Abstract
Sequential administration of anthracyclin and taxane for neoadjuvant chemotherapy (NAC) is the standard treatment for operable breast cancer. The pathological complete response (pCR) is a significant predictor of overall survival (OS), regardless of treatment. In this study, the pCR rate was retrospectively examined and compared with the treatment efficacy and the characteristics of pCR patients were analyzed. A total of 54 female patients with operable breast cancer, treated with FEC 100 followed by weekly paclitaxel between December 2005 and May 2009 at the Osaka City University Hospital, Osaka, Japan, were retrospectively reviewed. A total of 21 patients (39%) achieved pCR. The overall response rate was 91%. Only one patient had progressive disease. The pCR rate was significantly higher in those patients with estrogen receptor (ER)- and progesterone receptor (PR)-negative tumors and in those patients who completed the treatment course. An NAC regimen incorporating FEC 100 followed by weekly paclitaxel is effective for treating operable breast cancer.
Keywords: operable breast cancer, neoadjuvant chemotherapy, FEC 100, weekly paclitaxel
Introduction
Preoperative neoadjuvant chemotherapy (NAC) is a promising method for improving the breast-conserving treatment of operable breast cancer (1,2). Based on large scale trials that clearly demonstrate the feasibility and efficacy of NAC, it is now the treatment of choice for these patients as it effectively improves prognoses in a practical clinical setting. In 2001, Wolmark et al demonstrated that NAC, four cycles of doxorubicin and cyclophosphamide (AC), had a similar effect on overall survival (OS) and disease-free survival (DFS) to that of chemotherapy administered post-operatively (1). Van der Hage et al compared the efficacy of four cycles of combination chemotherapy with cyclophosphamide, epirubicin and fluorouracil (CEF) that were administered preoperatively with that of the same chemotherapy regimen administered postoperatively in patients with operable breast cancer. The study identified no difference in terms of OS and progression-free survival (PFS) between patients treated with NAC and those treated with postoperative adjuvant chemotherapy (2).
NAC also has an advantage in that the response to anti-cancer agents can be precisely monitored by examining surgical specimens after treatment. In one study (NSABP B-27), preoperative chemotherapy with AC followed by docetaxel increased the pathological complete response rate (pCR; the disappearance of all clinical evidence of disease) compared with preoperative AC alone (26.1 vs. 13.7%, respectively) (3), and a significant improvement in prognosis was observed for patients demonstrating pCR (4). This suggests that pCR is a significant predictor of prognosis.
Currently, combination chemotherapy with anthracyclines and taxanes is considered to be the most effective method for achieving the maximal effect of adjuvant therapy in patients with operable breast cancer with lymph node metastasis (3,5–7). However, the best protocol/schedule for administering these drugs has not been identified. Similar questions also arise regarding NAC. Bonneterre et al revealed that the FEC 100 protocol yielded significant improvements over the FEC 50 protocol in terms of DFS and OS after 10 years of follow-up when used as adjuvant chemotherapy for patients with operable breast cancer with lymph node metastasis. The treatment had an acceptable rate of adverse events and no toxicity-related mortalities (8). Another trial demonstrated the efficacy of weekly paclitaxel followed by four cycles of fluorouracil/doxorubicin/cyclophosphamide in patients with stage I–III breast cancer. Patients receiving weekly paclitaxel had a higher pCR rate (28.2%) than those treated with a ‘once every 3 weeks’ protocol (15.7%; P=0.02) (9,10). Therefore, based on recent evidence, FEC 100 followed by weekly paclitaxel was selected as the standard NAC regimen most likely to have a maximal effect on locally advanced operable breast cancer. In this study, we present the treatment results and discuss the clinical significance of this NAC protocol for operable breast cancer patients.
Patients and methods
Patients
A total of 54 females with histologically confirmed non-inflammatory invasive ductal carcinoma of the breast with stage IIA to IIIA disease [according to the UICC Classification of Breast Cancer (6)] were included in the study. Patients with stage IIA disease without lymph node involvement were excluded. All patients received NAC as the initial treatment at the Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Osaka, Japan, between December 2005 and May 2009. The histological diagnosis for each patient was made by core needle biopsy of the tumor. Cancer cell expression of estrogen receptors (ERs), progesterone receptors (PRs) and the Her-2/neu protein was also evaluated before initiating therapy. A proportion of patients with tumors overexpressing Her-2/neu were analyzed separately as they had received anti-Her-2/neu therapy in addition to NAC after 2008. To confirm that they met the inclusion criteria, all patients underwent a whole body evaluation that included a complete medical history and physical examination, a complete blood count, a blood chemistry profile, an enhanced computed tomography scan of the lungs and liver and a bone scan. Patients who had been treated with other chemotherapeutic agents, those not suitable for NAC, pregnant patients and those with evidence of distant metastasis, ischemic heart disease or hepato-renal dysfunction were excluded from the study. Individual patient characteristics are shown in Table I. The age of the patients ranged from 26 to 68 years (mean, 50.2) and the tumor size ranged from 15 to 60 mm (mean, 30.4). Patients were followed-up for 6 to 55 months (average, 29). Written informed consent was obtained from the patients prior to the study. This study was conducted with the approval of the Ethical Committee of Osaka City University Graduate School of Medicine.
Table I.
Clinical and pathological responses to neoadjuvant chemotherapy.
| Factors | No. of patients (%) | cCR | P-value | pCR | P-value |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≤50 | 30 (55.6) | 6 (20) | 0.46 | 13 (43) | 0.45 |
| >51 | 24 (44.4) | 3 (13) | 8 (33) | ||
| Stage | |||||
| IIA | 9 (16.7) | 1 (11) | 0.03 | 1 (11) | 0.10 |
| IIB | 31 (57.4) | 3 (10) | 12 (39) | ||
| III | 14 (25.9) | 5 (36) | 8 (57) | ||
| T factor | |||||
| T1 | 3 (5.6) | 2 (67) | 0.16 | 2 (67) | 0.31 |
| T2 | 48 (88.9) | 7 (15) | 18 (38) | ||
| T3 | 3 (5.6) | 0 (0) | 1 (33) | ||
| N factor | |||||
| N0 | 8 (14.8) | 1 (13) | 0.03 | 1 (13) | 0.10 |
| N1 | 32 (59.3) | 3 (9) | 12 (38) | ||
| N2 | 14 (25.9) | 5 (36) | 8 (57) | ||
| Receptor status | |||||
| ER+/PR+ | 24 (44.4) | 3 (13) | 0.36 | 7 (29) | 0.08 |
| ER+/PR− | 9 (16.7) | 1 (11) | 4 (44) | ||
| ER−/PR+ | 4 (7.4) | 1 (25) | 1 (25) | ||
| ER−/PR− | 17 (31.5) | 4 (24) | 9 (53) | ||
| Her2 status | |||||
| Her2+ | 4 (7.4) | 1 (25) | 0.66 | 3 (75) | 0.13 |
| Her2− | 49 (90.7) | 8 (16) | 18 (36) | ||
| Combination | |||||
| HR+/HER2+ | 1 (1.9) | 1 (100) | 1 (100) | ||
| HR+/HER2− | 35 (64.8) | 4 (11) | 10 (29) | ||
| HR−/HER2+ | 3 (5.6) | 0 (0) | 2 (67) | ||
| HR−/HER2− | 15 (27.9) | 4 (27) | 8 (53) | ||
| Total | 54 | 9 (17) | 21 (39) |
cCR, clinical complete response; pCR, pathological complete response. ER, estrogen receptor; PR, progesterone receptor.
Chemotherapy regimen
Chemotherapy was administered in an outpatient setting before loco-regional therapy. The FEC 100 regimen consisted of intravenous administration of fluorouracil (500 mg/m2), cyclophosphamide (500 mg/m2) and epirubicin (100 mg/m2) on day 1. This regimen was administered every 21 days for four cycles, followed by a once-weekly intravenous infusion of paclitaxel (80 mg/m2) for 12 weeks. Patients administered a full dose of each chemotherapeutic agent over 26 weeks (with no delay greater than 2 weeks) were classified as the high-dose intensity (high-DI) group. Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used to assess treatment-related toxicity.
Loco-regional treatment
Surgery (total or partial mastectomy associated with axillary lymph node dissection) was scheduled 3 weeks following the termination of paclitaxel administration. The preserved breast tissue was treated with extra-beam radiation (50 Gy) after breast-conserving mastectomy.
Clinical and pathological evaluation of treatment responses
Surgical specimens were fixed in buffered formalin and cut into 5-mm sections. The tissue was then examined by a pathologist (K.W.) after conventional H&E staining. Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) was used to assess the chemotherapeutic response of each tumor. The size of the breast tumors and any axillary lymph node metastases were assessed by ultrasound (US) examination. Clinical complete response (cCR) was defined as the clinical absence of all cancerous lesions in the breast and lymph nodes as assessed by US. Pathological findings were evaluated in accordance with the ‘General Rules for Clinical and Pathological Recording of Breast Cancer’ 16th edition (9). For those patients that underwent breast-conserving surgery, surgical specimens were treated and evaluated as described above. For patients that underwent mastectomy, the area immediately surrounding the tumor (including at least 2 cm of marginal tissue) was examined pathologically and compared with photographs and images recorded before NAC. pCR was defined as no histopathological evidence of viable cancer cells in the breast or dissected axillary lymph nodes (1). Surgically resected breast and axillary node specimens were evaluated to assess the pathological tumor response. Patients with no evidence of invasive breast cancer were considered to have a pCR. This included patients in whom only non-invasive or in situ cancer identified in the breast specimen, as well as those in whom no residual cancer cells were identified.
Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH)
All tumors were examined for ER and PR expression by IHC. Patients were classified as having ER- and/or PR-positive tumors when >10% of cancer cells demonstrated positive staining by IHC. Her-2/neu expression was also investigated using IHC and/or FISH. Tumors were classified as Her-2/neu-positive if they were scored as 3+ by IHC, or if gene amplification (>2.2-fold increase in fluorescence compared with that in the centromere) was identified by FISH.
Statistical analysis
The χ2 test was used to compare the factors and the response rates between the subgroups. P<0.05 was considered to indicate a statistically significant difference.
Results
cCR and pCR of the patients
The cCR and pCR of the patients are shown in Table II. Nine (16.7%) of the 54 patients were considered to have no residual tumor upon preoperative US examination after NAC (i.e., a cCR) and, overall, 49 patients (90.7%) demonstrated a clinical response after NAC. Histological evaluation of the surgical specimens revealed that 21/54 (38.9%) of patients had a pCR.
Table II.
Response rates to neoadjuvant chemotherapy.
| Response | Complete response (%) | Partial response (%) | Stable disease (%) | Progressive disease (%) |
|---|---|---|---|---|
| Clinical | 9 (16.7) | 40 (74.1) | 4 (7.4) | 1 (1.9) |
| Pathological | 21 (38.9) | 28 (51.9) | 4 (7.4) | 1 (1.9) |
One patient (1.9%) still displayed evidence of progressive disease after the 3rd week of paclitaxel administration, and surgery was performed 33 days after NAC was terminated. The NAC regimen was amended in five patients due to adverse events. NAC was terminated in one patient who suffered drug-induced pneumonia, and the paclitaxel regimen was changed to ‘once every three weeks’ in four patients due to repeated episodes of grade 3 neutropenia caused by weekly administration. Overall, 48 patients (88.9%) completed the full treatment course. In three patients, one or more of the administration schedules had to be postponed for more than 2 weeks due to persistent grade 3 neutropenia. Thus, 45 patients (83.3%) completed the original regimen within the scheduled period (high-DI group). The pCR rate of patients administered weekly paclitaxel was 46.7% (21/45). By contrast, none of the nine patients that did not receive paclitaxel according to the original schedule demonstrated pCR (P<0.02). Breast-conserving surgery was performed in 24 patients (44.4%).
The annual pCR rates increased over the years (Fig. 1) and were significantly higher after 2008 compared to before 2007. The chemotherapeutic schedules were adhered to more completely each year, and the pCR rate in the high-DI group was significantly higher after 2008 than before 2007 (Fig. 1). There was no evidence of recurrence in the patients with pCR, while three patients who did not demonstrate pCR had a recurrence during the 6 to 55 month follow-up period.
Figure 1.
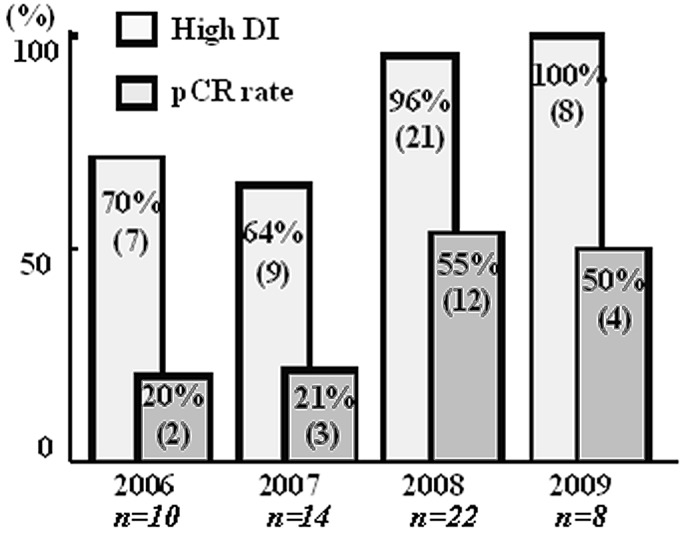
Annual rate of high-DI and pCR. Significantly higher high-DI and pCR rates are observed after 2008 compared with those prior to 2007. pCR, pathological complete response; high-DI, high dose intensity.
The factors influencing the pathological responses were also analyzed (Table I). Although not statistically significant, pCR was associated with a younger age, larger tumors and greater lymph node involvement. Thus, higher pCR rates were observed in those patients at a more advanced clinical disease stage (stage IIA, 11%; stage IIB, 39%; stage IIIA, 57%). Patients with ER- and PR-negative tumors had significantly higher pCR rates (56%) than those with ER- and PR-positive tumors (31%).
NAC toxicity
NAC toxicity is shown in Table III. No adverse events of grade ≤2 required treatment or a delay in treatment. Thirteen patients (24.1%) suffered grade 3 febrile neutropenia (FN) during the FEC 100 protocol. Three of these (23.1%) also suffered FN while receiving weekly paclitaxel. Nine of the 13 patients (76.9%) required hospitalization and recovered after treatment with antibiotics and granulocyte-colony stimulation factor (10). None of these patients required a postponement of FEC 100 or paclitaxel administration. Toxicities of grade >3 included neurotoxicity (3 patients; 5.6%), liver dysfunction (1 patient; 1.9%) and interstitial pneumonia (1 patient; 1.9%). Chemotherapy was terminated after the 11th dose of paclitaxel in the patient suffering interstitial pneumonia. The patient recovered immediately following methylpredonisolone infusion and underwent surgery with no complications 26 days later after recovery from respiratory symptoms, which included hypoxemia and coughing. The clinical and pathological responses of the patient's tumor were classified as cCR and pCR, respectively. In four patients, paclitaxel administration was delayed for 2 weeks due to grade 3 neutropenia during the 2nd, 3th, 4th and 9th weeks. The protocol was then changed to 175 mg/m2 paclitaxel once every three weeks to minimize further adverse events.
Table III.
Adverse effects of neo-adjuvant chemotherapy.
| Adverse event | Grade 2 or less (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|
| Febrile neutropenia | - | 13 (24.1) | 0 (0) |
| Neuropathy | 6 (11.1) | 3 (5.6) | 0 (0) |
| Hepatopathy | 3 (5.6) | 1 (1.9) | 0 (0) |
| Interstitial pneumonitis | 0 (0) | 1 (1.9) | 0 (0) |
Surgery was performed within 18 to 45 days (average, 23) after the final dose of NAC. None of the patients who completed the full NAC regimen demonstrated evidence of persistent adverse events for more than 3 weeks; therefore, none of the scheduled surgeries required postponing or cancellation.
Discussion
Several studies have examined the pathological responses of tumors after chemotherapy with NAC. According to several published prospective randomized case control studies, the pCR rates after anthracycline- and taxane-based NAC for locally advanced breast cancer were between 26.1 and 34% in a heterogeneous group of patients (3,12,13). The pCR rate in the present retrospective study was as high as 38.9%.
In this study, a considerable difference between cCR and pCR rate was observed (Table II). The clinical response to NAC is usually evaluated using US as the technique is non-invasive and inexpensive to perform repeatedly. However, it is difficult to distinguish small, shrinking cancer nests from non-cancerous scar tissue using imaging studies alone. Therefore, imaging studies tend to under-estimate response rates. For pathological examination, a method based on the NSABP B-18 protocol (1) was used, which is universally accepted. Although more long-term observations may be necessary to confirm the accuracy of our pCR data for predicting prognosis, the short-term results suggest a good prognosis for the pCR group, which indicates that our evaluation methods were appropriate.
A total of 18 patients (33.3%) developed adverse events of grade 3 or higher. However, all of these were managed using standard conservative treatments, and there were no persisting sequelae after NAC. Nine patients (16.7%) required hospital admission due to FN. However, all recovered within a week and continued NAC in the outpatient setting without any need to reduce the dose. FN accounts for 8–22% of all adverse events during chemotherapy (3–8,11), although it is a short-term event when managed appropriately (10), as demonstrated in the present study. Grade 3 neurotoxicity occurred in three patients during paclitaxel administration. One patient suffered from interstitial pneumonia, which required the termination of paclitaxel therapy. The patient recovered immediately after an infusion of methylpredonisolone and underwent surgery without complications. The incidence of interstitial pneumonia during paclitaxel administration is reported up be approximately 1% (14). Our results were in accordance with these observations. Overall, 83.3% of patients completed the full schedule in the present study, which demonstrates the feasibility and safety of the protocol. The high response and completion rates observed for this protocol are valuable parameters when considering a standard protocol for NAC.
The relatively low pCR rates observed for ER-positive tumors indicate that tumors of this subtype are resistant to chemotherapy. Similar results have been observed in other chemotherapeutic studies (15).
Notably, the pCR rate among patients with extensive lymphatic involvement (N2) was higher than that in patients with N0 or N1 involvement. Thus, the results revealed that patients with a higher disease stage responded better than those with a lower disease stage. In practice, adding taxane to anthracycline-based chemotherapy is only indicated for node-positive cases (6). At present, we have no logical explanation as to why the node-positive tumors demonstrated the best response rates in the present study. However, it would be of use to investigate the drug distribution and characteristics of the cancer cells that spread to the lymphatic system in these cases.
In the present study, an increasing pCR rate each year was observed, along with an increasing number of patients in the high-DI group. As reported by Citron et al, the dose-density of chemotherapy is an important factor for improving clinical outcomes (16). However, in a practical setting, the prevention of adverse effects should be the first consideration and, more importantly, the completion of the treatment protocol. At the same time, being too cautious due to fear of possible side-effects may impair the achievement of high-DI. During the early period of this study, the schedule was often postponed due to the fear of inducing repeated and life-threatening episodes of FN. This was less evident during the later period of the study. However, immediate therapy of FN using recommended treatments (9) resulted in rapid recovery without any marked delay in subsequent drug administrations. Moreover, it appears that severe FN does not occur during weekly administration of paclitaxel. Therefore, this protocol may be performed safely and without any delay to maintain high-DI. The results suggest that dose-intensity is the most significant factor for achieving a high pCR rate in the NAC setting.
This retrospective study demonstrates that a high pCR rate was achieved in patients with locally advanced operable breast cancer using a NAC regimen comprising FEC followed by paclitaxel. We consider that this will be a useful regimen, which is both safe and effective. Further prospective studies incorporating long follow-up periods are required to confirm the efficacy of this regimen. Moreover, further studies evaluating the addition of other anti-tumor drugs, including molecular targeting agents, may lead to enhanced efficacy (17,18).
References
- 1.Wolmark N, Wang J, Mamonas E, Fisher B. Preoperative chemotherapy in patients with operative breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 2.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 3.Bear HD, Anderson S, Brown A, et al. National Surgical Adjuvant Breast and Bowel Project Protocol B-27: The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 5.Herderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Mamonas EP, Bryant J, Lembersky BC, et al. Paclitaxel after doxorubicin puls cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3896. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 7.Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 8.Bonneterre J, Roché H, Kerbrat P, et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2005;23:2686–2993. doi: 10.1200/JCO.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 9.The Japanese Breast Cancer Society. General Rules for Clinical and Pathological Recording of Breast Cancer. 16th edition. 2008. p. 60. Kanehara Shuppan. [Google Scholar]
- 10.Morrison VA. An overview of the management of infection and febrile neutropenia in patients with cancer. Support Cancer Ther. 2005;2:88–94. doi: 10.3816/SCT.2005.n.002. [DOI] [PubMed] [Google Scholar]
- 11.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 13.Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20:1456–1466. doi: 10.1200/JCO.2002.20.6.1456. [DOI] [PubMed] [Google Scholar]
- 14.Khan A, McNally D, Tutschka PJ, Bilgrami S. Paclitaxel-induced acute bilateral pneumonitis. Ann Pharmacother. 1997;31:1471–1474. doi: 10.1177/106002809703101205. [DOI] [PubMed] [Google Scholar]
- 15.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 16.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 17.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, pacritaxel and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:1215–1221. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]


