Abstract
Photodynamic therapy (PDT) employs the triple combination of photosensitizers, visible light and ambient oxygen. When PDT is used for cancer, it has been observed that both arms of the host immune system (innate and adaptive) are activated. When PDT is used for infectious disease, however, it has been assumed that the direct antimicrobial PDT effect dominates. Murine arthritis caused by methicillin-resistant Staphylococcus aureus in the knee failed to respond to PDT with intravenously injected Photofrin®. PDT with intra-articular Photofrin produced a biphasic dose response that killed bacteria without destroying host neutrophils. Methylene blue was the optimum photosensitizer to kill bacteria while preserving neutrophils. We used bioluminescence imaging to noninvasively monitor murine bacterial arthritis and found that PDT with intra-articular methylene blue was not only effective, but when used before infection, could protect the mice against a subsequent bacterial challenge. The data emphasize the importance of considering the host immune response in PDT for infectious disease.
Keywords: bacterial arthritis, bioluminescence imaging, methicillin-resistant Staphylococcus aureus, methylene blue, neutrophils, photodynamic therapy, Photofrin®, preventative PDT
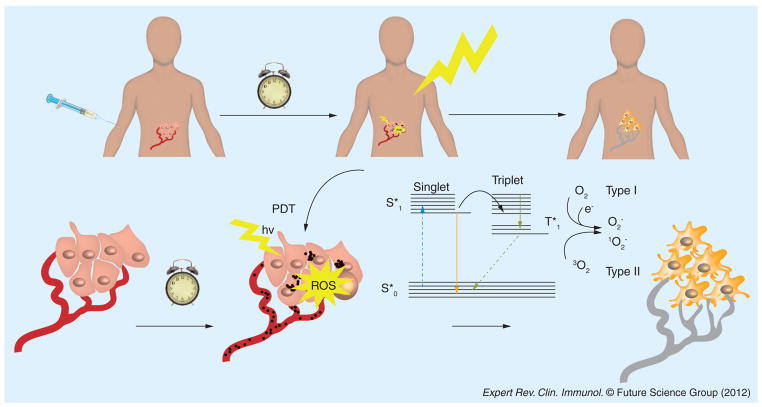
Photodynamic therapy (PDT) was discovered over 110 years ago by accidental observation of its antimicrobial effects [1]. Paramecia (a unicellular protozoan) were killed in the presence of a dye, acridine orange, only when exposed to strong daylight. In the intervening years since then, PDT has been investigated mainly as a cancer therapy and has found applications in ophthalmology and dermatology. PDT consists of three components: the dye or photosensitizer (PS), the light of the correct wavelength to be absorbed by the PS and ambient molecular oxygen [2]. For a reason not entirely understood, hyperproliferating cells (responsible for the three diseases listed above) have a preferential uptake of PS. After PS localization occurs, light excitation of the PS yields an electronically excited PS that can give a long-lived triplet state. The PS triplet can then react subsequently with molecular oxygen (O2) via energy transfer to produce singlet oxygen (1O2), a highly reactive and transient molecular species [3]. Additional reactions of the PS with oxygen caused by electron transfer from reactive oxygen species (ROS) includes: hydroxyl radicals, superoxide and hydrogen peroxide, where hydrogen peroxide may participate in the self-perpetuating Fenton reaction, yielding more hydroxyl radicals [4]. These ROS have been shown to destroy tumors by three distinct mechanisms (Figure 1): direct cytotoxicity towards tumor cells, causing apoptosis and necrosis; destruction of tumor capillaries and microvasculature mediated by PDT damage towards endothelial cells; and an acute inflammatory response that can activate host defense mechanisms involving neutrophils and dendritic cells (DCs).
Figure 1. Mechanisms of photodynamic therapy for cancer treatment.
The PS is usually injected intravenously followed after a suitable interval by illumination of the tumor with light of a suitable wavelength. The excited PS transitions to a long-lived triplet state that interacts with oxygen to form superoxide and hydroxyl radicals in a Type I process or to form singlet oxygen in a Type II process. These ROS produce direct killing of tumor cells by necrosis and apoptosis and shut down tumor blood vessels.
hv: Light; PDT: Photodynamic therapy; PS: Photosensitizer; ROS: Reactive oxygen species; S*: Singlet state.
In recent years PDT has been studied as an alternative antimicrobial strategy motivated by the worldwide increase in antibiotic resistance [5,6]. PSs have been designed in such a way that they will preferentially bind to microbial cells compared with host mammalian cells [7]. Here the idea is to locally introduce the PS into the infected area, followed after a short time by illumination of the infected tissue that will lead to killing of the microbes without unacceptable tissue damage. PDT has been applied to or proposed for many types of localized infections such as burns, wounds, abscesses, fungal infections of the skin or nails, periodontitis, endodontics, caries, sinusitis, otitis media, stomach infections, and so on [8,9]. The usual outcome measures are reduction of the number of colony-forming units (CFUs) in the infection and improved healing of the lesion (if applicable).
There does not seem to have been any connection made so far between the immune stimulating effects of PDT that have been widely studied in cancer models [10–12] and the antimicrobial effects of PDT when used as an infection treatment. This is somewhat surprising in light of the fact that the primary purpose of the immune system is to combat invasion by pathogens. We have discovered that in a mouse model of bacterial arthritis, PDT can not only produce a therapeutic effect when carried out after infection, but can also produce a protective effect if carried out before infection.
PDT & immune response
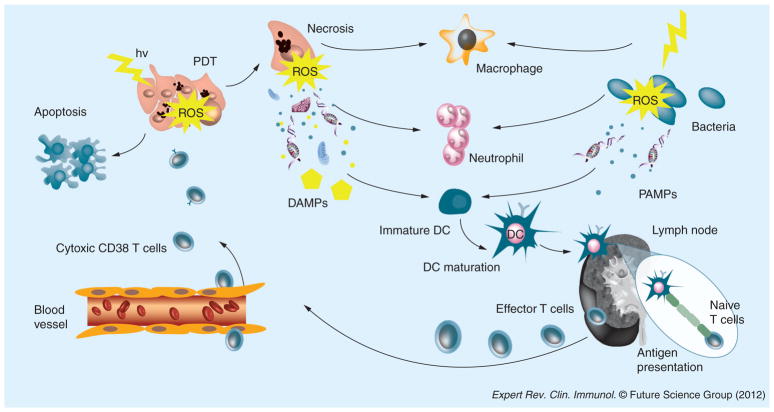
It was initially considered that PDT-induced damage to tumors was confined to the treated site. However, it is now accepted that the multifactorial effects of this therapy that include direct tumor cell killing, damage to the vasculature and activation of the immune system allow systemic effects to operate as well [13]. PDT triggers several cell-signaling cascades and the release of cell fragments, cytokines and inflammatory mediators that stimulate a complex interplay between the innate and the adaptive arms of the immune system. This aspect has been extensively investigated as part of the anticancer effect of PDT [10,11,14]. PDT alters tumor microenvironment by stimulating the release or expression of various pro-inflammatory and acute phase response mediators from the PDT-irradiated area. This reaction, in turn, activates systemic inflammation and innate and adaptive immunity. Figure 2 graphically illustrates some of the cells, signaling pathways and mediators responsible for activation of the immune system after PDT.
Figure 2. Stimulation of immune system after photodynamic therapy for cancer treatment.
The tissue damage to the tumor caused by the ROS leads to release of DAMPs that activate host immune cells such as macrophages, neutrophils and dendritic cells in an analgous manner to the PAMPs released by invading pathogens. The DCs can take up antigens released by apoptotic tumor cells and migrate to draining lymph nodes, where they activate naive T cells to become tumor-specific cytotoxic T cells that can return to destroy the tumor. hv represents light.
DAMP: Damage-associated molecular pattern; DC: Dendritic cell; hv: Light; PAMP: Pathogen-associated molecular pattern; PDT: Photodynamic therapy; ROS: Reactive oxygen species.
PDT & innate & adaptive immune systems
Defense against invading pathogens is provided by the immune system in two interlocking ways: the innate immune system, designed to protect the body from all invaders without pre-exposure; and the adaptive immune system, which can mount specific attacks against specific invaders. Although the innate and the adaptive immune systems play different roles in defending the body, both arms generally act together in a concerted manner.
The innate immune system is an antigen-non-specific defense mechanism that is activated very soon after infection. It is the first line of defense of the body and lacks immunologic memory, thus remaining unchanged regardless of how often the pathogen is encountered. Innate immunity is designed to recognize molecules/biochemical patterns exhibited by a foreign aggressor, for example ‘pathogen-associated molecular patterns’ (Figure 2). Most host defense cells express pattern-recognition receptors, a sub-class of which is known as Toll-like receptors, and so there is an immediate response against the invading microorganism. Researchers have recently discovered a second array of molecular motifs associated with host tissue stress or damage known as ‘damage-associated molecular patterns’ (DAMPs) [15–18]. These molecules are normally considered to be intracellular but tend to get exposed or secreted by damaged/dying cells acquiring immunostimulatory properties. The most frequently reported examples of DAMPs released after PDT are heat shock proteins (HSPs) HSP60, HSP70, HSP90 and GRP94 that are upregulated and translocated to the cell membrane [19,20]. It is likely that recognition of DAMPs is linked to expression of pattern-recognition receptors as has been found for CD14 [21] and TLR4 [22].
The innate immune response system includes humoral elements, which are soluble, and includes the complement system, antimicrobial proteins, acute phase proteins and cytokines and cellular elements such as innate leukocytes. The innate leukocytes include phagocytic cells (neutrophils, macrophages and DCs), mast cells and NK cells.
PDT regimes that prompt a powerful inflammatory response induce a rapid influx of neutrophils into the treated site leading to improved tumor response rates and enhanced immunity [23,24]. Neutrophils are among the first cells recruited to the illuminated area and their main function is to release enzymes for killing infectious organisms and secrete leukotrienes, prostaglandins, cytokines and other chemicals that promote inflammation. Neutrophilia begins immediately after PDT and it is still present 24 h later. Krosl et al. reported a 200-fold increase of neutrophils within 5 min after irradiation, followed by an increase in the levels of mast cells, myeloid cells and monocytes between zero and 2 h after PDT [25]. Sluiter and colleagues first observed that neutrophils adhere to the microvascular wall after PDT in vivo and provided essential information of their relevance in the anti-tumor response [26]. Cecic et al. investigated the impact of PDT on the systemic and local kinetics of neutrophil trafficking with SCCVII and EMT6 tumor models [23]. Significant neutrophilia was observed upon PDT treatment and was prevented by complement inhibition. The activation of the complement system in particular has emerged as a powerful mediator of PDT antitumor effects [27–29]. Complement not only acts as direct mediator of inflammation, but also stimulates cells to release secondary inflammatory mediators, including cytokines IL-1β, TNF-α, IL-6, IL-10, G-CSF, thromboxane, prostaglandins, leukotrienes, histamine and coagulation factors. The final complement activation is the membrane-attack complex that forms transmembrane channels, disrupting the integrity of the plasma membrane and leading to cell lysis and death.
Macrophages, as phagocytic cells, can directly kill microbes, infected cells and tumor cells. The activation and recruitment of macrophages induced by PDT potentiate immunity. Macrophages uptake antigens and present them to the T lymphocytes during the adaptive immune response. Macrophages, as well as DCs mentioned below, can therefore be referred to as APCs. Additionally, macrophages secrete cytokines, chemokines and other mediators essential in the development of PDT-mediated inflammation. Macrophages can be activated by low PDT doses (non-lethal) and secrete TNF-α [30,31]. The presence of HSP70 secreted by apoptotic and necrotic cells seems to be essential for macrophage activation [32]. Evidence also indicates that macrophages can show preferential toxicity towards treated tumor cells [33].
PDT-mediated enhancement of immunity is believed to be due, at least in part, to stimulation of DCs by DAMPs released/secreted by dying tumor cells [34,35]. DCs are the most potent APCs and a key element in the development of an immune response. Immature DCs are constantly sampling their environment for pathogens. Upon capturing antigens through phagocytosis and becoming activated by inflammatory cytokines, DCs migrate to lymph nodes. By the time they enter the lymph nodes, they have matured and are able to present antigen to T lymphocytes. Mature DCs express MHC proteins at the cell surface, as well as appropriate costimulatory molecules. This enables the T4 lymphocytes or T8 lymphocytes to become activated, proliferate and differentiate into effector cells, activating the adaptive immune response. PDT has the potential to create a favorable environment at the tumor site for antigen loading and DCs activation. PDT induces IL-1 and IL-6 expression [36] and upregulates HSP70 interaction with APCs such as DCs [37].
The adaptive immune response is triggered by recognizing endogenous and exogenous antigens. APCs express MHC proteins at the cell surface. This MHC antigen complex is recognized by T cells passing through the lymph node. Exogenous antigens, produced by exogenous pathogens such as bacteria, parasites or toxins, are usually displayed on MHC class II molecules, and mainly activate CD4+ helper T cells. The role of CD4+ helper T cells in PDT-mediated immunity remains unclear and they may have a supportive function [38,39], but the uncertainty may have been caused by inclusion of Tregs in the CD4+ population. Endogenous antigens, characteristic of viruses and tumor cells, are presented by MHC class I molecules and activate CD8+ cytotoxic T-cells. The first evidence of the specificity of the PDT antitumor effects came from Canti et al. [40], and the formation of immune memory was reported by Korbelik and Dougherty [41]. The control of the growth of tumors present outside the treatment field is mediated by CD8+ T cells and is accompanied by induction of antitumor immune memory responses [38]. In a recent study, Gollnick’s group showed that PDT induced the expression of MHC class I molecules activating NK cells and costimulating CD8+ T cells [42].
The PDT-induced immune response can be impaired by the mechanisms that the tumor employs to avoid or escape attack by the immune system, in particular by intratumoral accumulation of Tregs [43]. Tregs can be defined as a CD4+ T cell subpopulation that functionally suppresses an immune response. Tregs prevent autoimmunity, protect beneficial flora in the intestines and control established inflammation in tissues. On the downside, Tregs may sometimes help cancer cells to escape immune attack. Our laboratory was the first to realize that Tregs may play an important and negative role in PDT-induced antitumor immunity [44]. Tregs can be efficiently depleted by a low dose of cyclophosphamide, a traditional cytotoxic cancer drug that damages tumor DNA and can potentiate antitumor immunity. In this case, the depletion of Tregs potentiates benzoporphyrin derivative monoacid ring A(BPD)-mediated PDT, leading to significant long-term J774 tumor cures and memory immunity. Besides Tregs, it has been reported that PDT can also cause immunosuppression and that this phenomenon can occur in mice without tumors [45,46]. These reports have nearly all been concerned with the suppression of the contact hypersensitivity reaction in mice [46,47], although the actual mechanisms behind this phenomenon are still poorly understood.
On the other hand, attention should be given to how PDT affects immune cells. Several investigators have pointed out that the cytotoxic reaction in PDT may induce functional damage in phagocytes and/or decrease the number of these cells hampering the PDT outcome [48,49]. As we review in the following sections, the preservation of neutrophils is especially important for optimal antibacterial PDT. The PDT susceptibility of lymphocytes has led to PDT being proposed as treatment of graft versus host disease, some forms of autoimmune disease and cutaneous T-cell lymphoma [50–53].
PDT & immune response against cancer
The ideal cancer therapy should cause local tumor regression and eradication, as well as induce a tumor-directed immune response capable of eliminating the primary tumor, distant metastases and establish long-term tumor control. PDT may meet these expectations since it triggers acute inflammation and potentiates the immune response [2].
One of the first reports highlighting the immune response against cancer in PDT was proposed by Evans et al., who investigated the role of TNF-α production in murine macrophages treated with PDT [31]. Korbelik et al. later demonstrated that activation of immune memory is necessary for the most effective tumor control [41,54]. The success of PDT-induced anti-tumor immunity seems to be dependent on the presence of tumor rejection antigens. Our laboratory showed long-term cures and rejection of rechallenge of green fluorescent protein-expressing RIF tumor while no immune effect was observed in its wild-type counterpart [55]. Besides local immune responses, PDT was able to lead to the destruction of distant, untreated, established, antigen-expressing tumors as we demonstrated using CT26.CL25 tumor-bearing mice [56]. The role of tumor antigen in PDT has recently been investigated in the clinical setting. Kabingu et al. demonstrated that PDT of basal cell carcinoma in patients increased systemic immune responses against Hip1, a known tumor antigen in basal cell carcinoma [57]. Two other reports discussed the induction of antitumor immunity after PDT for angiosarcoma [58,59]. Many factors that affect this process are not completely understood, especially concerning the correct PDT dosimetry for optimizing the antitumor immunity effect. For instance, is high-fluence or low-influence PDT best? Is high or low fluence rate best? Is cellular or vascular targeting best?
The administration of immunostimulants or adjuvants and combination therapies has been used to sustain and amplify the PDT-induced immunity against tumors [12,60]. Many of these combination adjuvants are derived from microbial stimulators. Their role is to activate TCRs or similar pattern-recognition receptors present on the surface of macrophages and DCs. OK-432 is a preparation derived from killed streptococcal bacteria that increased the tumor-free time in mice with NR-S1 squamous cell carcinoma when it was injected intra-tumorally 3 h before irradiation [61]. Another study has shown that low doses of Corynebacterium parvum led to a delayed response of immune system that prevent the phototoxic killing of immune cells attracted to the tumor [62]. The majority of immunostimulants are delivered by intratumoral injection. Systemic activation of the innate immune system and uncontrolled secretion of cytokines would be toxic and even fatal.
A related approach that takes advantage of immunostimulatory effects of PDT is the PDT-generated cancer vaccines [35,63]. Instead of the standard PDT of solid tumors, tumor tissue is treated by PDT ex vivo to produce the vaccine material that is injected to the subject with a tumor. Although it is in a very early phase, recent research indicates that PDT vaccines have clinical potential to become beneficial adjuvants or primary therapy in the treatment of various cancers [64].
PDT in arthritis
Arthritis is a form of joint disorder that involves inflammation of one or more joints that can lead to long-term work incapacity [65,66]. During the course of the disease, peripheral joints are commonly destroyed by pathological proliferations of synovial membrane. In the early stages of the pathology, the treatment involves a combination of splintage and medical therapy. The patients with persistent joint inflammation are candidates for synovectomy, but this procedure can have an uncertain outcome [67]. PDT is known for the treatment of selected tumors in many countries and could be an alternative approach for arthritis treatment (Figure 3) [68]. Pathogenic synovial tissue and solid tumors present some similarity especially in the hyperproliferative nature and the induction of neovascularization. The microvasculature is often disorganized but it plays a key role in the progression of the pathology. One study has demonstrated that inhibition of angiogenesis, in a rabbit model of arthritis, prevented the development of disease [69].
Figure 3. Photodynamic therapy for arthritis.
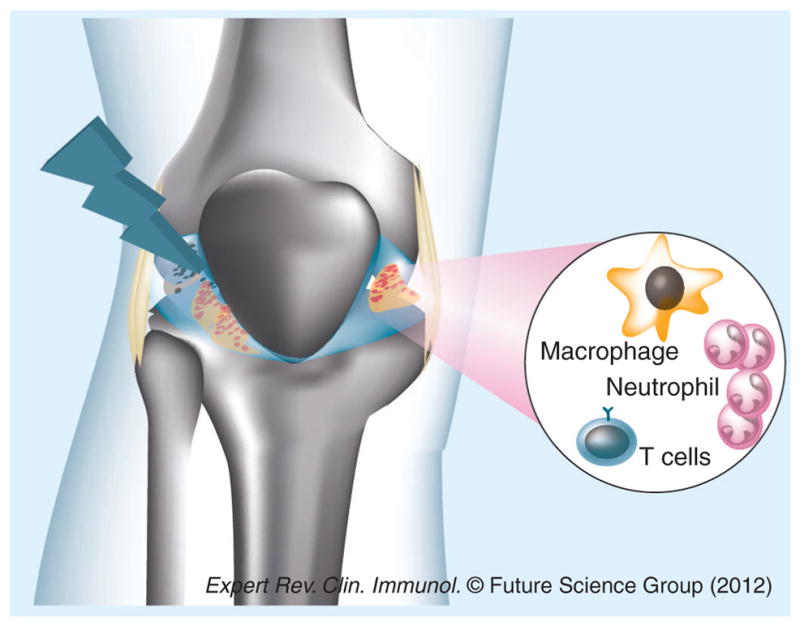
Systemic or intra-articular injection of photosensitizer followed by illumination of the affected joint can destroy hyperplasic synovium, shut down neovasculature and kill host inflammatory cells such as macrophages, T lymphocytes or neutrophils responsible for the arthritic pathology.
Several studies have been performed to verify the efficacy of PDT as an arthritis treatment. Many animal models of rheumatoid arthritis have been studied and different PSs, locally or systemically administered, were employed to test the optimal conditions for treatment. The choice of PS, the dose of the compound, the route of administration, the times, dose and kind of illumination have been investigated.
In 1994 Ratkay et al. performed a study to compare the effect of PDT and other immunomodulatory treatments in adjuvant-enhanced arthritis in MRL-lpr mice [70]. The arthritis was induced with two intradermal injections of Freud’s adjuvant supplemented with heat-inactivated, lyophilized Mycobacteria tubercolosis. The PS, BPD, was injected intravenously and activated with red light (690 nm) by transcutaneous PDT, 80 J/cm2 light dose on 0, 10 and 20 days after arthritis induction. The results of this study demonstrated that PDT gave no signs of skin photosensitivity and prevented clinical arthritis signs (such as cartilage erosion and bone destruction) even in more severe stages. Another effect noted was a transitory decrease in circulating leukocytes.
Later, Trauner et al. developed an antigen-induced arthritis model (AIA) in rabbits to investigate time-dependent distribution of BPD in articular and periarticular tissues after systemic administration and to evaluate photodynamic synovectomy to produce pathologic tissue destruction [71]. A bilateral synovitis was developed in rabbits that were sensitized with two intra-cutaneous injections of ovalbumin over a 6-week period. After 6 weeks both knees of each animal were intra-articular injected with ovalbumin in sterile saline solution. Light of wavelength 690 nm, 100 J/cm2 was applied by using of intra-articular optical fibers. The first results showed that significant quantities of BPD were localized in pathological synovium, and with intra-articular light administration a selective synovial destruction was observed. The knee treated with light showed a transient erythema that resolved by 24 h in all animals.
In two more studies the authors used the same animal model (AIA) and the same PS (BPD) used by Trauner’s group [72,73]. In the first one, Ratkay et al. [72] investigated the mechanism of action and local efficacy of transdermal PDT, 50 J/cm2. While the untreated animals showed a pronounced synovitis in synovial and subsynovial tissue with infiltration of inflammatory cells (in particular leukocytes, T cells and monocyte/macrophages), in transdermal PDT treatment, moderate signs of inflammation were still evident, but the treatment prevented pannus formation, cartilage and bone erosion. Moreover, apoptotic cells that expressed T-cell and macrophage markers were observed in histological examination. This study demonstrated that transdermal PDT prevented inflammation and subsequent severe stages of arthritis and also suggested a possible mechanism of action of PDT. In the second paper, Chowdhary et al. studied the distribution and quantification of BPD administered intravenously and intra-articularly [73]. They demonstrated that the biodistribution and the selective uptake of the compound in target cells depended on the dose and route of administration (intra-articular or intravenous) and also the efficacy of PDT by light delivery. A study on biodistribution of a different PS, hematoporphyrin (HpD), was performed by Beischer et al. [67]. They used a rabbit AIA model and animals, after intravenous injection of HpD, and delivered total light doses of 50, 100 and 200 J/cm2 with a 630-nm laser. Significant accumulation of HpD was evident in periarticular inflammatory tissue and in pannus on the femoral condyle margins. Small quantities of compound were revealed in cartilage whereas no traces were showed in muscle tissue. Moreover, the authors did not observe histological damage due to PDT in periarticular tissue. Small areas of focal necrosis of bone marrow in femoral epiphysis were observed when higher light doses were used. They demonstrated that activation of HpD by intra-articular injection provided a selective ablation of inflamed synovium.
Some studies have been conducted to verify the effect of PDT using different PSs at various doses to optimize the treatment of arthritis. Funke et al. used tetrahydroporphyrin tetratosylate in a model in severe combined immunodeficient mice [74]. Arthritis was induced by injection of murine fibroblast cell line (LS48) in the mouse knee joint. Tetrahydroporphyrin tetratosylate presented some advantages, such as minimal dark toxicity, high phototoxicity, high water solubility, rapid clearance from the body and it was able to localize in inflamed tissue [75,76]. PDT was performed using laser light coupled into a fiber-bundle. The laser system emitted light at 761 ± 3 nm, the affected knee was exposed to 100 J/cm2 for 200 s. This model of treatment, using a second generation PS with near infrared absorbance, offered the opportunity to penetrate deeper into tissue [76,77]. The treatment was well tolerated by animals and results of this study demonstrated that PDT reduced the duration and severity of the characteristic swelling of the joint, induced by LS48 cell proliferation. Moreover, signs of inflammation were not observed in the immediate vicinity of the treated lesion. Skin and intra-articular tissue, such as ligaments, menisci and cartilage remained undamaged. The unwanted effect observed in this study was the development of some necrosis in adjacent, well-vascularized skeletal muscle with paralysis of the treated extremity. The occurrence of paralysis could be due to the small size of the knee joints of the mice. One more PS studied was talaporfin sodium in a rat collagen-induced arthritis model and rheumatoid arthritis membrane [78]. Talaporfirin has been tested in PDT in vivo and in vitro studies in many different tumor models [79–83]. It was accumulated selectively in tumors and showed rapid elimination from normal tissue. In this study the authors performed PDT with intra-articular injection of talaporfin sodium and intra-articular irradiation. The authors demonstrated that PDT with talaporfin sodium was effective for the synovial membrane of patients with rheumatoid arthritis both in vitro and in vivo as well as on the synovial membrane of rats with collagen-induced arthritis. The effectiveness depended on the concentration of talaporfin sodium and the laser irradiation energy. The results of these studies suggested PDT could be a potential new clinical treatment for arthritic joints.
Antimicrobial PDT & PDT for infection
Over 100 years ago, Raab observed the killing of Paramecium caudatum when a harmless dye (acridine) was combined with visible light. A few years later Tappeiner demonstrated the involvement of light and oxygen in the process observed by Raab and he coined the name ‘photodynamic action’. Though PDT in the field of microbiology was discovered a century ago, it has mainly been applied for cancer treatment and ophthalmology but its application in the field of infectious diseases has been limited. The discovery of antibiotics in 1940 revolutionized the treatment of infectious diseases. The recent increase of antibiotic resistance, probably due to inappropriate and excessive use of antibiotics, social factors related to compliance and mechanisms adopted by bacterial cells to increase their resistance to external assaults, has given rise to the growth of interest in different therapeutic strategies for the treatment of infections. PDT can act on a broad spectrum of pathogens such as bacteria, parasitic protozoa, fungi, yeasts and viruses. Moreover, it is a noninvasive method and lacks the ability to induce resistance itself. Nitzan et al. in 1992 demonstrated that the difference of bacterial walls between Gram-positive and Gram-negative bacteria led to a fundamental difference of sensitivity to PDT treatment [84]. Antimicrobial PDT with a wide range of PS is more effective in inactivating Gram-positive bacterial species. The cytoplasmic membrane of these bacteria is surrounded by a porous layer of peptidoglycan and lipotechoic acid that provides more permeability to PS [85]. In contrast the wall membrane of Gram-negative bacteria consists of a double layer, an inner cytoplasmic membrane and an outer membrane that are separated by the peptidoglycan-containing periplasm. The outer membrane restricts the binding and penetration of PS [86]. Fungal cell walls, instead, have a relatively thick layer of β-glucan and chitin that leads to a permeability barrier intermediate between Gram-positive and Gram-negative bacteria. Several studies have been carried out to overcome the issue of permeability of the membrane [84,87] to improve the efficacy of PDT treatment against Gram-negative bacteria. Moreover, it has been observed that imparting positive charge to PS can also produce an increase spectrum PS that could inactivate all classes of microorganisms: Gram-positive and Gram-negative bacteria [5], fungi [88], viruses [89], parasites [90] and even highly resistant life forms such as bacterial spores [91] and the cystic stage of Acanthamoeba [92]. The choice of PS is a key factor for the treatment with PDT. In addition to optimized photophysical characteristics (such as high quantum yields for the generation of long-lived triplet state and the cytotoxic singlet oxygen species), important features of antimicrobial PS should be high-affinity for microbial cells compared with host mammalian cells providing the ability to induce microbial cell inactivation while minimizing the risk of inducing mutagenic processes and damage to the host tissue in the area of infection. Ideally PDT should prevent the regrowth of the pathogens after the treatment [93]. Many studies aimed to optimize these compounds to improve the selectivity for microbial cells over host mammalian cells [94], maximizing absorption in the near infrared regions of the spectrum [95] and reducing photobleaching [96]. Phenothiazinium dyes such as toluidine blue O and methylene blue [97–99] and azure dyes [100] have been widely tested and employed in PDT against Gram-positive, Gram-negative and fungal cells. Other PSs, such as porphyrins [101,102], phthalocyanines [103–105] and C60 fullerenes [88], have been synthesized and studied. These studies have proven PDT to be very effective in in vitro and in vivo models of infection. The importance of selectivity of PS in the treatment of infections has been demonstrated in a study where the authors investigated two different PSs. The use of a conjugate poly-L-lysine and chlorin (pL-ce6) and free chlorin(e6) (ce6) in an established soft tissue infection caused by S. aureus in the mouse thigh [106] and demonstrated that when each pL-ce6 and c were injected into the infected area and illuminated with the same red light, the conjugate pL-ce6 produced much more bacterial killing than ce6; moreover, it produced less damage to the host tissue. Other in vitro and in vivo studies have been carried out to verify the efficacy in many different kinds of infections produced by different bacterial strains. Numerous animal studies have been designed to verify the efficacy of PDT in infection models in mice and rats. Positive results were obtained in a model of severe combined immunodeficient mice with oral candidiasis [107]. Another study investigated wound infections inoculated with Pseudomonas aeruginosa and treated with PDT using pL-c. It was found that the healing was faster after PDT treatment rather than an alternative topical antimicrobial, silver nitrate application [108]. The effect of TBO-PDT on Vibrio vulnificus infections in a mouse excisional wound model was studied by Wong et al. and was also found to be beneficial as mice were saved from death due to sepsis [109]. Zolfaghari et al. [110] used MB-PDT to treat methicillin-resistant S. aureus (MRSA) in two models of mouse wound infection (excisional and partial thickness wounds) and found a 25-fold reduction in the number of viable MRSA. Animal models to study PDT effect on infection presented some limitations and difficulties in monitoring the development of infection; usually it was necessary to kill the animals, to remove the infected area and count the CFUs. To monitor the microbial infections, Hamblin et al. developed a new approach based on the use of genetically engineered bacteria that emit bioluminescence [108]. In this way, the infection could be detected in real-time using an intensified charge-coupled-device camera. Using this method the effect of PDT in many different conditions has been investigated. The efficacy of PDT was demonstrated on Escherichia coli and P. aeruginosa in excisional wounds infected in mice models [108,111] using polycationic PS conjugate. Also, in burn infections, Dai et al. [112] and Lambrechts et al. [113] demonstrated the positive effect of PDT against A. baumannii and S. aureus. Moreover, the effect of PDT has been investigated in animal models of oral and dental infections [107,114–118], soft tissue infections [106,119], viral infections [120,121] and leishmaniasis [122–124]. Some clinical trials for PDT for infectious disease have been carried out on viral lesions, dermatological infections such as acne and rosacea, and also in dental and gastric infections. The subject of clinical applications and clinical trials of PDT for infections has recently been reviewed in detail [9].
PDT for bacterial arthritis
Bacterial arthritis presents several difficulties in treatment. There is less blood flow to bone and cartilage tissue and this lack of perfusion sharply reduces the bioavailability of systemic antibiotics. The frequent use of artificial implants in joints raises the chance of biofilm formation and the ‘foreign body response’ predisposes to infection. The issue of multidrug resistant bacteria has emerged as an exacerbating factor in orthopedic infections, necessitating surgery, continuous irrigation and prolonged antibiotic regimes for patients with such intractable bacterial infections. An alternative therapeutic approach to bacterial arthritis using PDT has been investigated with Photofrin®, a PS that has proven effective in antitumor applications. The fact that Photofrin is known to be effective in killing Gram-positive bacterial species in vitro [125] suggested that Photofrin may be active in PDT of arthritis caused by MRSA, a Gram-positive pathogenic species.
Intravenous Photofrin
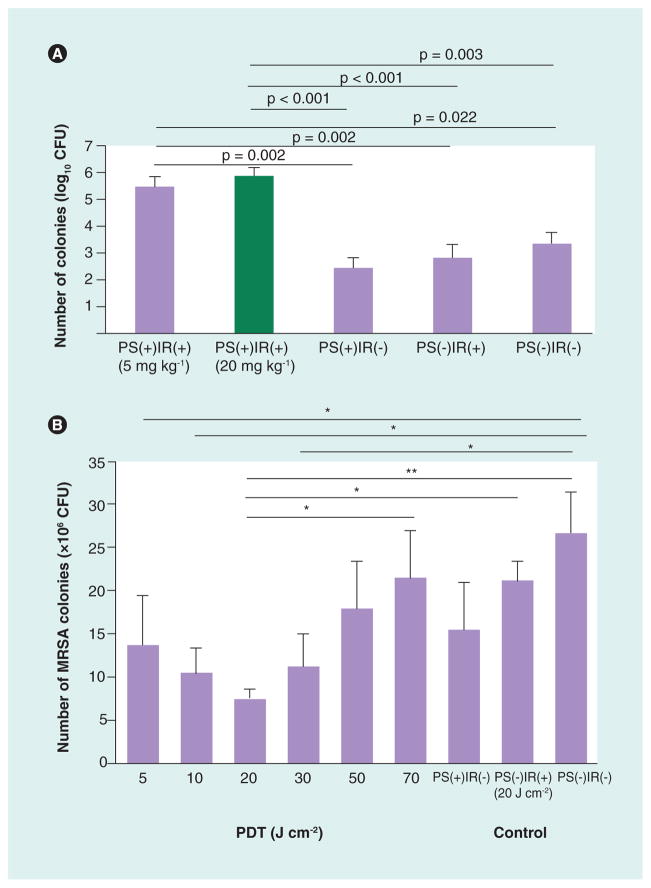
The testing of intravenous Photofrin to mediate PDT for bacterial arthritis caused by MRSA was first reported by Tanaka et al. [126]. MRSA causes many problematic infections in humans that respond poorly to standard therapies and tend to recur and become chronic and intractable. Photofrin can kill Gram-positive bacteria such as MRSA but is inactive against most Gram-negative species. Although PDT with Photofrin resulted in high levels of cytotoxicity for cultured MRSA, the therapy had a low efficacy in a murine MRSA arthritis model in the knee joint. Intravenously injected Photofrin accumulated well in the infected joint as shown by fluorescence imaging. However, when the infected joint was illuminated the number of MRSA CFUs extracted from the joint was actually higher than the controls that received PS alone, light alone or no treatment (Figure 4a). This undesired outcome was attributed to the PDT killing of intra-articular neutrophils that otherwise could have controlled the bacterial infection. Nearly 30% of intra-articular leukocytes, primarily neutrophils, were killed immediately during or following PDT. Intra-articular neutrophil counts further decreased after 24 h, and in the meantime, isolated peripheral neutrophils with morphological damage from PDT were on the path towards cell death. It was inferred that this unwanted side effect of PDT with Photofrin was responsible for the increase in MRSA CFUs in the mouse model. Thus, when applying PDT to clinical treatment of bacterial arthritis, it was important and necessary to consider not only the PDT-mediated cytotoxicity towards the bacteria, but also towards the immune defense cells in the host tissue.
Figure 4. Photodynamic therapy for methicillin-resistant Staphylococcus aureus arthritis with Photofrin®.
Colony-forming units extracted from synovial fluid at sacrifice of mice 24 h after PDT. (A) Photofrin was injected intravenously in mice with MRSA arthritis and 24 h later the affected knee was illuminated. (B) Photofrin was injected intra-articularly into the affected knee, which was immediately illuminated.
IR: Irradiation (635-nm light); MRSA: Methicillin-resistant Staphylococcus aureus; PDT: Photodynamic therapy; PS: Photosensitizer (Photofrin®).
(A) Reproduced with permission from [126].
(B) Reproduced with permission from [128].
Intra-articular Photofrin
Considering the ineffectiveness of PDT using intravenous injection of Photofrin for murine MRSA bacterial arthritis, an alternative strategy to administer Photofrin was necessary that would hopefully decrease the cytotoxic effect on host neutrophils. It was hypothesized that local administration of PS and immediate irradiation at the local infection site could dramatically reduce PDT-induced damage to mammalian host cells [127]. Therefore we redesigned the Photofrin PDT protocol to include direct administration in the form of intra-articular rather than systemic intravenous injection and followed that local injection with immediate photoirradiation to increase the chances that the PS would rapidly bind to bacteria, while binding to host neutrophils would be slower [128]. In this way, it was proposed to concentrate the PS at the local infection site and limit diffusion to other areas, minimizing the uptake and hence cytotoxicity of Photofrin to the host leukocytes. To further optimize the approach, we evaluated a range of light doses ranging from 5 to 70 J/cm2 using a 635-nm laser. The results showed a biphasic light dose response with maximum MRSA killing (determined by CFUs extraction) at 20 J/cm2, with lower bacterial reduction values at 5 and 10 J/cm2 and also lower bacterial reduction at 30, 50 and 70 J/cm2 (Figure 4b). Furthermore maximum neutrophil accumulation was also found at a fluence of 20 J/cm2. At this fluence, in contrast to the results from the intravenous Photofrin study [126], there was no immediate decrease in neutrophil counts or alteration of morphology, and in addition, neutrophil accumulation after 24 h was actually facilitated by PDT. The conclusion was that at light doses below this optimum level, insufficient amounts of bacteria were eliminated, and at levels above 20 J/cm2, neutrophil and tissue damage was significant, with both cases leading to bacterial regrowth after treatment. Direct intra-articular injection of Photofrin at the bacterial arthritis site with immediate PDT at the optimal light dosage could lead to optimal PDT therapeutic effects.
Finding a selective PS for PDT of bacterial arthritis
From the studies discussed above concerning the biphasic PDT effects of Photofrin on MRSA arthritis, we learned that the possibility of a cytocidal effects against neutrophils must be taken into account in designing antimicrobial PDT protocols. Therapeutic the beneficial effect of PDT for localized microbial infections could be exerted both by direct bacterial killing and also by the bactericidal effects of host neutrophils stimulated by PDT. Therefore, PDT-induced damage to neutrophils must be minimized, while direct photoinactivation of bacteria is maintained to maximize the therapeutic efficacy of antimicrobial PDT in vivo.
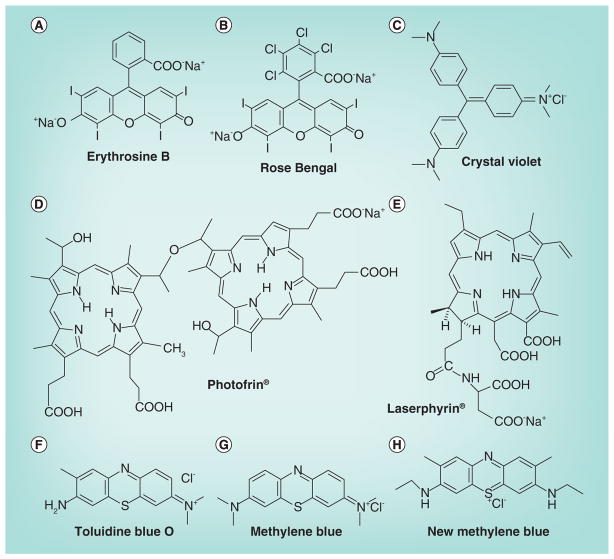
In a recent study [129], we evaluated and compared the cytocidal effects of PDT on MRSA bacteria and on murine peripheral-blood neutrophils using eight different PSs whose structures are given in Figure 5. These PSs were erythro sine B [130], rose bengal [91], crystal violet [131], Photofrin [126], Laserphyrin [132], toluidine blue-O [91], methylene blue (MB) [91] and new MB [91]. The results suggested that PDT using TB or MB could preserve host neutrophils (>80% viability) while killing substantial amounts of MRSA (5–6 logs of killing). While TB and MB are hydrophilic with a negative easured (P) value [133,134], crystal violet, erythrosine B, NPe6, new MB, rose bengal and PHP are amphiphilic with progressively higher positive log (P) values [129]. Both mammalian cell and bacteria membrane are composed of double layered phospholipids, but the bacterial cell membrane is encased in a cell wall [135]. It is likely that more hydrophilic PSs (high log (P) value) are relatively better at inactivating bacteria, perhaps because they can at least bind to the cell wall if not penetrate the cell membrane, while more hydrophobic PSs (high log (P) value) can penetrate the cell membrane of the much bigger mammalian cells but are excluded by the cell wall of bacteria. The peak absorption wavelength of MB is 664 nm and that of TB is 596 nm; these longer wavelengths might enable deeper treatment due to better light penetration compared with other PSs as found for MB [136]. In conclusion, PDT using TB or MB showed minimal damage to neutrophils under optimal concentrations of the PS for extensive killing MRSA.
Figure 5. Structures of photosensitzers screened for selectivity for killing bacteria while preserving neutrophils.
(A) Erythrosine B; (B) Rose bengal; (C) Crystal violet; (D) Photofrin®; (E) Laserphyrin®; (F) Toluidne blue O; (G) Methylene blue; (H) New methylene blue.
Therapeutic PDT for bacterial arthritis using intra-articular MB
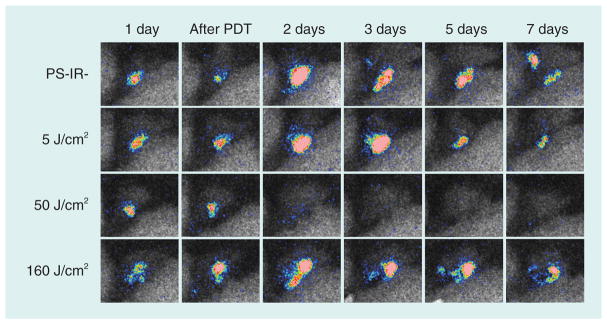
Based on the previous studies [129], we selected MB as an optimal PS for antimicrobial PDT in a model of bacterial arthritis in conjunction with direct intra-articular injection and immediate photoirradiation [137]. To carry out detailed examination of the PDT effects in the knee joint and demonstrate longitudinal progress of infection in the same mouse in an efficient manner, we established a murine intractable MRSA arthritis model using a combination of genetically engineered luciferase-expressing bioluminescent MRSA (lux-MRSA) [138] and artificial resin microparticles. The artificial resin microparticles injected into the mouse knee joint along with the bacteria enabled the establishment of biofilm formation, which leads to severe and intractable arthritis.
We initially studied a therapeutic PDT (Th-PDT) protocol in which MB (10 μl of 100 μM solution) was injected into the infected joint 24 h after establishment of infection, followed by immediate illumination of the knee joint with 660-nm light from a lamp fitted with a band-pass filter. Using an effective light dose ranging from 50 to 80 J/cm2, there was a reduction in bioluminescent signal of lux-MRSA from the knee joint starting from day 1 after PDT and lasting for the succeeding 7 days (Figure 6). Quantification of the therapeutic effect showed a 2-log10 reduction of bacterial bioluminescence signal. These results indicated that the therapeutic window of PDT using intra-articular MB was broader than that of PDT using Photofrin [128].
Figure 6. Therapeutic photodynamic therapy with intra-articular methylene blue.
Series of representative bioluminescence images captured from the same mouse for each group at time points up to 7 days post-PDT. Mice received an intra-articular injection of methylene blue (10 μl of 100 μM solution) followed by immediate illumination with different fluences of 660-nm light.
PDT: Photodynamic therapy.
Adapted with permission from [137].
The bioluminescent intensity did not decrease immediately after Th-PDT, which suggested that Th-PDT did not exert a direct bacterial killing effect as expected. This finding might be due to biofilm formation on the surface of the resin microparticles that inhibited the binding of methylene MB to lux-MRSA cells and therefore led to the failure to exert a direct bacterial killing. To further study the mechanism of Th-PDT effect for bacterial infection using MB, an anti-GR-1 (antineutrophil) antibody was administered. The therapeutic effect of PDT was eliminated by the administration of anti-GR-1 antibody, indicating that the therapeutic effect of PDT led to reduction of bacterial infection via an attraction and accumulation of neutrophils into the infectious region rather than by PDT-mediated bacterial killing.
Preventive PDT regimen
The crucial role of neutrophils in the therapeutic response of tumors to various PDT regimens has been reported several times [23,26,139]. Although the role of PDT-activated/stimulated neutrophils in the therapeutic effects of PDT against cancer is established, the role of neutrophils in the therapeutic effects of antimicrobial PDT had not been previously reported before our studies in murine bacterial arthritis [126,128,129].
The previous findings concerning the therapeutic effect of PDT indicated that PDT using MB could strongly activate innate host defense mechanisms against a microbial infection. Therefore, we hypothesized that PDT might suppress bacterial growth and inhibit the establishment of infection when performed as a protective pre-conditioning regimen. To test this hypothesis we devised a preventive PDT (Pre-PDT) protocol, consisting of carrying out PDT on a murine normal knee joint (non-infected) and then examined the protective effect of Pre-PDT on the subsequent development of infection after PDT [137]. The dose level chosen was similar to that shown to be optimal in the Th-PDT study (i.e., killed the bacteria without undue harm to host cells and tissues).
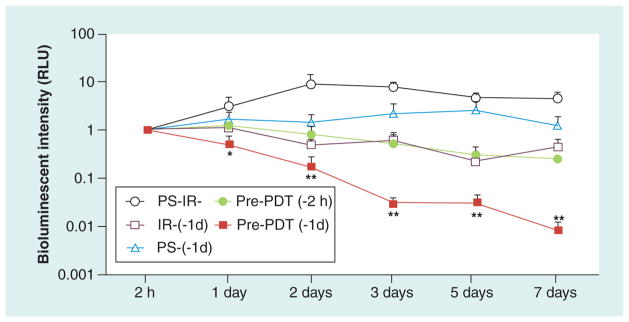
Pre-PDT was carried out at different time points. Pre-PDT performed on normal mouse knee joints 24 h before (but not 2 h before) the bacterial inoculation strongly inhibited the subsequent bacterial growth and suppressed the development of the infection as measured by bioluminescence imaging (Figure 7). Light alone or MB alone 24 h before infection had no such protective effect. Our results showed that in the Pre-PDT group, neutrophils immediately migrated into the tissue around microvessels and the number of leukocytes, mainly neutrophils, in the knee joint began to increase within 2 h after bacterial inoculation. This finding suggested that a certain preparatory state of neutrophil priming was achieved, because generally, neutrophil accumulation into the regions of bacterial infection needs at least 6 h from bacterial invasion because time is needed for the expression of chemotactic factors on vascular endothelium and surrounding tissues [140,141]. Moreover, the effect was eliminated by administration of an intravenous antineutrophil antibody (anti-GR-1), indicating that neutrophil activity played an important role in the protection against infection by Pre-PDT as well as the resolution of infection by the Th-PDT regimen. It should be noted that anti-GR1 is not absolutely specific for neutrophils and therefore other leukocytes could be implicated as well. If pre-PDT protects by stimulating neutrophils, what chemotactic factors were involve in this stimulating process? We performed Pre-PDT with a set of neutralizing antibodies for chemotactic factors and other pathways, which are responsible for neutrophil activation and accumulation. These were IL-1β, TNF-α, IL-6, macrophage inflammatory protein 2 (MIP-2), antikeratinocyte-derived chemokine, ICAM-1 and E-selectin. In addition, mice were given SN50, which is an inhibitor of NF-κB [142]. The results suggested that all the tested chemotactic factors (except for IL-6) were involved in the effect of Pre-PDT to stimulate innate immunity. IL-6 is not normally considered a chemotactic factor and does not act on neutrophils. Therefore, Pre-PDT might activate the whole sequential cascade for the accumulation, migration and activation of neutrophils into the local infectious site.
Figure 7. Preventative photodynamic therapy with intra-articular methylene blue.
Time course of quantification of bioluminescence signals from mice treated with preventative methylene blue-photodynamic therapy with 50 J/cm2 of 660-nm light (and controls) administered either at 2 or 24 h before infection with bacteria.
IR: Irradiation; PDT: Photodynamic therapy; PS: Photosensitizer.
Adapted with permission from [137].
PDT could be applied as a preventive (prophylactic) strategy for a surgical-site infection after high-risk orthopedic surgery such as total knee arthroplasty, as well as a therapeutic modality for a traumatic or a post-surgical infection in orthopedics. PDT could be a new strategic application for treatment and prevention of bone and joint bacterial infections as well as intractable arthritis caused by multiple-drug-resistant bacteria.
Conclusion
This review has presented data that show for the first time that activation of the immune response after antimicrobial PDT is an important process. It had previously been assumed that in PDT for infection, the PS bound to the infecting microorganism, and the ROS, produced after illumination, killed the pathogen without excessively damaging the host tissue. In the case of bacterial arthritis in the knee the chief elements of the host response system that respond to the bacteria are neutrophils.
In retrospect it should not have been surprising that PDT of bacterial arthritis by intravenously administered Photofrin was ineffective. Intravenously injected PS delivered by blood flow in the capillaries are designed to be targeted to host cells and tissue, not to be targeted to invading microbial cells. Furthermore, one type of host cell that is highly susceptible to PDT, namely neutrophils, are the primary mediators of host defense to invading bacteria. If PDT kills the main defenders it is unlikely to be beneficial for the infection. When the Photofrin was injected into the infected area (intra-articular) the effects on the infection were better than after intravenous injection. Even here, however, there was a noticeable biphasic dose response, whereby low or high doses of light were less effective than a modest dose that allowed bacterial reduction without killing the host neutrophils. However Photofrin is a PS designed to treat cancer and could be expected to bind preferentially to host cells such as neutrophils, even though it is also known to kill Gram-positive bacteria such as MRSA. In an effort to identify a more selective PS that would kill bacteria without killing neutrophils, we screened a panel of potential PS and selected MB as a lead compound.
When intra-articular MB was tested in a MRSA arthritis model with bioluminescent bacteria, there was again a beneficial response with modest doses of light. However, the reduction in bioluminescence was blocked by antibodies against neutrophils, suggesting that PDT was stimulating the host response rather than killing the actual bacteria. This observation prompted us to test the novel concept of carrying out PDT before initiating the infection. The success of the preventative regimen suggests that PDT could be used in a prophylactive manner in high-risk orthopedic procedures, where infection is a real risk. Taken together the data covered in this review show that activation of the host immune response in the course of PDT for infection can no longer be ignored.
Expert commentary
This is the first demonstration that the activation of the host immune response after PDT, a phenomenon that has been frequently studied in cancer applications, is also involved in the therapeutic effects of PDT for infections. It had previously been assumed that all the therapeutic benefit of PDT in infection models was due to direct killing of bacteria and fungi by the ROS produced in PDT. The known activation of neutrophils after PDT, their mobilization from the bone marrow and their attraction to the site of inflammation caused when PDT is carried out in tissue, suggests that these mechanisms could also be responsible for significant antibacterial effects. Many questions remain. Do these mechanisms centered on host cell activation apply in other infection models besides bacterial arthritis? Do these mechanisms apply to other classes of pathogen such as Gram-negative bacteria or fungal cells? Our study focused on identifying a PS that preserved the viability of neutrophils while killing bacteria, rather than identifying different PS that could possibly better carry out the priming of neutrophils to respond to bacterial infection, so further PS screening investigation may be necessary.
Five-year view
It is expected that the discoveries covered in this review could have implications in the broader field of PDT for infection. Investigators will have to ask themselves to what extent the reduction in pathogen numbers that is observed after PDT to localized infections is really due to PDT-mediated killing of the microorganisms and to what extent the reduction is due to activation of the host innate immune system after PDT. Furthermore the use of PDT as a preventative measure in high-risk surgical procedures may need to be given serious consideration. Since no overt damage appears to be caused by preventative PDT and, moreover, low-dose PDT has been shown to increase the wound healing response in several models as well as in non-healing ulcers in humans, the use of preventative PDT may need to be investigated further.
Key issues.
Photodynamic therapy (PDT) is known to activate the host immune response in cancer treatments.
PDT has been studied in some arthritis models particularly of rheumatoid arthritis.
PDT has been used for many models of localized infection based on direct PDT killing of the microorganisms.
PDT for bacterial arthritis using intravenous Photofrin fails due to killing of host neutrophils.
PDT for bacterial arthritis using intra-articular Photofrin shows a biphasic dose response due to neutrophil damage at high dose.
Methylene blue was the optimal photosensitizer for killing bacteria while preserving the viability of neutrophils.
PDT with intra-articular methylene blue showed a beneficial effect in a model with bioluminescent methicillin-resistant Staphylococcus aureus arthritis that was dependent on host neutrophils.
Preventative PDT (administered before infection) protected against development of methicillin-resistant S. aureus arthritis via priming of host neutrophils.
Footnotes
Financial & competing interests disclosure
Research in the Hamblin laboratory is supported by US NIH grant R01AI050875 M. Tanaka and Y Morimoto were supported by a Grant-in-Aid for Challenging Exploratory Research (23659628) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Hamblin MR, Mroz P. History of PDT - the first hundred years. In: Hamblin MR, Mroz P, editors. Advances in Photodynamic Therapy: Basic, Translational and Clinical. Artech House, MA, USA: 2008. pp. 1–12. [Google Scholar]
- 2•.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. doi: 10.3322/caac.20114. Authoritative and up to date review of photodynamic therapy (PDT) for cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B Biol. 1997;39(1):1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 4.St Denis TG, Dai T, Izikson L, et al. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2(6):509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Hamblin MR, Jori G. Photodynamic inactivation of microbial pathogens: medical and environmental applications. In: Hader GP, Jori G, editors. Comprehensive Series in Photochemical and Photobiological Sciences. RSC Publishing; Cambridge, UK: 2011. Recently published textbook containing 17 chapters on all aspects of antimicrobial PDT from in vitro, through animal studies to clinical reports. [Google Scholar]
- 7.Sharma SK, Dai T, Kharkwal GB, et al. Drug discovery of antimicrobial photosensitizers using animal models. Curr Pharm Des. 2011;17(13):1303–1319. doi: 10.2174/138161211795703735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections – state of the art. Photodiagnosis Photodyn Ther. 2009;6(3–4):170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43(7):755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. Comprehensive review on the simulation of immune response that has been observed after PDT for cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7(1):75–91. doi: 10.1586/eci.10.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Denis TG, Aziz K, Waheed AA, et al. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photochem Photobiol Sci. 2011;10(5):792–801. doi: 10.1039/c0pp00326c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollnick SO, Owczarczak B, Maier P. Photodynamic therapy and anti-tumor immunity. Lasers Surg Med. 2006;38(5):509–515. doi: 10.1002/lsm.20362. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 16.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010;15(9):1050–1071. doi: 10.1007/s10495-010-0479-7. [DOI] [PubMed] [Google Scholar]
- 18.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown. Photochem Photobiol Sci. 2011;10(5):670–680. doi: 10.1039/c0pp00294a. [DOI] [PubMed] [Google Scholar]
- 19.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65(3):1018–1026. [PubMed] [Google Scholar]
- 20.Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56(10):2355–2360. [PubMed] [Google Scholar]
- 21.Chun KH, Seong SY. CD14 but not MD2 transmit signals from DAMP. Int Immunopharmacol. 2010;10(1):98–106. doi: 10.1016/j.intimp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Lee KM, Seong SY. Partial role of TLR4 as a receptor responding to damage-associated molecular pattern. Immunol Lett. 2009;125(1):31–39. doi: 10.1016/j.imlet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem Photobiol. 2001;74(5):712–720. doi: 10.1562/0031-8655(2001)074<0712:iosnri>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Cecic I, Stott B, Korbelik M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int Immunopharmacol. 2006;6(8):1259–1266. doi: 10.1016/j.intimp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br J Cancer. 1995;71(3):549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vree WJ, Essers MC, de Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56(13):2908–2911. [PubMed] [Google Scholar]
- 27.Cecic I, Korbelik M. Deposition of complement proteins on cells treated by photodynamic therapy in vitro. J Environ Pathol Toxicol Oncol. 2006;25(1–2):189–203. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.110. [DOI] [PubMed] [Google Scholar]
- 28.Korbelik M, Cecic I. Complement activation cascade and its regulation: relevance for the response of solid tumors to photodynamic therapy. J Photochem Photobiol B Biol. 2008;93(1):53–59. doi: 10.1016/j.jphotobiol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Cheng Y, Lu J, Hu R, Wan Q, Feng H. Photodynamic therapy boosts anti-glioma immunity in mice: a dependence on the activities of T cells and complement C3. J Cell Biochem. 2011;112(10):3035–3043. doi: 10.1002/jcb.23228. [DOI] [PubMed] [Google Scholar]
- 30.Steubing RW, Yeturu S, Tuccillo A, Sun CH, Berns MW. Activation of macrophages by Photofrin II during photodynamic therapy. J Photochem Photobiol B Biol. 1991;10(1–2):133–145. doi: 10.1016/1011-1344(91)80218-7. [DOI] [PubMed] [Google Scholar]
- 31.Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990;82(1):34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F, Xing D, Chen WR. Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells. Int J Cancer. 2009;125(6):1380–1389. doi: 10.1002/ijc.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korbelik M, Krosl G. Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy. Photochem Photobiol. 1994;60(5):497–502. doi: 10.1111/j.1751-1097.1994.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 34.Preise D, Oren R, Glinert I, et al. Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol Immunother. 2009;58(1):71–84. doi: 10.1007/s00262-008-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62(6):1604–1608. [PubMed] [Google Scholar]
- 36.Kushibiki T, Tajiri T, Tomioka Y, Awazu K. Photodynamic therapy induces interleukin secretion from dendritic cells. Int J Clin Exp Med. 2010;3(2):110–114. [PMC free article] [PubMed] [Google Scholar]
- 37.Etminan N, Peters C, Lakbir D, et al. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br J Cancer. 2011;105(7):961–969. doi: 10.1038/bjc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96(12):1839–1848. doi: 10.1038/sj.bjc.6603792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrzak-Henion JA, Knisely TL, Cincotta L, Cincotta E, Cincotta AH. Role of the immune system in mediating the antitumor effect of benzophenothiazine photodynamic therapy. Photochem Photobiol. 1999;69(5):575–581. [PubMed] [Google Scholar]
- 40.Canti G, Lattuada D, Nicolin A, Taroni P, Valentini G, Cubeddu R. Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anticancer Drugs. 1994;5(4):443–447. doi: 10.1097/00001813-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999;59(8):1941–1946. [PubMed] [Google Scholar]
- 42.Belicha-Villanueva A, Riddell J, Bangia N, Gollnick SO. The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules. Lasers Surg Med. 2012;44(1):60–68. doi: 10.1002/lsm.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 44.Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci USA. 2008;105(14):5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mroz P, Hamblin MR. The immunosuppressive side of PDT. Photochem Photobiol Sci. 2011;10(5):751–758. doi: 10.1039/c0pp00345j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews YJ, Damian DL. Topical photodynamic therapy is immunosuppressive in humans. Br J Dermatol. 2010;162(3):637–641. doi: 10.1111/j.1365-2133.2009.09562.x. [DOI] [PubMed] [Google Scholar]
- 47.Hayami J, Okamoto H, Sugihara A, Horio T. Immunosuppressive effects of photodynamic therapy by topical aminolevulinic acid. J Dermatol. 2007;34(5):320–327. doi: 10.1111/j.1346-8138.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 48.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin Investig Drugs. 1998;7(1):57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 49.Hunt DW, Jiang H, Granville DJ, Chan AH, Leong S, Levy JG. Consequences of the photodynamic treatment of resting and activated peripheral T lymphocytes. Immunopharmacology. 1999;41(1):31–44. doi: 10.1016/s0162-3109(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 50.Boumédine RS, Roy DC. Elimination of alloreactive T cells using photodynamic therapy. Cytotherapy. 2005;7(2):134–143. doi: 10.1080/14653240510027109. [DOI] [PubMed] [Google Scholar]
- 51.Lavie G, Meruelo D, Aroyo K, Mandel M. Inhibition of the CD8+ T cell-mediated cytotoxicity reaction by hypericin: potential for treatment of T cell-mediated diseases. Int Immunol. 2000;12(4):479–486. doi: 10.1093/intimm/12.4.479. [DOI] [PubMed] [Google Scholar]
- 52.Favre L, Borle F, Velin D, et al. Low dose endoluminal photodynamic therapy improves murine T cell-mediated colitis. Endoscopy. 2011;43(7):604–616. doi: 10.1055/s-0030-1256382. [DOI] [PubMed] [Google Scholar]
- 53.Rook AH, Wood GS, Duvic M, Vonderheid EC, Tobia A, Cabana B. A Phase II placebo-controlled study of photodynamic therapy with topical hypericin and visible light irradiation in the treatment of cutaneous T-cell lymphoma and psoriasis. J Am Acad Dermatol. 2010;63(6):984–990. doi: 10.1016/j.jaad.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 54.Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56(24):5647–5652. [PubMed] [Google Scholar]
- 55.Castano AP, Liu Q, Hamblin MR. A green fluorescent protein-expressing murine tumour but not its wild-type counterpart is cured by photodynamic therapy. Br J Cancer. 2006;94(3):391–397. doi: 10.1038/sj.bjc.6602953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mroz P, Szokalska A, Wu MX, Hamblin MR. Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response. PLoS ONE. 2010;5(12):e15194. doi: 10.1371/journal.pone.0015194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a basal cell carcinoma associated antigen following photodynamic therapy. Clin Cancer Res. 2009;15(13):4460–4466. doi: 10.1158/1078-0432.CCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thong PS, Olivo M, Kho KW, et al. Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J Environ Pathol Toxicol Oncol. 2008;27(1):35–42. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i1.40. [DOI] [PubMed] [Google Scholar]
- 59.Thong PS, Ong KW, Goh NS, et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8(10):950–952. doi: 10.1016/S1470-2045(07)70318-2. [DOI] [PubMed] [Google Scholar]
- 60.Canti G, Calastretti A, Bevilacqua A, Reddi E, Palumbo G, Nicolin A. Combination of photodynamic therapy + immunotherapy + chemotherapy in murine leukiemia. Neoplasma. 2010;57(2):184–188. doi: 10.4149/neo_2010_02_184. [DOI] [PubMed] [Google Scholar]
- 61.Uehara M, Sano K, Wang ZL, Sekine J, Ikeda H, Inokuchi T. Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma. Cancer Immunol Immunother. 2000;49(8):401–409. doi: 10.1007/s002620000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myers RC, Lau BH, Kunihira DY, Torrey RR, Woolley JL, Tosk J. Modulation of hematoporphyrin derivative-sensitized phototherapy with Corynebacterium parvum in murine transitional cell carcinoma. Urology. 1989;33(3):230–235. doi: 10.1016/0090-4295(89)90399-3. [DOI] [PubMed] [Google Scholar]
- 63.Korbelik M. Cancer vaccines generated by photodynamic therapy. Photochem Photobiol Sci. 2011;10(5):664–669. doi: 10.1039/c0pp00343c. [DOI] [PubMed] [Google Scholar]
- 64.Gollnick SO, Brackett CM. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol Res. 2010;46(1–3):216–226. doi: 10.1007/s12026-009-8119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allaire S, Wolfe F, Niu J, Lavalley MP. Contemporary prevalence and incidence of work disability associated with rheumatoid arthritis in the US. Arthritis Rheum. 2008;59(4):474–480. doi: 10.1002/art.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lacaille D. Arthritis and employment research: where are we? Where do we need to go? J Rheumatol Suppl. 2005;72:42–45. [PubMed] [Google Scholar]
- 67.Beischer AD, Bhathal P, de Steiger R, Penn D, Stylli S. Synovial ablation in a rabbit rheumatoid arthritis model using photodynamic therapy. ANZ J Surg. 2002;72(7):517–522. doi: 10.1046/j.1445-2197.2002.02451.x. [DOI] [PubMed] [Google Scholar]
- 68•.Trauner KB, Hasan T. Photodynamic treatment of rheumatoid and inflammatory arthritis. Photochem Photobiol. 1996;64(5):740–750. doi: 10.1111/j.1751-1097.1996.tb01829.x. Review discussing the application of PDT for various forms of arthritis. [DOI] [PubMed] [Google Scholar]
- 69.Peacock DJ, Banquerigo ML, Brahn E. A novel angiogenesis inhibitor suppresses rat adjuvant arthritis. Cell Immunol. 1995;160(2):178–184. doi: 10.1016/0008-8749(95)80025-e. [DOI] [PubMed] [Google Scholar]
- 70.Ratkay LG, Chowdhary RK, Neyndorff HC, Tonzetich J, Waterfield JD, Levy JG. Photodynamic therapy; a comparison with other immunomodulatory treatments of adjuvant-enhanced arthritis in MRL-lpr mice. Clin Exp Immunol. 1994;95(3):373–377. doi: 10.1111/j.1365-2249.1994.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trauner KB, Gandour-Edwards R, Bamberg M, Shortkroff S, Sledge C, Hasan T. Photodynamic synovectomy using benzoporphyrin derivative in an antigen-induced arthritis model for rheumatoid arthritis. Photochem Photobiol. 1998;67(1):133–139. [PubMed] [Google Scholar]
- 72.Ratkay LG, Chowdhary RK, Iamaroon A, et al. Amelioration of antigen-induced arthritis in rabbits by induction of apoptosis of inflammatory cells with local application of transdermal photodynamic therapy. Arthritis Rheum. 1998;41(3):525–534. doi: 10.1002/1529-0131(199803)41:3<525::AID-ART19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 73.Chowdhary RK, Ratkay LG, Canaan AJ, Waterfield JD, Richter AM, Levy JG. Uptake of verteporfin by articular tissues following systemic and intra-articular administration. Biopharm Drug Dispos. 1998;19(6):395–400. doi: 10.1002/(sici)1099-081x(199809)19:6<395::aid-bdd117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 74.Funke B, Jungel A, Schastak S, Wiedemeyer K, Emmrich F, Sack U. Transdermal photodynamic therapy–a treatment option for rheumatic destruction of small joints? Lasers Surg Med. 2006;38(9):866–874. doi: 10.1002/lsm.20391. [DOI] [PubMed] [Google Scholar]
- 75.Oertel M, Schastak SI, Tannapfel A, et al. Novel bacteriochlorine for high tissue-penetration: photodynamic properties in human biliary tract cancer cells in vitro and in a mouse tumour model. J Photochem Photobiol B Biol. 2003;71(1–3):1–10. doi: 10.1016/s1011-1344(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 76.Schastak S, Jean B, Handzel R, et al. Improved pharmacokinetics, biodistribution and necrosis in vivo using a new near infra-red photosensitizer: tetrahydroporphyrin tetratosylat. J Photochem Photobiol B Biol. 2005;78(3):203–213. doi: 10.1016/j.jphotobiol.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol. 1981;77(1):13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 78.Torikai E, Kageyama Y, Kohno E, et al. Photodynamic therapy using talaporfin sodium for synovial membrane from rheumatoid arthritis patients and collagen-induced arthritis rats. Clin Rheumatol. 2008;27(6):751–761. doi: 10.1007/s10067-007-0794-8. [DOI] [PubMed] [Google Scholar]
- 79.Taber SW, Fingar VH, Coots CT, Wieman TJ. Photodynamic therapy using mono-L-aspartyl chlorin e6 (Npe6) for the treatment of cutaneous disease: a Phase I clinical study. Clin Cancer Res. 1998;4(11):2741–2746. [PubMed] [Google Scholar]
- 80.Nakamura H, Suzuki Y, Takeichi M, Saito T, Takayama M, Aizawa K. Morphologic evaluation of the antitumor activity of photodynamic therapy (PDT) using mono-L-aspartyl chlorin e6 (NPe6) against uterine cervical carcinoma cell lines. Int J Gynecol Cancer. 2002;12(2):177–186. doi: 10.1046/j.1525-1438.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 81.Mori K, Yoneya S, Anzail K, et al. Photodynamic therapy of experimental choroidal neovascularization with a hydrophilic photosensitizer: mono-L-aspartyl chlorin e6. Retina (Philadelphia, PA) 2001;21(5):499–508. doi: 10.1097/00006982-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 82.Nagae T, Aizawa K, Uchimura N, et al. Endovascular photodynamic therapy using mono-L-aspartylchlorin e6 to inhibit Intimal hyperplasia in balloon-injured rabbit arteries. Lasers Surg Med. 2001;28(4):381–388. doi: 10.1002/lsm.1066. [DOI] [PubMed] [Google Scholar]
- 83.Kato H, Furukawa K, Sato M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer. 2003;42(1):103–111. doi: 10.1016/s0169-5002(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 84.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of Gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55(1):89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 85.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B Biol. 1992;14(3):262–266. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 86.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44(3):522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valduga G, Bertoloni G, Reddi E, Jori G. Effect of extracellularly generated singlet oxygen on Gram-positive and Gram-negative bacteria. J Photochem Photobiol B Biol. 1993;21(1):81–86. doi: 10.1016/1011-1344(93)80168-9. [DOI] [PubMed] [Google Scholar]
- 88.Tegos GP, Demidova TN, Arcila-Lopez D, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12(10):1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohr H, Lambrecht B, Selz A. Photodynamic virus inactivation of blood components. Immunol Invest. 1995;24(1–2):73–85. doi: 10.3109/08820139509062763. [DOI] [PubMed] [Google Scholar]
- 90.Kassab K, Ben Amor T, Jori G, Coppellotti O. Photosensitization of Colpoda inflata cysts by meso-substituted cationic porphyrins. Photochem Photobiol Sci. 2002;1(8):560–564. doi: 10.1039/b201267g. [DOI] [PubMed] [Google Scholar]
- 91.Demidova TN, Hamblin MR. Photodynamic inactivation of Bacillus spores, mediated by phenothiazinium dyes. Appl Environ Microbiol. 2005;71(11):6918–6925. doi: 10.1128/AEM.71.11.6918-6925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferro S, Coppellotti O, Roncucci G, Ben Amor T, Jori G. Photosensitized inactivation of Acanthamoeba palestinensis in the cystic stage. J Appl Microbiol. 2006;101(1):206–212. doi: 10.1111/j.1365-2672.2006.02893.x. [DOI] [PubMed] [Google Scholar]
- 93.Jori G, Fabris C, Soncin M, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38(5):468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 94.Harris F, Chatfield LK, Phoenix DA. Phenothiazinium based photosensitisers–photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr Drug Targets. 2005;6(5):615–627. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 95.Huang L, Huang YY, Mroz P, et al. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother. 2010;54(9):3834–3841. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuznetsova N, Makarov D, Yuzhakova O, et al. Photophysical properties and photodynamic activity of octacationic oxotitanium(IV) phthalocyanines. Photochem Photobiol Sci. 2009;8(12):1724–1733. doi: 10.1039/b9pp00054b. [DOI] [PubMed] [Google Scholar]
- 97.Usacheva MN, Teichert MC, Biel MA. The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J Photochem Photobiol B, Biol. 2003;71(1–3):87–98. doi: 10.1016/j.jphotobiol.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against Gram-positive and Gram-negative microorganisms. Lasers Surg Med. 2001;29(2):165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 99.Wainwright M. Methylene blue derivatives – suitable photoantimicrobials for blood product disinfection? Int J Antimicrob Agents. 2000;16(4):381–394. doi: 10.1016/s0924-8579(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 100.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160(2):177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 101.Lazzeri D, Rovera M, Pascual L, Durantini EN. Photodynamic studies and photoinactivation of Escherichia coli using meso-substituted cationic porphyrin derivatives with asymmetric charge distribution. Photochem Photobiol. 2004;80(2):286–293. doi: 10.1562/2004-03-08-RA-105. [DOI] [PubMed] [Google Scholar]
- 102.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49(4):1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Segalla A, Borsarelli CD, Braslavsky SE, et al. Photophysical, photochemical and antibacterial photosensitizing properties of a novel octacationic Zn(II)-phthalocyanine. Photochem Photobiol Sci. 2002;1(9):641–648. doi: 10.1039/b202031a. [DOI] [PubMed] [Google Scholar]
- 104.Kussovski V, Mantareva V, Angelov I, et al. Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity. FEMS Microbiol Lett. 2009;294(2):133–140. doi: 10.1111/j.1574-6968.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- 105.Simonetti O, Cirioni O, Orlando F, et al. Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/Cl in an experimental model of Staphylococcus aureus wound infection. Br J Dermatol. 2011;164(5):987–995. doi: 10.1111/j.1365-2133.2011.10232.x. [DOI] [PubMed] [Google Scholar]
- 106.Gad F, Zahra T, Francis KP, Hasan T, Hamblin MR. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem Photobiol Sci. 2004;3(5):451–458. doi: 10.1039/b311901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(2):155–160. doi: 10.1067/moe.2002.120051. [DOI] [PubMed] [Google Scholar]
- 108.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187(11):1717–1725. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wong TW, Wang YY, Sheu HM, Chuang YC. Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob Agents Chemother. 2005;49(3):895–902. doi: 10.1128/AAC.49.3.895-902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zolfaghari PS, Packer S, Singer M, et al. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111•.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75(1):51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. The first description of the use of genetically engineered bioluminescent bacteria to monitor antimicrobial PDT of infections. [DOI] [PubMed] [Google Scholar]
- 112.Dai T, Tegos GP, Lu Z, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53(9):3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem Photobiol Sci. 2005;4(7):503–509. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shibli JA, Martins MC, Nociti FH, Jr, Garcia VG, Marcantonio E., Jr Treatment of ligature-induced peri-implantitis by lethal photosensitization and guided bone regeneration: a preliminary histologic study in dogs. J Periodontol. 2003;74(3):338–345. doi: 10.1902/jop.2003.74.3.338. [DOI] [PubMed] [Google Scholar]
- 115.Kömerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47(3):932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernandes LA, de Almeida JM, Theodoro LH, et al. Treatment of experimental periodontal disease by photodynamic therapy in immunosuppressed rats. J Clin Periodontol. 2009;36(3):219–228. doi: 10.1111/j.1600-051X.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- 117.de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG. Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol. 2008;79(11):2156–2165. doi: 10.1902/jop.2008.080103. [DOI] [PubMed] [Google Scholar]
- 118.de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG. Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol. 2007;78(3):566–575. doi: 10.1902/jop.2007.060214. [DOI] [PubMed] [Google Scholar]
- 119.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology (NY) 1994;12(7):703–706. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 120.Smetana Z, Malik Z, Orenstein A, Mendelson E, Ben-Hur E. Treatment of viral infections with 5-aminolevulinic acid and light. Lasers Surg Med. 1997;21(4):351–358. doi: 10.1002/(sici)1096-9101(1997)21:4<351::aid-lsm6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 121.Moore C, Wallis C, Melnick JL, Kuns MD. Photodynamic treatment of herpes keratitis. Infect Immun. 1972;5(2):169–171. doi: 10.1128/iai.5.2.169-171.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gardlo K, Horska Z, Enk CD, et al. Treatment of cutaneous leishmaniasis by photodynamic therapy. J Am Acad Dermatol. 2003;48(6):893–896. doi: 10.1067/mjd.2003.218. [DOI] [PubMed] [Google Scholar]
- 123.Sohl S, Kauer F, Paasch U, Simon JC. Photodynamic treatment of cutaneous leishmaniasis. J Dtsch Dermatol Ges. 2007;5(2):128–130. doi: 10.1111/j.1610-0387.2007.06177.x. [DOI] [PubMed] [Google Scholar]
- 124.González U, Pinart M, Reveiz L, Alvar J. Interventions for old world cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008;4:CD005067. doi: 10.1002/14651858.CD005067.pub3. [DOI] [PubMed] [Google Scholar]
- 125.Maisch T, Baier J, Franz B, et al. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci USA. 2007;104(17):7223–7228. doi: 10.1073/pnas.0611328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126•.Tanaka M, Kinoshita M, Yoshihara Y, et al. Influence of intra-articular neutrophils on the effects of photodynamic therapy for murine MRSA arthritis. Photochem Photobiol. 2010;86(2):403–409. doi: 10.1111/j.1751-1097.2009.00658.x. Shows that PDT of bacterial arthritis with intravenously injected Photofrin fails due to PDT killing of neutrophils. [DOI] [PubMed] [Google Scholar]
- 127.Hamblin MR, Dai T. Can surgical site infections be treated by photodynamic therapy? Photodiagnosis Photodyn Ther. 2010;7(2):134–136. doi: 10.1016/j.pdpdt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128•.Tanaka M, Kinoshita M, Yoshihara Y, et al. Photodynamic therapy using intra-articular Photofrin for murine MRSA arthritis: biphasic light dose response for neutrophil-mediated antibacterial effect. Lasers Surg Med. 2011;43(3):221–229. doi: 10.1002/lsm.21037. Demonstrates that PDT of bacterial arthritis with intra-articular injected Photofrin exhibits a biphasic dose response and suggests that the beneficial effects are due to neutrophil mediated killing of bacteria rather than direct PDT bacterial killing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129•.Tanaka M, Kinoshita M, Yoshihara Y, et al. Optimal photosensitizers for photodynamic therapy of infections should kill bacteria but spare neutrophils. Photochem Photobiol. 2012;88(1):227–232. doi: 10.1111/j.1751-1097.2011.01005.x. Reports the screening of a panel of eight photosensitizers to determine candidates that can mediate PDT killing of bacteria without killing host neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57(4):680–684. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 131.Docampo R, Moreno SN, Muniz RP, Cruz FS, Mason RP. Light-enhanced free radical formation and trypanocidal action of gentian violet (crystal violet) Science. 1983;220(4603):1292–1295. doi: 10.1126/science.6304876. [DOI] [PubMed] [Google Scholar]
- 132.Kato H, Usuda J, Okunaka T, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med. 2006;38(5):371–375. doi: 10.1002/lsm.20346. [DOI] [PubMed] [Google Scholar]
- 133.Tseng SP, Teng LJ, Chen CT, et al. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Lasers Surg Med. 2009;41(5):391–397. doi: 10.1002/lsm.20765. [DOI] [PubMed] [Google Scholar]
- 134.Hirao A, Sato S, Saitoh D, Shinomiya N, Ashida H, Obara M. In vivo photoacoustic monitoring of photosensitizer distribution in burned skin for antibacterial photodynamic therapy. Photochem Photobiol. 2010;86(2):426–430. doi: 10.1111/j.1751-1097.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 135.Renkin EM. Cellular and intercellular transport pathways in exchange vessels. Am Rev Respir Dis. 1992;146(5 Pt 2):S28–S31. doi: 10.1164/ajrccm/146.5_Pt_2.S28. [DOI] [PubMed] [Google Scholar]
- 136.König K, Bockhorn V, Dietel W, Schubert H. Photochemotherapy of animal tumors with the photosensitizer methylene blue using a krypton laser. J Cancer Res Clin Oncol. 1987;113(3):301–303. doi: 10.1007/BF00396390. [DOI] [PubMed] [Google Scholar]
- 137.Tanaka M, Mroz P, Dai T, Huang L, Morimoto Y, et al. Photodynamic therapy can induce a protective innate immune response against murine bacterial arthritis via neutrophil accumulation. PLoS ONE. 2012;7(6):e39823. doi: 10.1371/journal.pone.0039823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42(1):38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002;183(1):43–51. doi: 10.1016/s0304-3835(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 140.Montefort S, Gratziou C, Goulding D, et al. Bronchial biopsy evidence for leukocyte infiltration and upregulation of leukocyte-endothelial cell adhesion molecules 6 hours after local allergen challenge of sensitized asthmatic airways. J Clin Invest. 1994;93(4):1411–1421. doi: 10.1172/JCI117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kielian T, Hickey WF. Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development. Am J Pathol. 2000;157(2):647–658. doi: 10.1016/S0002-9440(10)64575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun YX, Dai DK, Liu R, et al. Therapeutic effect of SN50, an inhibitor of nuclear factor-κB, in treatment of TBI in mice. Neurol Sci. 2012 doi: 10.1007/s10072-012-1007-z. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]