Abstract
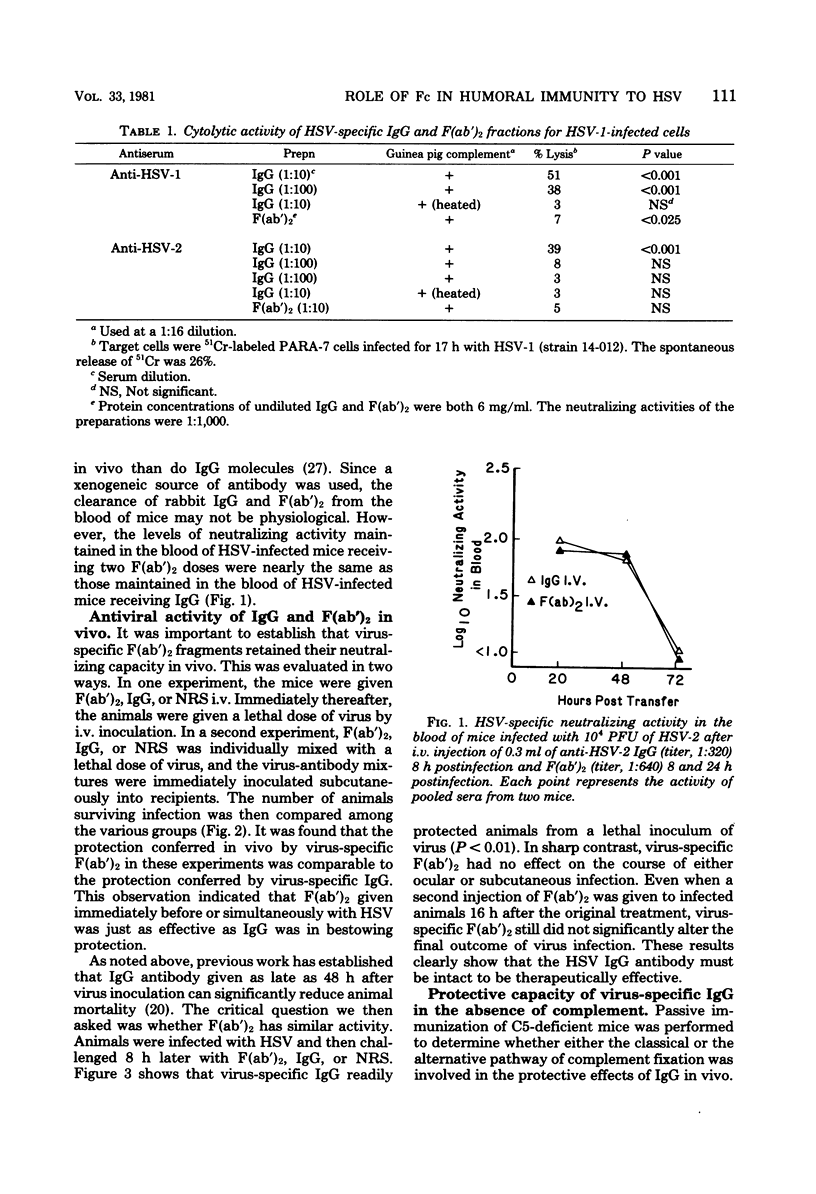
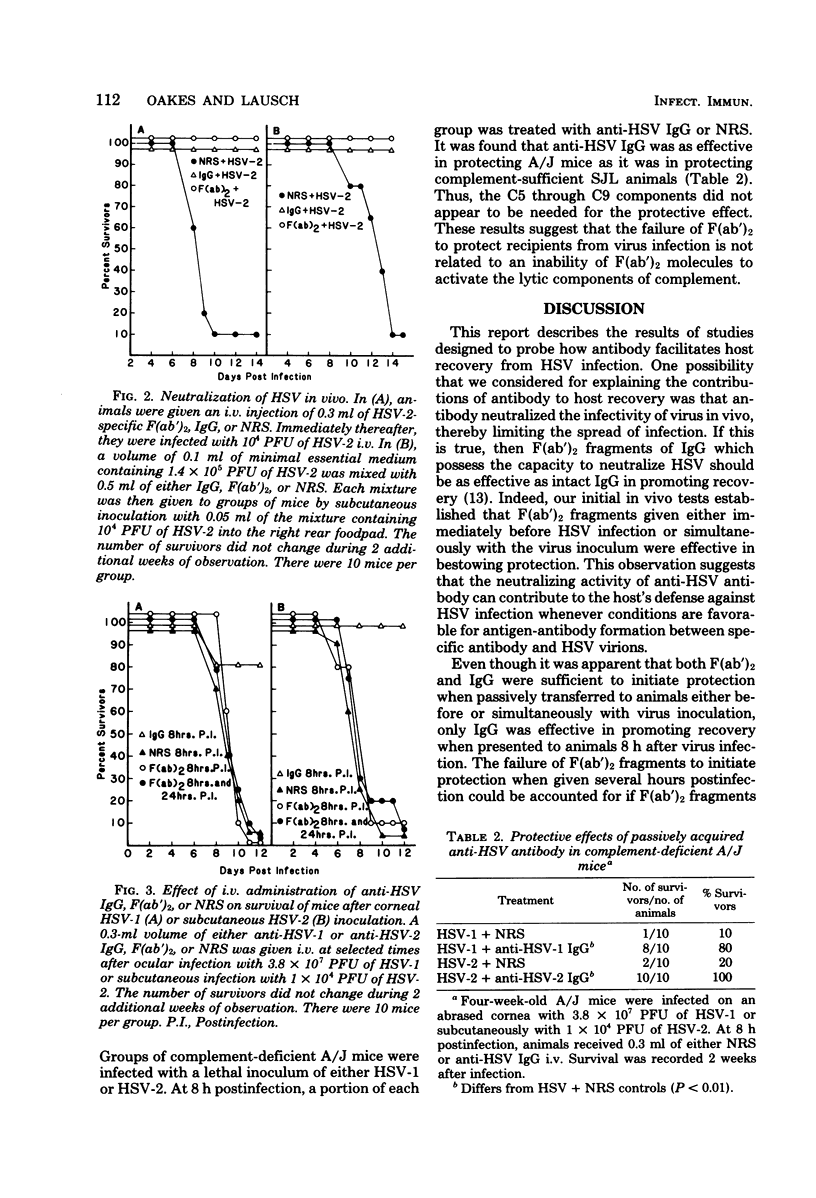
The contributions of the Fc fragment of virus-specific antibody in the resistance of mice to peripheral herpes simplex virus infection were investigated. Rabbit anti-herpes simplex virus-specific F(ab')2 fragments prepared by pepsin digestion of immune immunoglobulin G (IgG) were found to be inactive in complement-mediated cytolysis while retaining their capacity to neutralize virus infectivity in vitro. When F(ab')2 fragments were passively transferred either before or simultaneously with virus inoculation, they were as efficient as intact IgG was in protecting animals from virus challenge. However, if passive transfer was delayed until 8 h after herpes simplex virus infection, only IgG antibody was protective. The loss of protective activity could not be attributed to a rapid disappearance of F(ab')2 fragments, because comparable levels of F(ab')2 fragments and IgG antibody were maintained in the blood of recipients during the time that antibody mediated its protective effects. The inability of F(ab')2 subunits to activate complement was also not a factor, because complement-deficient A/J mice and complement-sufficient SJL/J mice recovered from herpes simplex virus infection after the passive transfer of IgG. We concluded that the Fc component of the antibody molecule is needed to resolve intracellular infection and that the mechanism by which antibody mediates recovery remains undefined but does not appear to involve virus neutralization or complement activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., Taylor J. A., Oakes J. E. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J Infect Dis. 1979 Oct;140(4):534–540. doi: 10.1093/infdis/140.4.534. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. I. VIRUS PATHWAYS TO THE NERVOUS SYSTEM OF SUCKLING MICE DEMONSTRATED BY FLUORESCENT ANTIBODY STAINING. J Exp Med. 1964 Feb 1;119:343–356. doi: 10.1084/jem.119.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Cahall D. L., Walters D. L., Schaffner V. E. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J Immunol. 1979 Jul;123(1):25–30. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsson A., Perlmann P. Study of Fab and F(ab') 2 from rabbit IgG for capacity to induce lymphocyte-mediated target cell destruction in vitro. Int Arch Allergy Appl Immunol. 1972;43(1):80–88. doi: 10.1159/000230823. [DOI] [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall R. R., Klassen T., Baringer J. R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979 Feb;23(2):305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melewicz F. M., Shore S. L., Ades E. W., Phillips D. J. The mononuclear cell in human blood which mediates antibody-dependent cellular cytotoxicity to virus-infected target cells. II. Identification as a K cell. J Immunol. 1977 Feb;118(2):567–573. [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Davis W. B., Taylor J. A., Weppner W. A. Lymphocyte reactivity contributes to protection conferred by specific antibody passively transferred to herpes simplex virus-infected mice. Infect Immun. 1980 Aug;29(2):642–649. doi: 10.1128/iai.29.2.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E. Invasion of the central nervous system by herpes simplex virus type 1 after subcutaneous inoculation of immunosuppressed mice. J Infect Dis. 1975 Jan;131(1):51–57. doi: 10.1093/infdis/131.1.51. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. The proteins specified by herpes simplex virus. I. Time of synthesis, transfer into nuclei, and properties of proteins made in productively infected cells. Virology. 1968 Dec;36(4):545–555. doi: 10.1016/0042-6822(68)90186-4. [DOI] [PubMed] [Google Scholar]
- Worthington M., Conliffe M. A., Baron S. Mechanism of recovery from systemic herpes simplex virus infection. I. Comparative effectiveness of antibody and reconstitution of immune spleen cells on immunosuppressed mice. J Infect Dis. 1980 Aug;142(2):163–174. doi: 10.1093/infdis/142.2.163. [DOI] [PubMed] [Google Scholar]



