Abstract
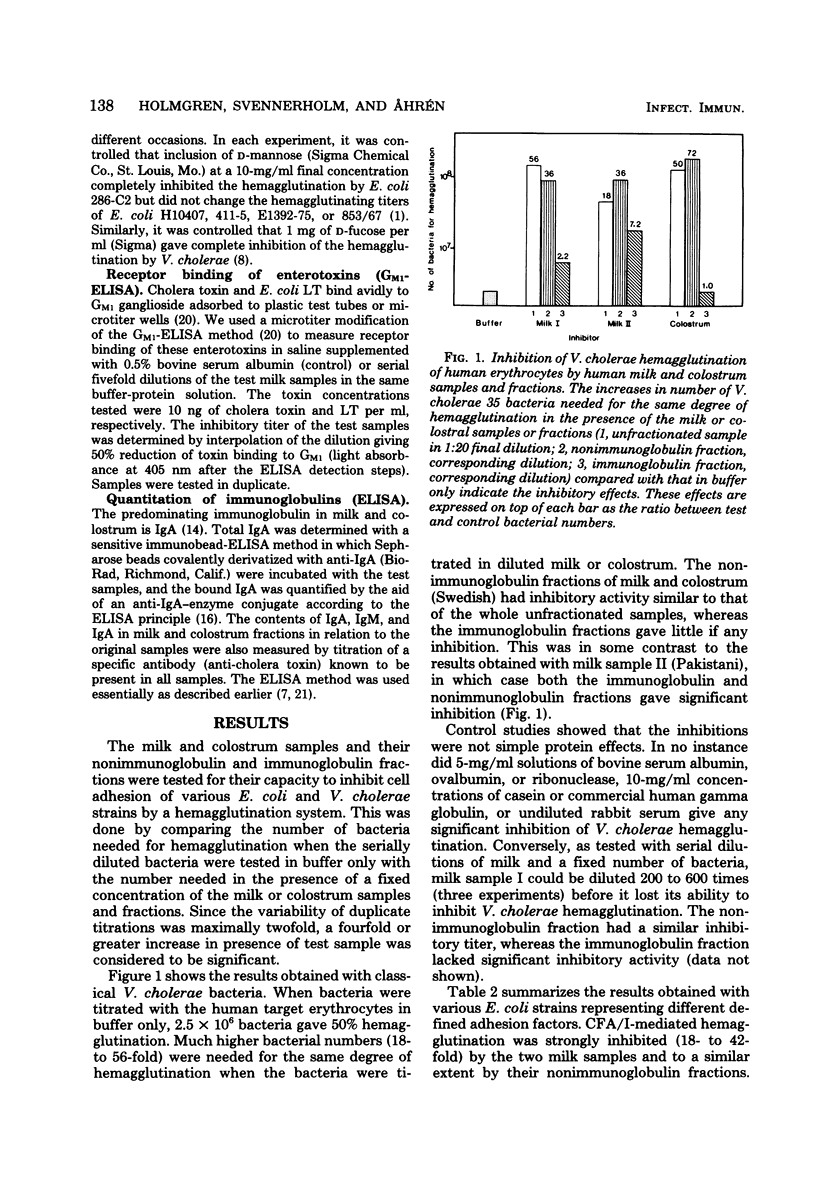
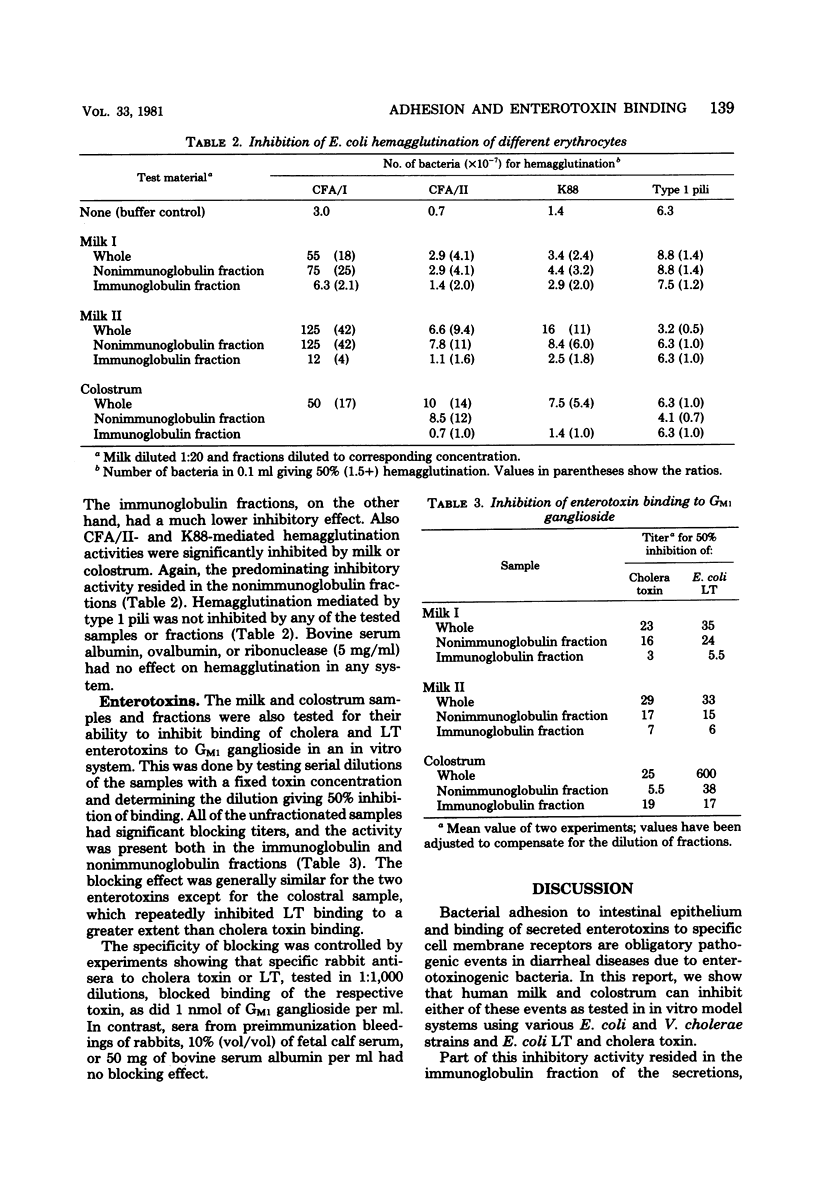
Human milk and colostrum samples were divided into an immunoglobulin and a nonimmunoglobulin fraction by immunosorbent chromatography. The ability of these fractions to inhibit bacterial cell adhesion and enterotoxin receptor binding of Vibrio cholerae and various Escherichia coli isolates was then tested by in vitro assays. The strongest effect was generally seen with the nonimmunoglobulin fractions, which were shown to significantly inhibit E. coli cell adhesion (hemagglutination) mediated by CFA/I, CFA/II, or K88 fimbriae (but not type 1 pili) and V. cholerae hemagglutination, as well as the binding of cholera toxin and E. coli heat-labile enterotoxin to GM1 ganglioside. Also, the immunoglobulin fractions had significant inhibitory activity in some of these systems. The results are interpreted to suggest that human milk and colostrum may contain secreted structure analogs of the cell receptors for some bacterial adhesions and enterotoxins; this might contribute to the protective effect of milk against enteric infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. W. Breast-feeding: second thoughts. Pediatrics. 1974 Dec;54(6):757–764. [PubMed] [Google Scholar]
- Hanson L. A., Winberg J. Breast milk and defence against infection in the newborn. Arch Dis Child. 1972 Dec;47(256):845–848. doi: 10.1136/adc.47.256.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Strannegård O., Svennerholm L. Gangliosides as receptors for bacterial toxins and Sendai virus. Adv Exp Med Biol. 1980;125:453–470. doi: 10.1007/978-1-4684-7844-0_40. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Hanson L. A., Carlson B., Lindblad B. S., Rahimtoola J. Neutralizing antibodies against Escherichia coli and Vibrio cholerae enterotoxins in human milk from a developing country. Scand J Immunol. 1976;5(6-7):867–871. doi: 10.1111/j.1365-3083.1976.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Otnaess A. B., Orstavik I. The effect of human milk fractions on rotavirus in relation to the secretory IgA content. Acta Pathol Microbiol Scand C. 1980 Feb;88(1):15–21. doi: 10.1111/j.1699-0463.1980.tb00067.x. [DOI] [PubMed] [Google Scholar]
- SVIRSKY-GROSS S. Pathogenic strains of coli (0,111) among prematures and the use of human milk in controlling the outbreak of diarrhea. Ann Paediatr. 1958 Feb;190(2):109–115. [PubMed] [Google Scholar]
- Sack D. A., Neogi P. K., Alam M. K. Immunobead enzyme-linked immunosorbent assay for quantitating immunoglobulin A in human secretions and serum. Infect Immun. 1980 Jul;29(1):281–283. doi: 10.1128/iai.29.1.281-283.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoliar O. A., Pelley R. P., Kaniecki-Green E., Kkaus M. H., Carpenter C. C. Secretory IgA against enterotoxins in breast-milk. Lancet. 1976 Jun 12;1(7972):1258–1261. doi: 10.1016/s0140-6736(76)91735-9. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J., Hanson L. A., Lindblad B. S., Quereshi F., Rahimtoola R. J. Boosting of secretory IgA antibody responses in man by parenteral cholera vaccination. Scand J Immunol. 1977;6(12):1345–1349. doi: 10.1111/j.1365-3083.1977.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Immunoglobulin and specific-antibody synthesis in vitro by enteral and nonenteral lymphoid tissues after subcutaneous cholera immunization. Infect Immun. 1977 Feb;15(2):360–369. doi: 10.1128/iai.15.2.360-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayot J. L., Holmgren J., Svennerholm L., Lindblad M., Tardy M. Receptor-specific large-scale purification of cholera toxin on silica beads derivatized with lysoGM1 ganglioside. Eur J Biochem. 1981 Jan;113(2):249–258. doi: 10.1111/j.1432-1033.1981.tb05060.x. [DOI] [PubMed] [Google Scholar]



