Abstract
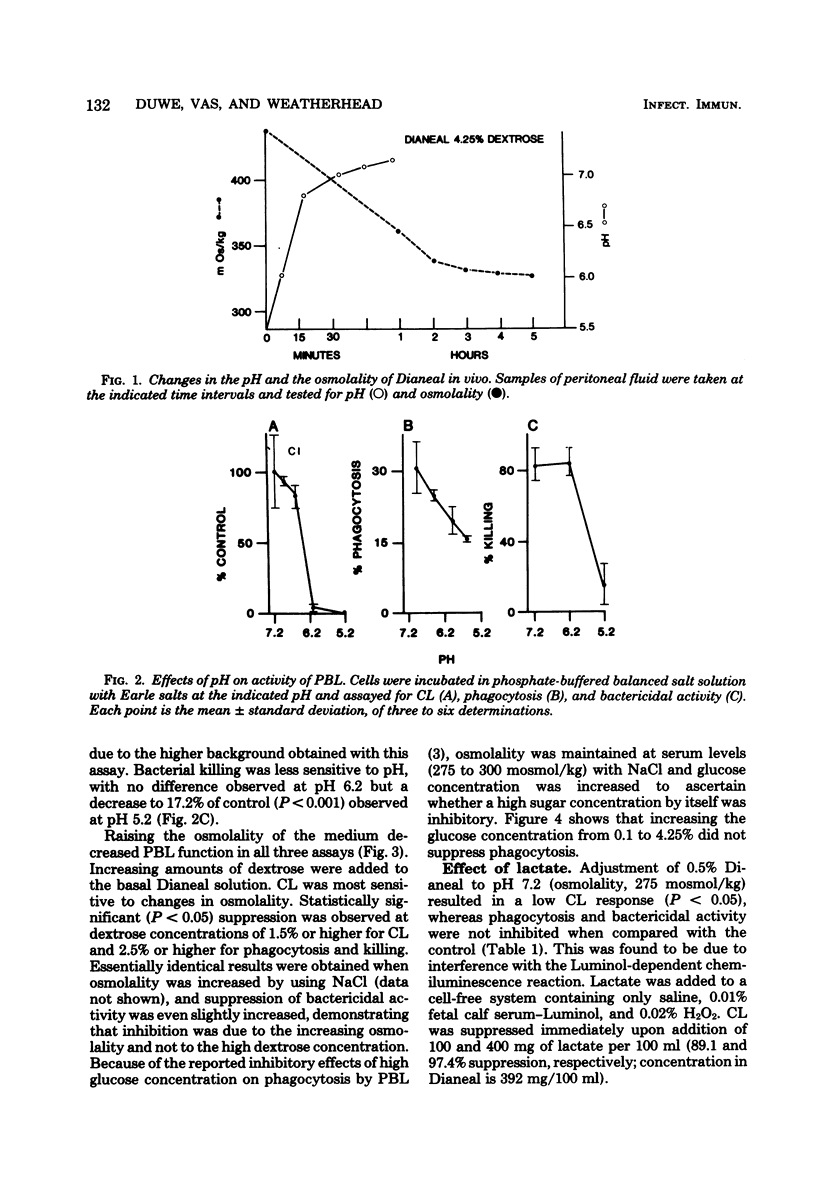
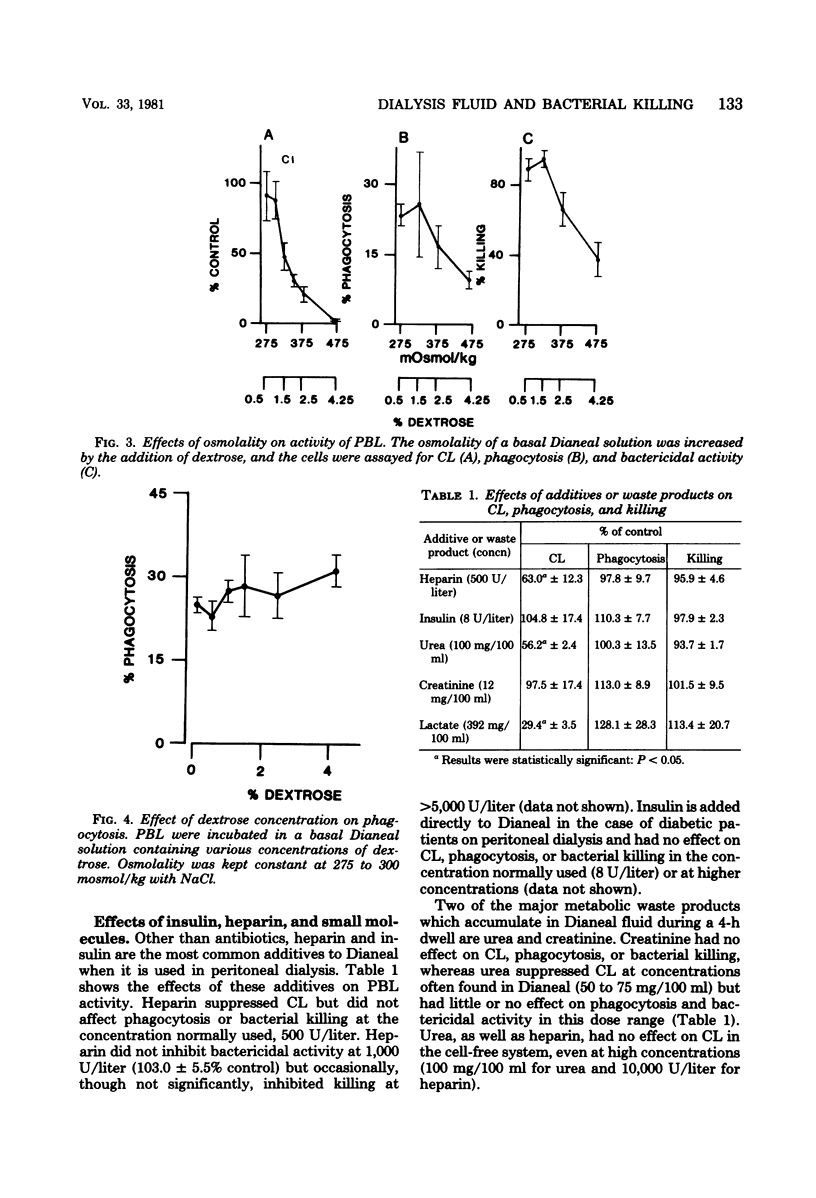
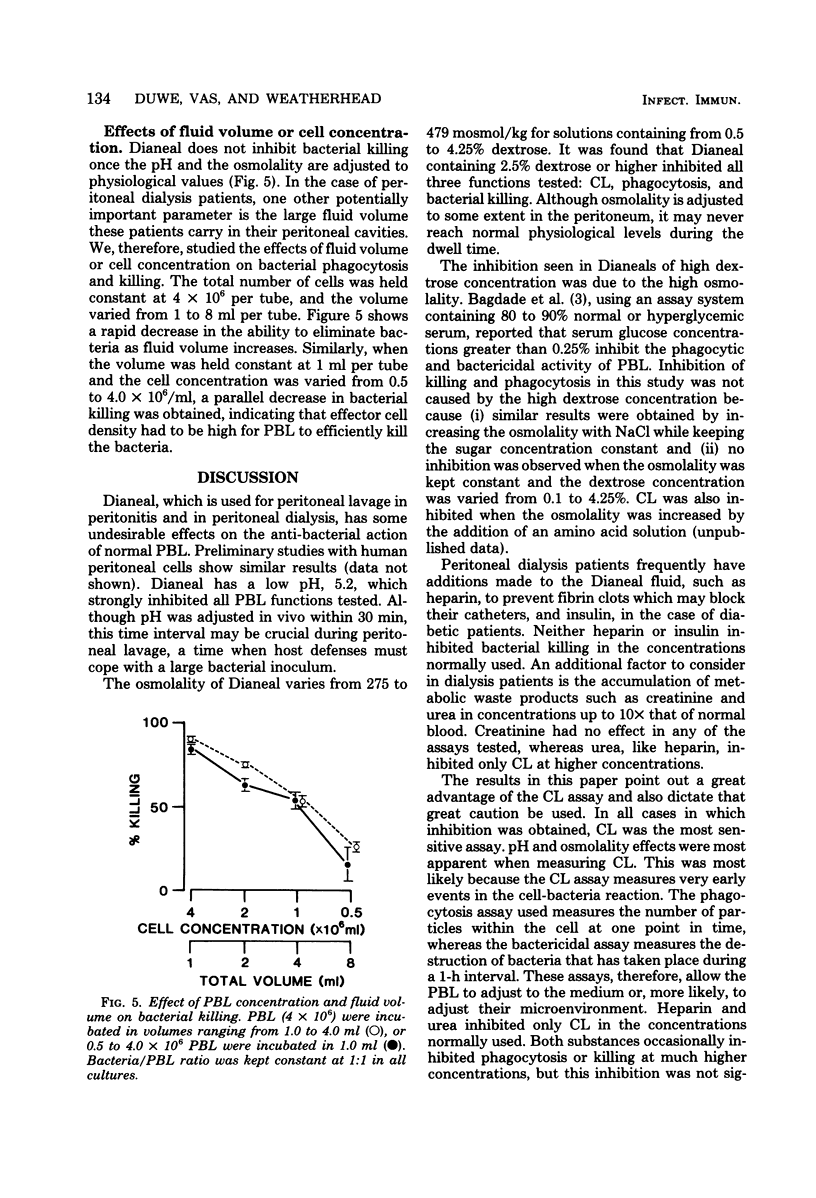
Commercial fluid used for peritoneal lavage in peritonitis and in peritoneal dialysis suppressed the activity of peripheral blood leukocytes as measured by chemiluminescence, phagocytosis, and bacterial killing. Suppression was found to be due to the low pH and high osmolality of the fluid. The pH was adjusted to noninhibitory levels in vivo within 30 min, whereas osmolality changes were less rapid and remained at inhibitory levels for fluids of high dextrose concentration (4.25%). Chemiluminescence was the most sensitive assay for inhibitory effects of pH and osmolality, as well as for urea and heparin. The metabolic waste product urea at levels normally found in dialysate and heparin at concentrations routinely added to fluid inhibited only chemiluminescence, whereas creatinine and added insulin were not inhibitory. High fluid volume also resulted in a decrease in efficiency of bacterial killing. These results suggest some changes to be made in the treatment of peritonitis and in peritoneal dialysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Root R. K., Bulger R. J. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974 Jan;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Allred C. D., Solberg C. O., Hill H. R. Chemiluminescence by polymorphonuclear leukocytes from patients with active bacterial infection. J Infect Dis. 1980 Jan;141(1):14–26. doi: 10.1093/infdis/141.1.14. [DOI] [PubMed] [Google Scholar]
- Hau T., Ahrenholz D. H., Simmons R. L. Secondary bacterial peritonitis: the biologic basis of treatment. Curr Probl Surg. 1979 Oct;16(10):1–65. doi: 10.1016/s0011-3840(79)80011-8. [DOI] [PubMed] [Google Scholar]
- Hooke A. M., Oeschger M. P., Zeligs B. J., Bellanti J. A. Ideal target organism for quantitative bactericidal assays. Infect Immun. 1978 May;20(2):406–411. doi: 10.1128/iai.20.2.406-411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley R. M., Muogbo D., Wilson G. W., Ali M. A. Cellular composition of peritoneal effluent: response to bacterial peritonitis. Can Med Assoc J. 1977 Nov 5;117(9):1061–1062. [PMC free article] [PubMed] [Google Scholar]
- Montgomerie J. Z., Kalmanson G. M., Guze L. B. Renal failure and infection. Medicine (Baltimore) 1968 Jan;47(1):1–32. doi: 10.1097/00005792-196801000-00001. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., Jr, SMITH M. R., PERRY W. D., BERRY J. W. Studies on the cellular immunology of acute bacteremia. I. Intravascular leucocytic reaction and surface phagocytosis. J Exp Med. 1951 Dec 1;94(6):521–534. doi: 10.1084/jem.94.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S., Demus A. Antibody-dependent cytolysis of chicken erythrocytes by an in vitro-established line of mouse peritoneal macrophages. J Immunol. 1975 Feb;114(2 Pt 2):765–769. [PubMed] [Google Scholar]



