rapid response of Sus1 mRNA levels to hypoxia (3% O2) than anoxia (0% O2), and a difference in duration dependent on genetic background.
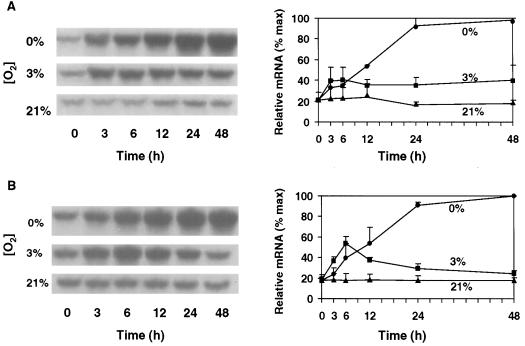
Sh1 mRNA Levels Respond Markedly to Anoxia
Figure 2 shows that although Sh1 mRNA levels did not rise significantly above those of aerobic controls until 12 h of anoxia, they ultimately increased by more than 5-fold during a 24-h period in both maize genotypes. Sh1 mRNA abundance continued to rise slowly thereafter. Other studies have reported anaerobic responses as great or greater for Sh1 at the mRNA level in 24-h experiments (McCarty et al., 1986; Springer et al., 1986; Bailey-Serres et al., 1988; McElfresh and Chourey, 1988; Rowland et al., 1989) and also in shorter-term experiments (S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication). In contrast, hypoxic conditions had a rapid but less marked effect on mean Sh1 mRNA levels. The increase was variable in the NK508 hybrid, and although a significant rise was observed within the first 6 h in W22, the mRNA level declined thereafter. Comparison of these hypoxic and anoxic responses defines Sh1 as an anaerobic gene that is distinctly less sensitive to hypoxia than to anoxia (unlike Sus1), and demonstrates the extended time course that can be involved in the Sh1 response.
Figure 2.
Time course and extent of change in Sh1 mRNA levels in root tips of intact hybrid (NK508) (A) and inbred (W22) (B) maize seedlings under 0% O2 (anoxic), 3% O2 (hypoxic), or 21% O2 (aerobic) conditions. Blots were identical to those probed with Sus1 in Figure 1, except that mRNA was hybridized with a cDNA for Sh1. Visualization and quantification were also as in Figure 1. Error bars represent the means ± se of two to three experiments.
Enzyme Responses Include Changes in Protein Abundance and Activity
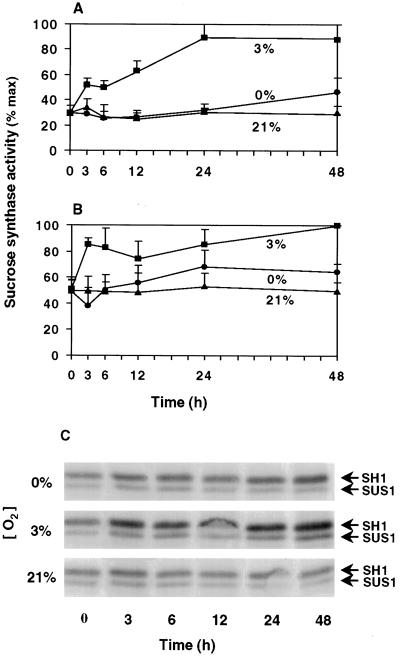
Figure 3 shows that total Suc synthase activity rose rapidly in response to hypoxia, approximately doubling within the first 3 h for both genotypes. Activity persisted at the elevated levels throughout the 48-h time course, increasing more slowly after 6 h. In seedlings of both genetic backgrounds (NK508 and W22), little or no change in mean total activity was observed after 48 h of anoxic treatment.
Figure 3.
Influence of low O2 on Suc synthase activity and protein levels. Time course of the change in relative Suc synthase activities in root tips of intact hybrid (NK508) (A) and inbred (W22) (B) maize seedlings under 0% O2 (anoxic), 3% O2 (hypoxic), or 21% O2 (aerobic) conditions. Data are the means ± se of two to three experiments, and values are plotted as a percentage of the maximum activity (137 and 152 μmol Suc g−1 fresh weight h−1 for NK508 and W22, respectively). Profiles were similar if expressed per unit of protein. C, Protein gel-blot analysis of SH1 and SUS1 proteins from NK508 seedlings. Five micrograms of protein was loaded in each lane, separated via SDS-PAGE, transferred to a nitrocellulose membrane, and hybridized with antibody cross-reactive to both SH1 and SUS1 proteins, but preferential for SH1 (Koch et al., 1992). Results were similar for the W22 inbred line (data not shown).
Western-blot analyses were conducted to determine the degree to which changes in SH1 and SUS1 protein abundance may have contributed to the differences in Suc synthase activity under 0, 3, and 21% O2 conditions. Figure 3C indicates that despite the smaller effects of hypoxia compared with anoxia on levels of Sh1 mRNA (Fig. 2), SH1 protein was more abundant under hypoxia than under either anaerobic or aerobic conditions. As with the Sh1 mRNA, an increase in SH1 protein levels was evident after as little as 3 h of hypoxia. SUS1 protein levels were also enhanced by hypoxia, and although responses appeared to be less pronounced than for SH1, direct comparison between the two is difficult due to a slight antibody preference for SH1 (Koch et al., 1992). Together, these results suggest that changes in relative isozyme levels and protein abundance may have contributed at least partially to the observed changes in Suc synthase activity.
Sus1 Gene and SUS1 Protein Responses to Hypoxia and Anoxia in the sh1-Null Mutant
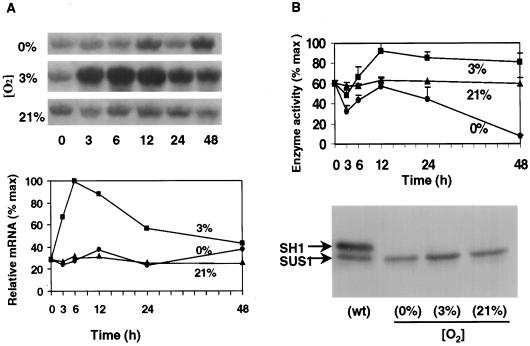
To appraise the individual contributions from the Sh1 and Sus1 genes to hypoxic and anoxic responses, comparative studies were conducted using a sh1-null mutant in which the sh1 gene has been deleted, but is otherwise isogenic to the W22 wild type. Results shown in Figure 4A confirm that in the sh1-null mutant, the low-O2 responses of Sus1 mRNA were similar to those shown for the W22 wild type shown in Figure 1B (note quantified values in both figures). This comparison is consistent with earlier work (Chourey and Talercio, 1994) indicating that Sus1 does not fully compensate in the absence of Sh1.
Figure 4.
Time course of changes in Sus1 mRNA levels and SUS1 enzyme activity in the sh1-null mutant under 0% O2 (anoxic), 3% O2 (hypoxic), or 21% O2 (aerobic) conditions. A, Relative abundance of Sus1 mRNA. Low-O2 treatments and sampling, as well as visualization and quantification of blots, were as in Figure 1. B, SUS1-Suc synthase activities and protein gel-blot analyses. Data are the means ± se of two to three experiments, and values are plotted as percentage of maximum activity (77 μmol Suc g−1 fresh weight h−1 for the sh1-null mutant). Profiles were similar if expressed per unit of protein. For the protein gel blot, 5 μg of protein was applied to each lane, separated with SDS-PAGE, transferred to a nitrocellulose membrane, and hybridized with antibody cross-reactive to both SH1 and SUS1 proteins, but preferential for SH1 (Koch et al., 1992). An aerobic, wild-type control from NK508 is shown for comparison with 12-h samples from the sh1-null mutant. Results from the sh1-null mutant were similar at 24 h (not shown).
However, Figure 4B showed that SUS1 activity in the sh1-null mutant responded differently to low O2 than did total Suc synthase activity (SUS1 plus SH1) in the isogenic wild type (Fig. 3B). Under hypoxic conditions there was a pronounced 6-h delay before increases in SUS1 activity could be detected in the sh1-null mutant, whereas in wild-type root tips, total activity had consistently risen in less than 3 h. Under more extreme anoxic conditions, SUS1 activity clearly decreased during the same period, consistently dropping to levels one-half that of aerobic controls. These reductions were statistically significant and remained below aerobic controls for the first 12 h of low O2. The decrease was not countered by rises in activity observed between 6 and 12 h in all genotypes and experiments. Significant long-term decreases in SUS1 activity were observed under anoxia but not hypoxia. In fact, the contrasting increases observed under hypoxia (Fig. 4B) indicate that SUS responses are strongly sensitive to differences in O2 availability. SUS activity in sh1-null mutants may thus differ markedly between treatments such as submersion (often hypoxic) and N2 gassing (Guglieminetti et al., 1996), and may be very sensitive to trace amounts of O2.
After 12 h, a decrease in mean SUS1 activity was observed under low O2, and although minimally evident under hypoxia, activities under anoxia had dropped to barely detectable levels by 48 h. This contrasted markedly to the capacity for maintenance and even increase in total Suc synthase activity (SUS1 plus SH1) in the isogenic wild type under anoxia and hypoxia, respectively (Fig. 3B). Increases in total activity under hypoxic conditions were greater when both SUS1 and SH1 proteins were present, but the greatest effect of SH1 appears to be in maintaining Suc synthase activity under anoxia. These results suggested that SH1 activity contributed significantly to both the very early and long-term responses of maize root tips under severe low-O2 stress.
The protein gel blots shown in Figure 4B verified an increase in SUS1 protein abundance that coincided with the elevated Suc synthase activity observed in the sh1-null mutant after 12 h of hypoxia (Fig. 4B). These and similar data from 24-h analyses (not shown) were consistent with responses of the same gene and protein in wild-type seedlings.
Adh1 as an Indicator of O2 Status
The known anaerobic up-regulation of Adh1 (Dennis et al., 1984, 1988; Paul and Ferl, 1991a, 1991b) was used here to verify the low-O2 status at the metabolic level in each experiment. The mRNA analyses shown in Figure 5 were from blots identical to those for experiments using NK508 shown in Figure 1. Responses to hypoxia and anoxia were rapid, rising approximately 5- to 6-fold within 6 h of treatment, and continuing for 24 h for 0% O2. The extent and kinetics of this increase were as reported earlier, although mRNA levels persisted longer in the present work (Andrews et al., 1994).
Figure 5.
Time course and extent of change in abundance of Adh1 mRNA in root tips of intact maize seedlings of NK508 as a marker for O2 status under 0% O2 (anoxic), 3% O2 (hypoxic), and 21% O2 (aerobic) conditions. Blots were identical to those shown in Figure 1, except that mRNA was hybridized with a cDNA for Adh1. Visualization and quantification were also as in Figure 1.
Contrasting Temporal Profiles for Hypoxic and Anoxic Contributions at the mRNA and Protein Accumulation Levels
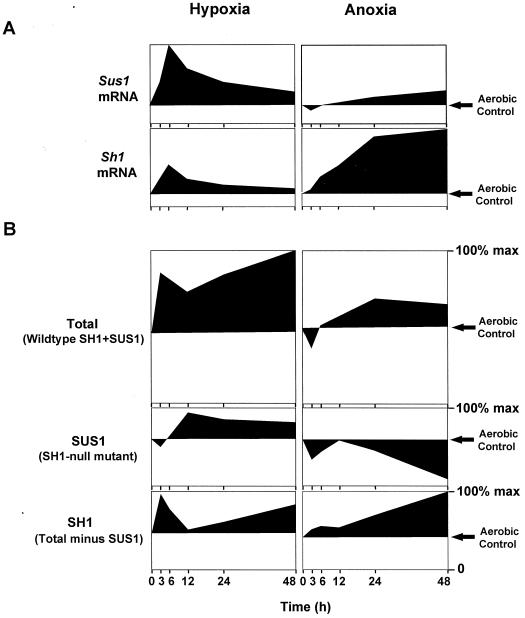
Figure 6 compares the temporal profiles of hypoxic and anoxic responses of the two Suc synthases at the mRNA and enzyme activity levels, and presents these relative to concurrent changes in SUS1- and SH1-dependent enzyme activities in the W22 inbred. SUS1 activity was assayed directly in the sh1-null mutant, and SH1-dependent activity was determined as the difference between the sh1-null mutant and its isogenic wild type. Collectively, these data indicate marked differences in responses depending on whether they occur in the first 3 to 6 h, during a second 6- to 12-h period, or as part of a long-term response over 24 to 48 h. In addition, the effects of hypoxia and anoxia have distinctly different temporal profiles that are typified at the mRNA level by rapid Sus1 and more prolonged Sh1 increases, respectively. Contrasts between profiles for mRNA and enzyme activity indicate that complementary modes of transcriptional and posttranscriptional regulation are operating under hypoxia and anoxia, and that these change over the duration of each stress.
Figure 6.
Dissection of contributions from Sus1 and Sh1 at the mRNA (A) and enzyme activity levels (B) under hypoxia (3% O2) and anoxia (0% O2) relative to aerobic controls (21% O2). Profiles at the mRNA level represent data from the isogenic W22 inbred line (derived from Figs. 1B and 2B) and are pictured for comparative purposes. Profiles at the enzyme activity level represent data from total Suc synthase (SH1 plus SUS1) in the wild-type W22 inbred line (derived from Fig. 3B), data from SUS1 alone in an isogenic sh1-null mutant (derived from Fig. 4B), and SH1-dependent activity (determined from comparison of SUS1 activity to total Suc synthase activity in the isogenic wild type). That Sus1 mRNA and SUS1 protein level responded similarly in wild-type or mutant material is indicated in Figures 1A, 3C, and 4B. Profiles are expressed as a percentage of the maximum activities for SUS1 and SH1 together, SUS1 alone, or SH1-dependent activity, which were 152, 77, and 75 μmol Suc g−1 fresh weight h−1, respectively. Decreases in activity relative to aerobic controls are shown as profiles extending below this aerobic reference line in each figure.
Profiles of the SUS1- and SH1-dependent activities are also shown in Figure 6. SUS1 activity was consistently lower in the sh1-null mutants during initial phases of either hypoxia or anoxia, whereas the same was not observed for total activity when SH1 was present in the isogenic wild type. The profile of SH1-dependent activity highlights the marked contribution by this form under low-O2 stress, and its capacity for two distinct response phases. The first is a rapid but transient increase, primarily at the enzyme activity level under hypoxia. The second is a prolonged rise in SH1-dependent activity under both low-O2 treatments, which correlates with Sh1 mRNA levels under the anoxic treatment. Between 24 and 48 h, this rise in SH1-dependent activity can account for about one-half of the total increase under hypoxia, and almost all of the activity maintained under anoxia.
DISCUSSION
This study provides evidence for Sus1 and Sh1 as predominantly hypoxic and anoxic genes, respectively (parallel to their induction by carbohydrate “feast and famine” conditions), and describes the contributions by each of these Suc synthase genes to low-O2 responses at the mRNA, protein, and enzyme levels.
The first portion of this study, focusing on gene expression at the mRNA level, has three implications: First, our data extend the contention by Drew and co-workers (Johnson et al., 1989, 1994; Andrews et al., 1994; He et al., 1996a, 1996b; Drew, 1997) that hypoxia and anoxia are distinctly different stresses involving different genes and signals. Second, temporal response profiles help clarify discrepancies between previous single-point studies in the literature, and suggest a means for sequential, transcriptional contributions by Sus1 and Sh1 during O2-depletion events. Third, differential regulation of Sus1 and Sh1 by O2 parallels closely the differential modulation of the same genes by sugar availability. Our data are consistent with recent suggestions that sugar signaling may be linked to glycolytic flux (Koch 1996; Jang et al., 1997), which is strongly O2 responsive (Bouny and Saglio, 1996).
The second portion of the present study, delineation of Sh1- and Sus1-dependent gene contributions at the enzyme level, provides a possible resolution of long-standing questions regarding the role of SH1 protein under low-O2 stress. Data presented here indicate that RNA and protein-level changes follow in slow succession for SH1 under anoxia, and are largely responsible for maintaining total Suc synthase activity during severe, long-term stress. In contrast, under hypoxia, SUS1-dependent activity has a more rapid but temporally limited involvement that depends on genotype. However, both of these instances of up-regulation involve rapid, protein-level responses likely to include posttranscriptional mechanisms.
This study thus tests hypotheses for differential regulation of the Suc synthase genes by separating the effects of hypoxia and anoxia and by comparing mutant and wild-type responses. In addition, temporal changes in expression are examined concurrently at the mRNA, protein, and enzyme activity levels.
Sus1 and Sh1 Are Up-Regulated Preferentially by Hypoxia and Anoxia, Respectively
Until relatively recently, hypoxia (3% O2) and anoxia (0% O2) were not widely viewed as distinctly different types of low-O2 stress. In fact, anaerobic genes have sometimes been used to describe a collective, broadly inclusive group of genes induced under varying degrees of O2 deprivation. Results shown here, however, demonstrate a preferential up-regulation of Sus1 under hypoxia and of Sh1 under anoxia.
Resolution of Sus1 and Sh1 as genes preferentially responsive to hypoxia and anoxia, respectively, provides a framework for integration of often differing results from earlier studies at both the protein (discussed later) and mRNA levels. Previous evidence indicated that both genes generally responded to low O2 (McCarty et al., 1986; Springer et al., 1986; McElfresh and Chourey, 1988; Rowland et al., 1989); however, effects of hypoxia and anoxia were not separated in this earlier work. During these studies, low O2 was generally imposed by submerging seedlings in buffer for 24 h, and although maize Sh1 mRNA levels typically rose, anaerobic effects could not be readily attributed to specific hypoxic or anoxic influences. Recently, Sh1 gene expression was demonstrated under defined anoxia in both long-term experiments (Guglielminetti et al., 1997) and short-term studies of run-on transcription and mRNA accumulation (S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication), although hypoxia was not tested in either study. In the present work, data further confirm anoxic induction of Sh1 over time, and in addition, contrast this to a markedly reduced Sh1 response under hypoxia.
Results have differed among studies of Sus1 responses to low O2 at the mRNA level, with a 2-fold increase after 24 h reported by McCarty et al. (1986), in contrast to little change observed by Springer et al. (1986), and decreases reported by McElfresh and Chourey (1988) and Rowland et al. (1989). Again, hypoxia and anoxia were not distinguished in these earlier works. Our data indicate that the extent of O2 deprivation and/or its duration may be an important contributor to factors affecting apparent discrepancies between results from different studies. Sus1 mRNA levels rose 4- to 5-fold within 6 h of hypoxic treatments, often declining slightly thereafter. Increases over 24 h of anoxia were about one-half of the level observed after 6 h of hypoxia. Recent analysis of run-on transcription of Sus1 under anoxia showed marked enhancement after 6 and 12 h, despite a lack of concurrent mRNA accumulation (S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication). This adjustment was considered a possible advantage for recovery if stress were transient. Sus1 mRNA levels responded rapidly to hypoxia in the present study, rising to peak abundance within 6 h. Duration of this elevation varied with genotype, but any possible relationship to hypoxic adjustment by inbred versus hybrid materials remains unclear.
Time-Course Profiles Suggest Different Roles for Sus1 and Sh1 mRNAs
Contrasts in the time course of the rapid hypoxic (Sus1) and more prolonged anaerobic (Sh1) response profiles observed here over 48-h periods (Figs. 1 and 2) indicate that different mechanisms may be involved in regulating mRNA levels by affecting synthesis and/or longevity. In addition, the temporal variations observed emphasize the extent to which results can differ if compared at a single point in time. Even measurements at 6 h can differ markedly from those at 12 h for mRNA levels and run-on transcriptional analyses of several genes responding to anoxia (Fennoy and Bailey-Serres, 1995; S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication). Furthermore, rapid readjustments in adenylate turnover that occur during initial low-O2 adjustments (Hochachka et al., 1996) could easily be linked to changes in C flux through glycolysis, a process recently implicated in altering expression of sugar-responsive genes such as Sus1 and Sh1 (Koch, 1996; Jang et al., 1997). Eventual depletion of sugars via the Pasteur effect would thus be separated in time from initial signals of sugar abundance associated with high flux. This provides one means of testing the degree to which sugar-sensing systems and Suc synthase gene responses can distinguish between supply and flux. Effects of the former could also be enhanced under anoxia relative to hypoxia by limited phloem transport (Saglio, 1985).
Temporal differences in the responses of Sus1 and Sh1 mRNA levels could perhaps mediate sequential up-regulation of these genes during low-O2 events. Hypoxia typically precedes anoxia during natural progressions of severe O2 depletion, and hypoxia alone may predominate during less extreme deprivation. Results are also consistent with the contrasts in metabolic states and acclimation mechanisms associated with hypoxia versus anoxia (Drew, 1997).
O2 and Sugar Availability Exert Similar Patterns of Differential Expression
Differential expression of the Sus1 and Sh1 Suc synthase genes may also contribute to the functional roles of isozymes that apparently have otherwise similar characteristics (Su and Preiss, 1978; Echt and Chourey, 1985) and phosphorylation sites (Huber et al., 1996). Expression of these genes differs markedly between tissues (Heinlein and Starlinger, 1989; Rowland et al., 1989; Nolte and Koch, 1993; Nolte et al., 1995) and also in response to sugar availability (Koch et al., 1992; Koch, 1996). Extension of these distinctions to hypoxic and anoxic conditions potentially enhances our collective understanding of both mechanisms.
Of primary importance are the similar patterns of differential expression by both sugars and O2. Sus1 and Sh1 respond to “feast and famine” conditions of carbohydrate availability, respectively. The additional association between each of these and their rapid hypoxic (Sus1) or prolonged anaerobic (Sh1) responses is unlikely to be coincidental, particularly in light of the tight regulation of glycolysis by adenylates (Farrar and Williams, 1991). In addition, the most prominent mechanism for sugar signal initiation in a range of organisms is currently considered to be flux through the first step in glycolysis, hexokinase (for review, see Koch, 1996), which in turn is markedly responsive to O2 levels (Bouny and Saglio, 1996). Initial adjustments of glycolytic C flow under low O2 thus have the potential to exert signals similar to those generated by sugar abundance and, therefore, up-regulation of the sugar-enhanced Sus1 in advance of Sh1 (feast before famine). This is also consistent with the induction of other glycolytic (sucrolytic) genes under low-O2 stress. If responsive to the same sugar-signaling system, the Sh1 gene would be up-regulated as glycolytic flux began to drop and sugar depletion became severe. This convergent line of thought is consistent with the existence of distinct phases of acclimation to both sugar and O2 deficits.
Both Suc Synthases Respond to Low O2 at the Protein/Enzyme Activity Level
In each of our experiments Suc synthase activity showed a consistent and statistically significant increase in response to hypoxic conditions from as early as 3 h after the start of treatment (Fig. 3, A and B). Little or no significant change in total activity was observed in response to more severe anoxic treatments. However, changes were also observed in both SH1 and SUS1 protein abundance by western-blot analysis, particularly after longer time periods (Figs. 3C and 4B). Results suggest Suc synthase gene expression responds to anoxia and hypoxia not only at the mRNA level, but also at the protein level. Previous reports based on analysis of activity and protein at 20 to 24 h suggest that maize Suc synthase may not be fully inducible under anaerobic conditions (McElfresh and Chourey, 1988; Taliercio and Chourey, 1989). The present work concurs with the earlier result for total activity at 24 h, but comparison of wild-type and sh1-null mutant responses (Figs. 3, 4, and 6B) indicated that there were significant increases in the SH1 portion of this activity. Although increases in Sh1 mRNA levels were markedly greater under anoxia than hypoxia (Figs. 2 and 6A), further analysis revealed SH1 involvement at the enzyme level under both conditions (Fig. 6B). Data presented here also concur to some degree with earlier evidence for SUS1 as an anaerobic protein (Bailey-Serres et al., 1988), since activities consistently increased between 6 and 12 h under both hypoxia and anoxia (Fig. 6B). However, SUS1 mRNA responses and enzyme contributions to total activity were far greater under hypoxia than anoxia, and decreases in activity under anoxia surpassed transient increases (Fig. 6B).
Previous studies by others are also consistent with this analysis. Both SUS1 and SH1 proteins are strongly labeled with 35S-Met in maize root tips under low O2 (Bailey-Serres et al., 1988). One of the anaerobic proteins in maize roots (ANP 87) was also identified as SH1 by Springer et al. (1986), and was later found to include SUS1 at a similar Mr (Bailey-Serres et al., 1988). In situ examinations by Rowland et al. (1989) also indicated a significant but less strong increase in Suc synthase protein under low O2 within 1 cm of the maize root apex. Recently, studies (S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication) have shown increased incorporation of Sh1 mRNA, and to a lesser extent Sus1 mRNA, into polyribosomes under anoxia, indicating a capacity for effective translation. Furthermore, the polysome profiles for Sh1, Adh, and Adh2 under anoxia in that study were indicative of efficient translational initiation and elongation. Guglielminetti et al. (1997) demonstrated a clear rise in Suc synthase enzyme activity in maize after 3 d of low-O2 treatment. Ricard et al. (1991) reported that in rice, anaerobic stress induced both transcription and translation of Suc synthase, with increases in activity detectable after as little as 6 h of anoxic treatment. Varying degrees of similar low-O2 responses have also been shown for Suc synthase in Arabidopsis (Martin et al., 1993).
Posttranscriptional regulation is indicated by differences in the speed and magnitude of low-O2 responses at the mRNA and enzyme activity levels. The first enzyme activity responses were rapid compared with overall rises in mRNA and protein levels, and in some instances also differed in direction (Figs. 3 and 6, A and B). Posttranscriptional control of Sh1 expression has also been suggested (Chourey and Taliercio, 1994). In addition, protein-level regulation of Suc synthases is receiving increased attention (Huber et al., 1996; Zhang and Chollet, 1997). Although the influence of low O2 remains unclear, phosphorylation of SUS1 has been documented (Huber et al., 1996), shown to occur under anoxia (Shaw et al., 1994), and may have effects that extend beyond observed changes in substrate affinities (Huber et al., 1996). SH1 has similar phosphorylation sites and potential for protein-level regulation via this mechanism. Finally, translational regulation may also underlie the protein-level responses observed here, as indicated by rapid shifts in translation and profiles of ribosome loading for SUS1, SH1, and several anaerobic proteins under low O2 (Fennoy and Bailey-Serres, 1995; S.L. Fennoy, T. Nong, and J. Bailey-Serres, personal communication).
Comparative Analyses of the sh1-Null Mutant Distinguishes Individual Contributions of SUS1 and SH1 under 0 and 3% O2
In addition to the comparisons discussed above, analysis of temporal response profiles shown in Figure 6 highlights the extent to which short-and long-term responses to low O2 can differ. The most rapid adjustments (< 3 h) are evident at the activity level and differ markedly for SUS1 and SH1. Within 12 h both return to the levels observed in aerobic controls, and more sustained responses related to mRNA accumulation gain prominence. Together, these data suggest that this combination of changes results in sequential, alternating contributions to low-O2 activity, first by very rapid increases in SH1 activity (probably posttranscriptional), followed by relatively rapid up-regulation of Sus1 expression, and finally superseded by sustained up-regulation of Sh1 at the mRNA and enzyme activity level.
Closing Comments
This research resolves and extends previous studies of maize Suc synthase responses to low O2 in several key respects: (a) distinct responses to hypoxia and anoxia are shown at the gene expression level, with Sus1 and Sh1 preferentially showing rapid hypoxic and prolonged anaerobic responses, respectively; (b) responses involved not only mRNA accumulation, but also enzyme activity and protein abundance; (c) the time-course analyses shown here indicate that for Suc synthases, these changes occur in three phases, the first of which appears to involve rapid, protein-level responses (< 3 h) and to be much like a similar phase proposed to aid rapid readjustment of adenylate levels in animal systems (Hochachka et al., 1996), and the second two are mediated, respectively, by a relatively rapid up-regulation of Sus1 mRNA accumulation, followed by a slower, more sustained increase in mRNA accumulation and translation of Sh1; (d) complementary patterns of regulation are indicated at transcriptional and posttranscriptional levels; and (e) the distinctly different responses of Sus1 and Sh1 genes to hypoxia and anoxia implied markedly different sensitivities to O2 and/or sugar signals. Such differences could provide an effective mechanism for optimizing sequential contributions by different Suc synthases (and possibly other genes) to progressively greater stress during natural episodes of O2 depletion.
Finally, in a broader context, this work further defines the interface between sugar- and O2-signaling systems. Our results are consistent with the prevailing hypothesis that one key to up-regulation of some sugar-responsive genes is C flow through the first enzyme of glycolysis (hexokinase).
Footnotes
This research was supported by the National Science Foundation and the Florida Agricultural Experiment Station (journal series no. R-06193).
LITERATURE CITED
- Andrews DL, Drew MC, Johnson JR, Cobb BG. The response of maize seedlings of different ages to hypoxic and anoxic stress. Changes in induction of Adh mRNA, ADH activity, and survival of anoxia. Plant Physiol. 1994;105:53–60. doi: 10.1104/pp.105.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Kloeckner-Gruissem B, Freeling M. Genetic and molecular approaches to the study of the anaerobic responses and tissue specific gene expression in maize. Plant Cell Environ. 1988;11:351–357. [Google Scholar]
- Bouny JM, Saglio P. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chourey PS, Taliercio EW. Epistatic interaction and functional compensation between the two tissue- and cell-specific sucrose synthase genes in maize. Proc Natl Acad Sci. 1994;91:7917–7921. doi: 10.1073/pnas.91.17.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourey PS, Taliercio EW, Kane EJ. Tissue-specific expression and anaerobically induced posttranscriptional modulation of sucrose synthase genes in Sorghumbicolor M. Plant Physiol. 1991;96:485–490. doi: 10.1104/pp.96.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ES, Gerlach WL, Pryor AJ, Bennetzen JL, Inglis A, Lewellyn D, Sachs MM, Ferl RJ, Peacock WJ. Molecular analysis of the alcohol dehydrogenase 1 (Adh1) gene of maize. Nucleic Acids Res. 1984;12:3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ES, Gerlach WL, Walker JC, Lavin M, Peacock WJ. Anaerobically regulated aldolase gene of maize: a chimeric origin? J Mol Biol. 1988;202:759–767. doi: 10.1016/0022-2836(88)90556-6. [DOI] [PubMed] [Google Scholar]
- Dennis ES, Sachs MM, Gerlach WL, Finnegan EJ, Peacock WJ. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985;13:727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R, Ellis M, Bruxelles GD, Trevaskis B, Hoeren F, Dennis ES, Peacock WJ. Strategies of gene action in Arabidopsis during hypoxia. Ann Bot. 1997;79:21–31. [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Echt CS, Chourey PS. A comparison of two sucrose synthase isozymes from normal and shrunken-1 maize. Plant Physiol. 1985;79:530–536. doi: 10.1104/pp.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J, Williams JHH. Control of the rate of respiration in roots: compartmentation, demand, and the supply of substrate. In: Emms M, editor. Compartmentation of Metabolism. London: Butterworths; 1991. pp. 167–188. [Google Scholar]
- Fennoy SL, Bailey-Serres J. Post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J. 1995;7:287–295. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Guglieminetti L, Alpi A, Perata P. Shrunken-1-encoded sucrose synthase is not required for the sucrose-ethanol transition in maize under anaerobic conditions. Plant Sci. 1996;119:1–10. [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiol. 1995;108:735–741. doi: 10.1104/pp.108.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Wu Y, Boschi E, Yamaguchi J. Effects of anoxia on sucrose degrading enzymes in cereal seeds. J Plant Physiol. 1997;150:251–258. [Google Scholar]
- Hake S, Kelley PM, Taylor WC, Freeling M. Coordinate induction of alcohol dehydrogenase 1, aldolase, and other anaerobic RNAs in maize. J Biol Chem. 1985;260:5050–5054. [PubMed] [Google Scholar]
- He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol. 1996a;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996b;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein M, Starlinger P. Tissue and cell-specific expression of the two sucrose synthase isozymes in developing maize kernels. Mol Gen Genet. 1989;215:441–446. [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Huber JL, Liao PC, Gage DA, McMichael RW, Chourey PS, Hannah LC, Koch KE. Phosphorylation of serine-15 of maize leaf sucrose synthase. Plant Physiol. 1996;112:793–802. doi: 10.1104/pp.112.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Cobb BG, Drew MC. Hypoxic induction of anoxia tolerance in root tips of Zea mays. Plant Physiol. 1989;91:837–841. doi: 10.1104/pp.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Cobb BG, Drew MC. Hypoxic induction of anoxia tolerance in root tips of Adh1-null Zea mays L. Plant Physiol. 1994;105:61–67. doi: 10.1104/pp.105.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley PM, Freeling M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J Biol Chem. 1984a;259:14180–14183. [PubMed] [Google Scholar]
- Kelley PM, Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J Biol Chem. 1984b;259:673–677. [PubMed] [Google Scholar]
- Kelly PM, Tolan DR. The complete amino acid sequence of the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 1986;100:1–6. doi: 10.1104/pp.82.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koch KE, Nolte KD, Duke ER, McCarty DR, Avigne WT. Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell. 1992;4:59–69. doi: 10.1105/tpc.4.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal SK, Johnson S, Conway T, Kelley PM. Characterization of a maize cDNA that complements an enolase-deficient mutant of Escherichia coli. Plant Mol Biol. 1991;16:787–795. doi: 10.1007/BF00015071. [DOI] [PubMed] [Google Scholar]
- Lal SK, Kelly PM, Elthon TE. Purification and differential expression of enolase from maize. Physiol Plant. 1994;91:587–592. [Google Scholar]
- Martin T, Frommer WB, Salanoubat M, Willmitzer L. Expression of an Arabidopsis sucrose synthase gene indicates a role in metabolization of sucrose both during phloem loading and in sink organs. Plant J. 1993;4:367–377. doi: 10.1046/j.1365-313x.1993.04020367.x. [DOI] [PubMed] [Google Scholar]
- McCarty DR. A rapid and simple method for extracting RNA from maize tissues. Maize Gen Coop News Lett. 1986;60:61. [Google Scholar]
- McCarty DR, Shaw JR, Hannah LC. The cloning, genetic mapping, and expression of the constitutive sucrose synthase locus of maize. Proc Natl Acad Sci USA. 1986;83:9099–9103. doi: 10.1073/pnas.83.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfresh KC, Chourey PS. Anaerobiosis induces transcription but not translation of sucrose synthase in maize. Plant Physiol. 1988;87:542–546. doi: 10.1104/pp.87.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte KD, Hendrix DL, Radin JW, Koch KD. Sucrose synthase localization during initiation of seed development and trichome differentiation in cotton ovules. Plant Physiol. 1995;109:1285–1293. doi: 10.1104/pp.109.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte KD, Koch KE. Companion-cell-specific localization of sucrose synthase in zones of phloem loading and unloading. Plant Physiol. 1993;101:899–905. doi: 10.1104/pp.101.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A-L, Ferl RJ. Adh1 and Adh2 regulation. Maydica. 1991a;36:129–134. [Google Scholar]
- Paul A-L, Ferl RJ. In vivo footprinting reveals unique cis-elements and different modes of hypoxic induction in maize Adh1 and Adh2. Plant Cell. 1991b;3:159–168. doi: 10.1105/tpc.3.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perata P, Alpi A. Plant responses to anaerobiosis. Plant Sci. 1993;93:1–17. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Mobilization of endosperm reserves in cereal seeds under anoxia. Ann Bot. 1997;79:49–56. [Google Scholar]
- Peschke VM, Sachs MM. Multiple maize pyruvate decarboxylase genes are induced by hypoxia. Mol Gen Genet. 1993;240:206–212. doi: 10.1007/BF00277058. [DOI] [PubMed] [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiol. 1991;95:669–674. doi: 10.1104/pp.95.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoal J, Thind S, Pradet, Richard B. Differential induction of pyruvate decarboxylase subunits and transcripts in anoxic rice seedlings. Plant Physiol. 1997;114:1021–1029. doi: 10.1104/pp.114.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LJ, Chen YC, Chourey PS. Anaerobic treatment alters the cell specific expression of Adh-1, Sh, and Sus1 genes in roots of maize seedlings. Mol Gen Genet. 1989;218:33–40. [Google Scholar]
- Russell DA, Sachs MM. Differential expression and sequence analysis of the maize glyceraldehyde-3-phosphate dehydrogenase gene family. Plant Cell. 1989;1:793–803. doi: 10.1105/tpc.1.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IN. Anaerobic gene expression and flooding tolerance in maize. J Exp Bot. 1996;47:1–15. [Google Scholar]
- Saglio PH. Effect of path or sink anoxia on sugar translocation in roots of maize seedlings. Plant Physiol. 1985;77:285–290. doi: 10.1104/pp.77.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta S. Physiology and genetics of submergence tolerance in rice. Ann Bot. 1997;79:67–77. [Google Scholar]
- Shaw JR, Ferl RJ, Baier J, St Clair D, Carson C, McCarty DR, Hannah LC. Structural features of the maize Sus1 gene and protein. Plant Physiol. 1994;106:1659–1665. doi: 10.1104/pp.106.4.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B, Werr W, Starlinger P, Bennett CD, Zokolica M, Freeling M. The shrunken gene on chromosome 9 of Zea mays L. is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986;205:461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Su J-C, Preiss J. Purification and properties of sucrose synthase from maize kernels. Plant Physiol. 1978;61:389–393. doi: 10.1104/pp.61.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliercio EW, Chourey PS. Post-transcriptional control of sucrose synthase expression in anaerobic seedlings of maize. Plant Physiol. 1989;90:1359–1364. doi: 10.1104/pp.90.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot (Suppl. A) 1997;79:3–20. [Google Scholar]
- Zhang XQ, Chollet R. Seryl-phosphorylation of soybean nodule sucrose synthase (nodulin-100) by a Ca+2-dependent protein kinase. FEBS Lett. 1997;410:126–130. doi: 10.1016/s0014-5793(97)00537-1. [DOI] [PubMed] [Google Scholar]