Abstract
It is not easy to induce cytotoxic T lymphocytes (CTLs) against cancer in in vitro culture. Regulatory T cells (Tregs) are considered to play a pivotal role in tumor immune escape. In this study, we analyzed the distribution of Tregs among tumor-infiltrating lymphocytes (TILs), regional lymph node lymphocytes (RLNLs) and peripheral blood lymphocytes (PBLs) in patients with lung cancer, and analyzed the effect of Tregs on the induction of CTLs in vitro. A total of 84 patients with non-small cell lung cancer underwent surgery between January 2003 and December 2004. The TILs, RLNLs and PBLs from these patients were subjected to a comparison analysis. The proportion of CD4+CD25+Foxp3+ cells in these lymphocytes was determined by flow cytometry. The effects of Tregs on the induction of CTLs was analyzed by the depletion of Tregs in mixed lymphocyte-tumor cell culture (MLTC). The average proportions of Tregs in the TILs, RLNLs and PBLs were 10.4±9.5, 4.4±2.4 and 2.8±2.1%, respectively. The proportion of Tregs in the RLNLs was significantly higher than that in the PBLs (P<0.001); furthermore, TILs contained a larger number of Tregs than RLNLs (P=0.034). These Tregs substantially suppressed the induction of CTLs against autologous tumor cells. The depletion of Tregs in the MLTC resulted in the successful induction of CTLs. Tregs were found at a higher frequency in the TILs and RLNLs than in the PBLs in lung cancer patients. Since Tregs inhibited the induction of CTLs, the depletion of Tregs may represent a new therapeutic strategy for lung cancer patients.
Keywords: non-small cell lung cancer, regulatory T cells, cytotoxic T lymphocytes, immune escape mechanism, regional lymph node lymphocytes
Introduction
Lung cancer is the most common malignant neoplasm and the leading cause of cancer mortality in industrialized countries (1). Although there have been advances in the diagnostic and therapeutic approaches against lung cancer, limited levels of improvement in the treatment outcome have been accomplished. Recent clinical studies on immunotherapy indicated favorable therapeutic effects, and suggested that such a strategy might represent an alternative treatment approach for lung cancer (2,3). A large number of tumor-associated antigens have been identified in various human cancers (4–6). A high level of CD8 T cell infiltration into tumor tissues has been reported to be associated with a better prognosis in colon carcinoma and in ovarian cancer, suggesting that antitumor immunity can be provoked in cancer patients (7,8). A large number of antigen-based immunotherapy studies have been conducted; however, Rosenberg et al reported that the overall response rate for patients with cancer (mainly melanoma) to vaccines was as low as 2.6% (9). Such low response rates may be associated with an immunological escape mechanism.
Overcoming these tumor immunological escape mechanisms is considered to be necessary to develop effective immunotherapy using tumor-associated antigens. Regulatory T cells (Tregs) play a pivotal role in various escape mechanisms, and it is necessary to suppress the effects of Tregs to provide efficient cancer immunotherapy. It has been shown that Tregs constitutively express high levels of the interleukin 2 receptor chain (CD25) and specifically express the forkhead/winged helix transcription factor (Foxp3), which inhibits the activation of both self-antigen and foreign-antigen reactive T cells (10,11). Tregs are known to have a critical physiological role in the suppression of autoimmune diseases (12,13). However, Tregs also play a critical role in suppressing antitumor immune responses, since most tumor-associated antigens are self-antigens.
The immunological implications of the population of Tregs in the local region of the tumor and regional lymph nodes have not been fully investigated. Therefore, the aim of the present study was to evaluate the frequency of CD4+CD25+Foxp3+ T cells in the tumor-infiltrating lymphocytes (TILs), regional lymph node lymphocytes (RLNLs) and peripheral blood lymphocytes (PBLs) of non-small cell lung cancer (NSCLC) patients, and to determine their influence on the induction of cytotoxic T lymphocytes (CTLs) against autologous tumor cells.
Materials and methods
Patients
The study protocol was approved by the Human and Animal Ethics Review Committee of the University of Occupational and Environmental Health, Japan, and a signed consent form was obtained from each patient before collecting the tissue samples used in this study. Between January 2003 and December 2004, 153 patients with NSCLC underwent surgery at the University of Occupational and Environmental Health. Of these, 84 patients were enrolled in this study, and their RLNLs and PBLs were collected at the time of surgery and stored at −80°C until they were analyzed. The patients' records, including their clinical data, preoperative examination results, details of surgery, histopathological findings and TNM staging were also reviewed. The characteristics of the patients are shown in Table I. The preoperative assessments included chest roentgenography, computed tomography (CT) of the chest and upper abdomen, magnetic resonance imaging (MRI) of the brain, bronchoscopy and bone scintigraphy. All resected specimens, including the primary tumor and the systematically dissected hilar and mediastinal lymph nodes, were examined pathologically to identify the extent of lymph node metastases. The histopathological findings were classified according to the World Health Organization criteria, and the TNM staging system of the International Union Against Cancer (UICC) was employed (14,15).
Table I.
Characteristics of the patients with non-small cell lung cancer.
| Characteristic | No. of patients |
|---|---|
| Age (years) | 67.8 (44–88) |
| Gender | |
| Male | 54 |
| Female | 30 |
| Histology | |
| Adenocarcinoma | 59 |
| Squamous cell carcinoma | 14 |
| Other | 11 |
| pStage | |
| IA | 38 |
| IB | 18 |
| IIA | 1 |
| IIB | 9 |
| IIIA | 10 |
| IIIB | 5 |
| IV | 3 |
pStage, pathological stage.
Antibodies and flow cytometric analysis to detect CD4, CD25 and Foxp3
Phycoerythrin-Cy5 (PE-Cy5)-conjugated anti-human CD4 (RPA-T4) and a mouse IgG2b isotype control (eBMG2b) were purchased from eBioscience (San Diego, CA, USA). A fluorescein isothiocyanate (FITC)-labeled monoclonal antibody against CD25 (2A3), IgG1 (X40) and phycoerythrin (PE)-labeled monoclonal antibody against IgG1 (X40) were purchased from BD Bioscience (San Jose, CA, USA). The PE-labeled monoclonal antibody to Foxp3 (259D) was purchased from BioLegend (San Diego, CA, USA). A FITC-labeled mouse IgG1 or PE-labeled mouse IgG1 was used as an isotype-matched control. To analyze the intracytoplasmic Foxp3 expression, a human regulatory T cell staining kit was purchased from eBioscience. The fresh and cultured PBL and RLNL cells were washed with phosphate-buffered saline (PBS; pH 7.4). Hanks' balanced salt solution containing 0.1% NaN3 and 1% fetal calf serum (FCS) was used as the staining buffer. Following incubation for 30 min with a monoclonal antibody against Foxp3 (259D) or isotype-matched controls, 1×105 labeled cells in each sample were analyzed on a FACSCalibur instrument (BD Bioscience).
In vitro system to induce CTLs against autologous tumor cells
The lung cancer cell lines, F1121L (adenocarcinoma cell line) and L1023L (squamous cell carcinoma cell line) were previously established in our department (16). The culture medium consisted of RPMI-1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated FCS (Equitech-Bio, Ingram, TX, USA), 10 mM HEPES, 100 IU/ml penicillin G and 100 mg/ml streptomycin sulfate. The RLNL cells were obtained at the time of surgery. Each lymph node was divided into two parts; one for the histological diagnosis and one for this study. Lymphocytes from each lymph node were mixed and stored at −80°C until use, as described previously (17). They were then rapidly thawed and stimulated with an irradiated (100 Gy) CD80-transfected autologous tumor cell line (F1121L and L1023L) weekly at a tumor-to-lymphocyte ratio of 1:10 in culture medium as described previously (17). The tumor-specific induction of CTLs was considered to be successful if the CTLs lysed more than 10% of the autologous tumor cells at an effector/target ratio of 30/1 and did not lyse autologous Epstein-Barr virus-transformed B cells during the 4-h standard 51Cr release assay performed on day 28 of the mixed lymphocyte-tumor cell culture (MLTC).
The tumor cells (F1121L and L1023L) were plated at 5×105 cells/25 cm2 flask (Falcon; Becton Dickinson, Oxnard, CA, USA) in culture medium, and were incubated at 37°C with 5% CO2. At 24 h after plating, almost all of the tumor cells were adherent to the bottom of the flask, and the culture medium was completely recovered, centrifuged to remove cellular debris, and then frozen at −80°C until the measurement of TGF-β was performed. The level of TGF-β in the supernatant was measured using an ELISA kit (Amersham Lifescience, Braunschweig, Germany).
Statistical analysis
The Mann-Whitney U test was used to determine the differences in the continuous variables between the two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
CD4+CD25+Foxp3+ Tregs are more common in the primary tumor and regional lymph nodes than in the peripheral blood
The RLNLs and PBLs of the 84 patients with lung cancer were analyzed. The Foxp3+/CD4+ cell ratios in the RLNLs and PBLs were 4.4±2.4 and 2.8±2.1%, respectively. TILs could be analyzed in 9 cases, and the Foxp3+ cell/CD4+ cell ratio was 10.4±9.5%. The frequency of Tregs in the RLNLs was significantly higher than that in the PBL, and the frequency in the TILs was higher still (Fig. 1). The frequencies of Tregs did not correlate with clinicopathological factors such as gender, histology or pathological stage (Table II).
Figure 1.
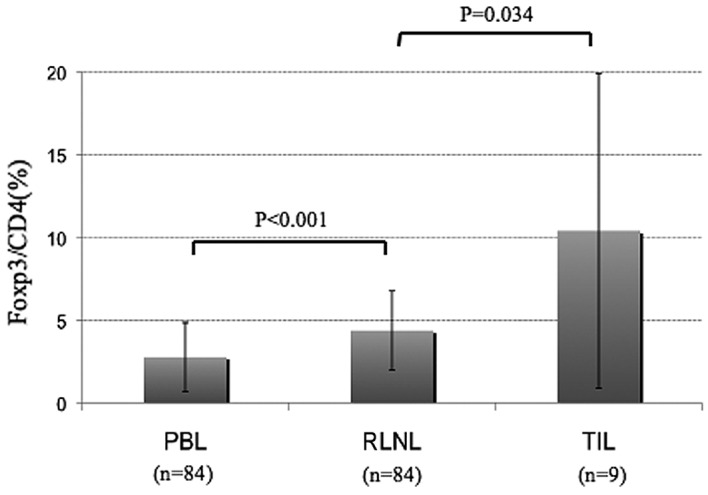
Average ratios of Foxp3+ cells/CD4+ cells in the PBLs, RLNLs and TILs in patients with non-small cell lung cancer. The average ratios of Foxp3+ cells/CD4+ cells in the PBLs, RLNLs and TILs were 2.8, 4.4 and 10.4%, respectively. The number of Tregs in the RLNLs was significantly higher than that in the PBLs, and the number of Tregs in the TILs was significantly higher than that in the RLNLs. PBL, peripheral blood lymphocyte; RLNL, regional lymph node lymphocyte; TIL, tumor-infiltrating lymphocyte.
Table II.
Frequency of Foxp3+ cells/CD4+ cells in the lymph nodes and peripheral blood in patients with non-small cell lung cancer.
| Characteristic | n | RLNL (%) | PBL (%) |
|---|---|---|---|
| Gender | |||
| Male | 54 | 4.5±2.5 | 2.9±2.1 |
| Female | 30 | 4.1±2.3 | 2.5±2.5 |
| Histology | |||
| Adenocarcinoma | 59 | 4.4±2.2 | 2.7±2.2 |
| Squamous cell carcinoma | 14 | 4.8±2.8 | 2.3±1.7 |
| Other | 11 | 3.9±2.7 | 3.7±1.7 |
| pStage | |||
| I | 56 | 4.6±2.6 | 2.6±2.0 |
| II–IV | 28 | 4.0±1.9 | 3.2±2.6 |
| pN | |||
| 0 | 61 | 4.6±2.5 | 2.6±2.0 |
| 1–3 | 23 | 3.8±1.9 | 3.2±2.3 |
RLNL, regional lymph node lymphocyte; PBL, peripheral blood lymphocyte.
Depletion of Tregs improves the efficiency of CTL induction
The induction of CTLs against L1023L could not be achieved from the RLNLs of patient L1023. In order to evaluate the effects of Tregs on the induction of CTLs, autologous MLTC was performed in the presence or absence of CD4+CD25+ T cells. The CD4+CD25+ T cells were isolated from RLNLs using the AutoMACS magnetic separation system with a human CD4+CD25+ isolation kit (Miltenyi Biotec, Auburn, CA, USA). Following depletion of the CD4+CD25+ T cells, the RLNLs (9×106 cells) were seeded into 9 wells (1×106/well in 2 ml culture medium). The 9 wells were used for 3 experimental groups as follows: no addition of CD4+CD25+ T cells, addition of 1×104 (1%) CD4+CD25+ T cells, and addition of 5×104 (5%) CD4+CD25+ T cells. The induction of tumor-specific CTLs was analyzed by a 51Cr release assay on day 28 of the MLTC.
The control group (Treg-depleted group), 1% Treg-added group and 5% Treg-added group were subjected to MLTC for 4 weeks. After 4 rounds of stimulation with cancer cells, the Tregs were analyzed in each group by flow cytometry. Although the Tregs were maintained for 4 weeks after MLTC in each group, the proportion of Tregs was higher in the 5% Treg-added group than in the Treg-depleted control group (Fig. 2A). The mean fluorescence intensity of Foxp3 in the CD4+CD25+ T cells was also higher in the 5% Treg-added group than in the Treg-depleted group (Fig. 2B). The CTL activity in each group (depletion, 1% and 5% Treg addition), was assessed by the cytolytic activity against the autologous tumor cell line, L1023L. One of 3 independent wells in the Treg-depleted group showed the successful development of CTL activity against the autologous tumor (L1023L) cells. In contrast, the addition of 1 and 5% Tregs completely inhibited the induction of CTL activity (Fig. 3).
Figure 2.

Proportion of residual Tregs 4 weeks after MLTC. Before MLTC, the Tregs were depleted using a human CD4+CD25+ isolation kit. Following Treg depletion, a control group (Treg-depleted group), 1% Treg-added group and 5% Treg-added group were subjected to MLTC for 4 weeks. At 4 weeks after MLTC, the populations of Tregs were analyzed by flow cytometry with a FACSCalibur instrument. (A) The proportion of Foxp3+/CD4+ cells is shown. (B) The relative ratio of the mean fluorescence intensity of Foxp3+CD4+CD25+/Foxp3+CD4+CD25− is shown. The Tregs remained for at least 4 weeks according to the initial proportion added following MLTC in each group. Tregs, regulatory T cells; MLTC, mixed lymphocyte-tumor cell culture; FACS, fluorescence-activated cell sorting.
Figure 3.
Effect of the deletion of Tregs on the induction of CTLs. The CTL activity in the Treg-depleted group, 1% Treg-added group and 5% Treg-added group was assessed based on the cytolytic activity against the autologous tumor cell line, L1023L, 4 weeks after MLTC. The CTL activity against L1023L cells was successfully induced by autologous RLNLs in the Treg-depleted group. By contrast, the addition of Tregs at 1 and 5% following depletion completely inhibited the induction of CTL activity. Tregs, regulatory T cells; CTL, cytotoxic T lymphocyte; E/T ratio; effector cells/target cell ratio; MLTC, mixed lymphocyte-tumor cell culture; RLNL, regional lymph node lymphocyte.
Addition of TGF-β inhibits CTL induction and increases the number of regulatory T cells
In order to evaluate the inhibitory effect of TGF-β on the induction of CTLs, autologous RLNLs were subjected to MLTC in the absence or presence of 100 or 400 pg/ml TGF-β (R&D Systems, Minneapolis, MN, USA). During the MLTC, various amounts of TGF-β (0, 100 or 400 pg/ml) were added every day as described previously (18). In both of the groups with added TGF-β, the induction of CTLs was inhibited. However, in the group cultured without TGF-β, CTL induction was achieved in 2 out of 3 wells, as described previously (18). In the same experiment, we analyzed the proportion of Tregs 28 days after MLTC, and the proportion of CD4+CD25+Foxp3+ Tregs in each sample was analyzed using a FACSCalibur instrument. A higher Treg population was observed in the groups treated with TGF-β than in the group without treatment (Table III).
Table III.
Increase of Treg population following addition of TGF-β during mixed lymphocyte-tumor cell culture.
| TGF-β (pg/ml) | CD4+CD25+/CD4+ (%) | Foxp3+/CD4+ (%) | MFI ratioa |
|---|---|---|---|
| 0 | 19.89±1.24 | 4.59±0.89 | 1.28±0.12 |
| 100 | 28.22±5.97 | 5.83±1.79 | 1.38±0.19 |
| 400 | 24.22±14.0 | 7.19±1.26 | 1.67±0.11 |
The relative ratio of the mean fluorescence intensity of Foxp3+CD4+CD25+/Foxp3+CD4+CD25− cells.
Amount of TGF-β in the supernatant after 4 weeks of MLTC
The level of TGF-β in the supernatant of cells treated with the autologous tumor cells (L1023L) alone was 115 pg/ml. In the 1% Treg-added group, the TGF-β level in the supernatant of the culture medium after MLTC was 173 pg/ml, whereas the TGF-β level in the supernatant of the culture medium after MLTC was 301 pg/ml in the 5% Treg-added group. The level of TGF-β in the supernatant was higher in the 1 and 5% Treg-added group than in the Treg-depleted group (Fig. 4).
Figure 4.
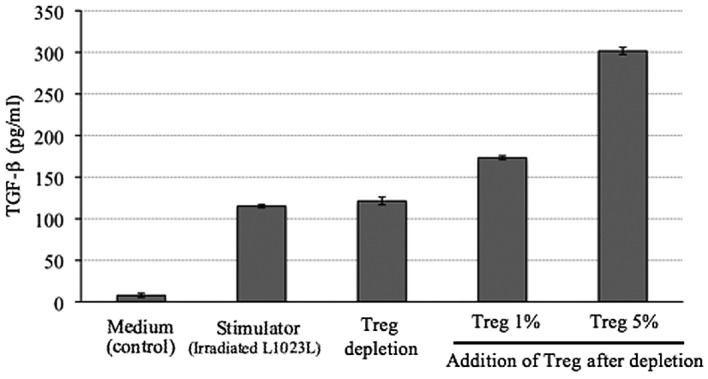
Level of TGF-β in the supernatants 4 weeks after MLTC. The TGF-β levels in the supernatants 4 weeks after MLTC were analyzed by ELISA. In both the 1 and 5% Treg-added group, the TGF-β level in the supernatant was higher than that in the Treg-depleted group. The TGF-β level in the supernatant in the autologous tumor cell culture (L1023L) was 115 pg/ml. Given this background for the autologous tumor, it was determined that the high level of TGF-β in the supernatant in the Treg-added groups was derived from the autologous Tregs. Treg, regulatory T cell. MLTC, mixed lymphocyte-tumor cell culture; ELISA, enzyme-linked immunosorbent assay.
Discussion
The accumulation of CD4+CD25+ immunosuppressive Tregs in tumors has been reported in various types of cancer (19–26). Tregs are known to be a key contributor to the maintenance of immune tolerance, preventing the emergence of organ-specific autoimmune diseases (19). These cells constitute 2–3% of the CD4+ T cells in the peripheral blood (20). Patients with various types of cancer exhibited higher numbers of Tregs in their peripheral blood than healthy donors (21), and high numbers of tumor-infiltrating Tregs have been reported in patients with hepatocellular, lung, ovarian, gastric, esophageal and breast cancer (22–25). Emerging evidence suggests that Tregs have a major effect on suppressing antigen-specific tumor immunity (24). Furthermore, three recent studies showed that the amount of tumor-infiltrating Tregs influenced the prognosis of ovarian and breast cancer, as well as gastrointestinal stromal tumors (22–26). Our present study showed that Tregs were present at a higher frequency in the TILs and RLNLs compared with the PBLs in patients with lung cancer.
There have been few investigations regarding the immunological role of Tregs in the RNLN of NSCLC patients. However, Tregs were considered to contribute to the suppression of antitumor immunity by inhibiting the induction of CTLs in the local region of the tumor and regional lymph nodes. In addition, Petersen et al reported that stage I NSCLC patients with a higher proportion of Tregs among their TILs had a significantly higher risk of recurrence (27).
We have previously reported that TGF-β production varied among lung cancer cell lines, and that the induction of CTLs tended to fail against tumor cell lines with a high level of TGF-β production (18). In that study, as a higher production of TGF-β in the tumor cells was noted, a lower induction of CTLs was observed. TGF-β was demonstrated to be necessary to maintain Tregs in in vitro culture (28). In the present study, the TGF-β levels in the supernatant of the culture medium following MLTC in the 1 and 5% Treg-added groups were higher than those in the Treg-depleted group. Taking into account the background level of TGF-β derived from the autologous tumor, the high level of TGF-β in the supernatant in the Treg-added groups was considered to be ascribable to its production from autologous Tregs. Furthermore, the efficiency of CTL induction was increased by the depletion of Tregs in MLTC.
Wieczorek et al reported that the proportion of Tregs is significantly increased in the peripheral blood of patients with interleukin 2-treated melanoma and in the tumor tissue of patients with lung and colon carcinomas (29). Conversely, they showed that immunosuppressive therapy, including the use of therapeutic antibodies against CD25 (IL-2Ra), leads to a significant reduction of Tregs from the peripheral blood of transplantation patients. In addition, the Treg numbers are elevated in the peripheral blood of patients with various solid tumors. Foxp3 is currently the only marker that is exclusively expressed in Tregs. However, Foxp3 is an intranuclear molecule, and therefore cannot be depleted using a monoclonal antibody. Further studies on the mechanism(s) of action for Foxp3 may identify molecular targets that could be useful to specifically suppress the function of Tregs.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is a co-inhibitory molecule expressed by Tregs (30–33). The CTLA-4 in Tregs plays a significant role in maintaining immune homeostasis by downregulating T cell signaling to inhibit the CD28-B7 co-stimulatory pathway, limiting T cell responses and contributing to the tolerance to self-antigens (34,35). A blockade of CTLA-4 signaling has been shown to augment tumor-specific T cell responses, and to induce tumor rejection in a number of animal models (36–38). Two monoclonal antibodies against human CTLA-4 were found to elicit objective and durable tumor responses in clinical trials (39–43). However, anti-CTLA-4 therapy had a major drawback, as adverse effects such as autoimmune enterocolitis and depigmentation occurred. These problems may be resolved by better dose control of the anti-CTLA-4 antibody and the design of an efficient drug delivery system for the tumor environment.
Yamaguchi et al reported that natural Tregs constitutively express high amounts of the folate receptor 4 (FR4), a subtype of the receptor for the vitamin folic acid, and this high expression of FR4 distinguishes them from other naïve or activated T cells (44). In addition, combinations of FR4 and CD25 may be used to distinguish between four functionally different CD4+ T cell subpopulations; i.e., natural Tregs, effector T cells, memory-like T cells and naïve T cells. The administration of an anti-FR4 monoclonal antibody specifically reduced Treg cells, provoking effective tumor immunity in tumor-bearing animals, whereas a similar treatment of normal young mice elicited autoimmune disease (44). The minimum dose required for tumor control or the development of a delivery system that specifically targets the tumor environment should be investigated in further studies.
In conclusion, the results of this study suggest that Tregs are present at a high frequency in RLNLs and TILs, and they are considered to contribute to the suppression of antitumor immunity by inhibiting the induction of CTLs in the regional lymph nodes. The TGF-β production of Tregs was associated with the suppression of CTL induction, and the depletion of Tregs could result in a more efficient induction of CTLs.
Acknowledgments
This study was supported in part by a UOEH Research Grant for the Promotion of Occupational Health and a Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Yukari Oshibuchi, Misako Fukumoto and Aya Katayama for their expert technical assistance.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007;13:4652–4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 3.Vansteenkiste J, Zielinski M, Linder A, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled Phase II study to assess the efficacy of MAGE-A3 immuno-therapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;(Suppl 25):398s. [Google Scholar]
- 4.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 5.Ichiki Y, Takenoyama M, Mizukami M, et al. Simultaneous cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol. 2004;172:4844–4850. doi: 10.4049/jimmunol.172.8.4844. [DOI] [PubMed] [Google Scholar]
- 6.Takenoyama M, Baurain JF, Yasuda M, et al. A point mutation in the NFYC gene generates an antigenic peptide recognized by autologous cytolytyic T lymphocytes on a human squamous cell lung carcinoma. Int J Cancer. 2006;118:1992–1997. doi: 10.1002/ijc.21594. [DOI] [PubMed] [Google Scholar]
- 7.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+) cd4(+) t regulatory cells suppress naïve and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:41–46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, non proliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read S, Powrie F. CD4(+) regulatory T cells. Curr Opin Immunol. 2001;13:644–649. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs AR, Thunnissen FB. Histological typing of lung and pleural tumours: third edition. J Clin Pathol. 2001;54:498–499. doi: 10.1136/jcp.54.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 16.Sugaya M, Takenoyama M, Osaki T, et al. Establishment of 15 cancer cell lines from patients with lung cancer and the potential tools for immunotherapy. Chest. 2002;122:282–288. doi: 10.1378/chest.122.1.282. [DOI] [PubMed] [Google Scholar]
- 17.Takenoyama M, Yoshino I, Eifuku R, et al. Successful induction of tumor-specific cytotoxic T lymphocytes from patients with non-small cell lung cancer using CD80-transfected autologous tumor cells. Jpn J Cancer Res. 2001;92:309–315. doi: 10.1111/j.1349-7006.2001.tb01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuyama T, Ichiki Y, Yamada S, et al. Cytokine production of lung cancer cell lines: correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci. 2007;98:1048–1054. doi: 10.1111/j.1349-7006.2007.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and auto-immune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 22.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 23.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 24.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 25.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 26.Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-h-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 28.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta 1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieczorek G, Asemissen A, Model F, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 30.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 34.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: A negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 36.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 37.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Elsas A, Sutmuller RP, Hurwitz AA, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12:873–883. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]



