Abstract
Non-steroidal nuclear receptors play a major role in breast cancer development. A correlation among, and possible prognostic function of, the members of the nuclear receptor superfamily has been discussed controversially over the years. Hence, we conducted a quantification of the different expression levels of the thyroid receptor (TR), retinoid X receptor (RXR), peroxisome proliferator-activated receptor (PPAR) and vitamin D receptor (VDR) in malignant breast tumour tissue samples. Patients diagnosed and treated for breast cancer between 1990 and 2000 were included. Receptor expression was detected by immunohistochemical staining. Correlation analyses for the expression of the receptors were performed for the clinical and histopathological data. The paraffin-embedded tissue from 82 breast cancer patients was available. The different steroid receptors showed varying results when correlated with known histopathological markers. TRα2 demonstrated the most significant correlations with steroid hormone receptors. Significant correlations between the major isoforms of TR, and between RXR, PPAR and VDR, were demonstrated in the patient sample. The immunohistochemical association of these receptors may provide the first proof of an interaction on the molecular level. This assumption awaits confirmation in studies with larger cohorts.
Keywords: thyroid receptor, retinoid X receptor, peroxisome proliferator-activated receptor, vitamin D receptor, breast cancer
Introduction
Although the involvement of the oestrogen receptor (ER) and progesterone receptor (PR) in breast cancer development and growth is well-established, little is known about the relevance and correlation of steroid hormone receptors with other members of the related non-steroidal nuclear receptor family. The latter is divided into two subfamilies (1), the first including the oestrogen, androgen, progesterone and mineralocorticoid receptors and the second including the thyroid receptor (TR), vitamin D receptor (VDR), retinoic acid receptor (RAR), peroxisome proliferator-activated receptor (PPAR) and retinoid X receptor (RXR). The second group of receptors is able to form heterodimers with each other, function through appropriate ligands (2) and interact at the genetic level (3).
The immunohistochemical expression of these receptors in breast cancer cells is known (4) and their expression levels are higher than in normal breast tissue or benign breast lesions (5–8).
The hormone dependency of the mammary gland and the similarity of TR and ER/PR have led to the hypothesis that TR may be a prognostic marker in breast cancer patients (9). The ER has two isoforms (α and β), which are differentiated by their molecular construction yet identical in their basic effect (10). In this study, which focuses on ER detected at the time of first diagnosis of breast cancer, ER isoform α expression was measured since this is the main isoform for which the most authentic histopathological data have been shown in previous studies (11). PR was also detected at the time of the first breast cancer diagnosis.
In the case of the TRs, immunohistochemical staining of the best known isoforms was conducted. The three main isoforms are TRα1, TRα2 and TRβ1 (12), which show high homology in amino acid composition.
Synthetic ligands of RXR have been reported to induce arrest of growth and differentiation in breast cancer cells in vitro and in animal models (13,14). Ligand activation of RXR and PPAR induces antitumour effects in breast cancer cells (15). For RXR, three isoforms exist (α, β and γ). The best data on their detection in malignant breast tumours are available for RXRα (8). For PPAR, most studies refer to the γ isoform (13,16).
VDR is expressed in epithelial, stromal and immune cells of the normal mammary gland and is dynamically regulated in the epithelial compartment during hormonal changes (17). Furthermore, the receptor exists in malignant dividing cell types which respond to 1,25 vitamin D3 (18).
The present study is an evaluation of the potential correlations among different steroid hormone receptors following their immunohistochemical detection.
Materials and methods
Patients and ethics
Patients with an initial diagnosis of anamnestic sporadic breast cancer who received treatment in the Department of Obstetrics and Gynaecology of the Ludwig-Maximilians-University (Munich, Germany) and whose tissue samples were obtained at the surgery in our institution between 1990 and 2000 were included. Patients were stratified into groups according to lymph node involvement, grading and histopathological type, as described previously (19).
Ethical approval was obtained from the local ethics committee at the University of Munich (Project No. 048-08). The participants provided written informed consent. The study was carried out according to the guidelines of the 1975 Declaration of Helsinki. All samples and clinical information were used anonymously.
TNM classification was conducted according to the WHO criteria (20). The histological grading classification proposed by Bloom and Richardson was determined according to a modification of the Elston and Ellis grading system (21). Further clinical and histopathological parameters collected included age, year of breast cancer diagnosis, tumour size, histopathological type, axillary node involvement, histological grading and oestrogen/progesterone receptor status. At the time of the tissue extraction, Her-2/neu was not regularly investigated in Germany. As far as possible, it has now been determined for the existing slides. Values of 0 and 1 were considered to be negative, values of 3+ were classified as positive and in cases of 2+, a fluorescence in situ hybridisation (FISH) assay was performed.
Histological diagnostic evaluation and staging were performed by two experienced gynecologic pathologists.
Clinical data on the patients’ diseases were available from patients’ charts, aftercare files and tumour registry database information.
Immunohistochemistry
Immunohistochemistry was performed using a combination of pressure cooker heating and the standard streptavidin-biotin-peroxidase complex with the use of the mouse/rabbit-IgG-Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). The antibodies used for staining are listed in Table I.
Table I.
Antibodies and working concentrations.
| Antibody | Species isotype | Working dilution | Source |
|---|---|---|---|
| TRα1/2 | Polyclonal rabbit IgG | 1:200 | Abcam, Cambridge, MA, USA |
| TRα1 | Polyclonal rabbit IgG | 1:1000 | AbD Serotec Oxford, UK |
| TRα2 | Monoclonal rabbit IgG1 | 1:200 | AbD Serotec, Oxford, UK |
| TRβ1/2 | Polyclonal rabbit IgG | 1:200 | Zytomed, Berlin, Germany |
| TRβ1 | Polyclonal rabbit IgG | 1:200 | Millipore, Schwalbach, Germany |
| TRβ2 | Polyclonal rabbit IgG | 1:100 | Millipore, Schwalbach, Germany |
| RXRα | Mouse monoclonal IgG | 1:150 | Perseus Proteomics Inc., Tokyo, Japan |
| PPARγ | Rabbit polyclonal IgG | 1:1000 | Abcam, Cambridge, MA, USA |
| VDR | Mouse monoclonal IgG2a | 1:100 | AbD Serotec, Oxford, UK |
TR, thyroid receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; VDR, vitamin D receptor.
Briefly, paraffin-fixed tissue sections were dewaxed with xylol for 15 min and then rehydrated in descending concentrations of alcohol (100, 75 and 50%). Endogenous peroxidase activity was quenched by dipping the slides into 3% hydrogen peroxide (Merck, Darmstadt, Germany) in methanol for 20 min. For epitope retrieval, the sections were then incubated in a pressure cooker using sodium citrate buffer (pH 6.0) containing 0.1 M citric acid and 0.1 M sodium citrate in distilled water for 10 min. After cooling, the slides were washed in phosphate-buffered saline (PBS) twice. Non-specific binding of the primary antibodies was blocked by incubating the sections with diluted normal serum (10 ml PBS containing 150 μl horse/goat serum and 50 μl secondary antibody; Vector Laboratories) for 20 min at room temperature. Sections were incubated in diluted biotinylated secondary antibody (10 ml PBS containing 150 μl horse/goat serum and some secondary antibody; Vector Laboratories) for 30 min and the avidin-biotin peroxidase complex (diluted in 10 ml PBS; Vector Laboratories) for 30 min. Visualisation was performed using the substrate and the chromogen 3,3′-diaminobenzidine (DAB; Dako, Glostrup, Denmark). Sections were counterstained with Mayer’s acidic haematoxylin, dehydrated in an ascending series of alcohol concentrations and then covered. The determination of the different receptors is shown in Fig. 1A–D. Negative and positive controls (placental tissue) were used to assess the specificity of the immunoreactions. For negative controls (coloured blue), isotype-matched control antibodies of the same species (Dako, Hamburg, Germany) were applied to the breast cancer tissue. The control tissue showed neither nuclear nor cytoplasmic staining. Negative controls and unstained cells were blue (Fig. 1E). Positive cells were brown (Fig. 1F).
Figure 1.
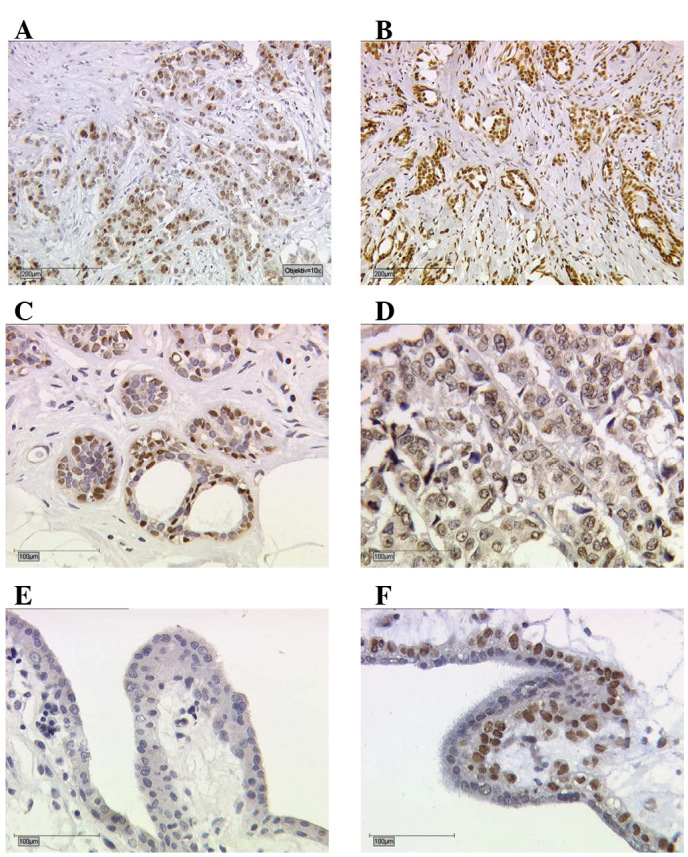
Immunohistochemical staining of (A) TR, (B) RXR, (C) PPAR and (D) VDR in human breast cancer tissue. The images show immunoreactions following incubation of tumour cells with the primary antibody (×10 and ×25 lens). (E and F) Placental tissue serves as negative and positive controls for the receptors (here RXR). (E) For negative controls (blue), the isotypes matching the control antibodies of the same species were used. (F) Positive control shows TR staining of villous trophoblast cells. TR, thyroid receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; VDR, vitamin D receptor.
Slides were evaluated and digitalised with a Zeiss photomicroscope (Axiophot; AxioCam, Zeiss, Jena, Germany). The immunoreactive score (IRS) was assigned according to Remmele and Stegner (22). The intensity and distribution patterns of specific immunohistochemical staining were evaluated using the semi-quantitative assay (23,24). The IRS score was calculated by multiplying the optical staining intensity (graded as 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) with the percentage of positively stained cells (0, no staining; 1, <10% of cells stained; 2, 11–50% of cells stained; 3, 51–80% of cells stained; 4, >81% of cells stained). Microscopic analysis was performed by two independent observers.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (PASW Statistics; SPSS Inc., IBM, Chicago, IL, USA). Correlation analysis of the receptor expression was performed using the non-parametric Mann-Whitney U test and the non-parametric Spearman’s rho. All statistical tests were two-sided and P<0.05 was considered to indicate a statistically significant result.
Results
Patient characteristics
The paraffin-embedded tissues of 82 patients were available for analyses. The age at primary diagnosis ranged from 54–95 years. All patients had received an initial diagnosis of breast cancer and had an invasive ductal histopathological type. Patient characteristics are detailed in Table II.
Table II.
Baseline characteristics of participants.
| Factor | n | % |
|---|---|---|
| Tumour size | 82 | 100 |
| pT1a | 1 | 1 |
| pT1b | 15 | 18 |
| pT1c | 44 | 54 |
| pT2 | 17 | 21 |
| pT3 | - | - |
| pT4 | 5 | 6 |
| LNI | 82 | 100 |
| Yes | 38 | 46 |
| No | 44 | 54 |
| Grading | 82 | 100 |
| 1 | 9 | 11 |
| 2 | 40 | 49 |
| 3 | 33 | 40 |
| TR | 82 | 100 |
| α1/2 | 78 | 95 |
| α1 | 78 | 95 |
| α2 | 79 | 96 |
| β1/2 | 77 | 94 |
| β1 | 79 | 96 |
| β2 | 76 | 93 |
| RXRα | 78 | 95 |
| PPARγ | 78 | 95 |
| VDR | 75 | 91 |
LNI, lymph node involvement; TR, thyroid receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; VDR, vitamin D receptor.
The detection of TR, RXR and PPAR expression was limited to the nuclei. However, VDR expression was also found in the cytoplasm of the tumours (Fig. 1A–D). Positive immunohistochemical results (Table III) and correlations with known histopathological markers were identified (Table IV).
Table III.
Immunohistochemical staining results of all receptors.
| Antigen | IRS negative (0–1) n (%) |
IRS positive (2–12) n (%) |
|---|---|---|
| TR | ||
| α1 | 23 (29) | 55 (71) |
| α2 | 25 (32) | 54 (78) |
| α1/2 | 59 (76) | 19 (24) |
| β1 | 36 (46) | 43 (54) |
| β2 | 16 (21) | 60 (79) |
| β1/2 | 44 (57) | 33 (43) |
| RXRα | 11 (14) | 74 (86) |
| PPARγ | 33 (42) | 45 (58) |
| VDR | 6 (8) | 89 (92) |
IRS, immunoreactive score; TR, thyroid receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; VDR, vitamin D receptor.
Table IV.
Correlations of antibodies with histopathological data.
| Antigen | Tumour size (pT) | LNI | Differentiation grade | ER/PR | Her-2/neu |
|---|---|---|---|---|---|
| TRα1/2 | ns | ns | ns | ns | ns |
| TRα1 | cc=−0.357, P=0.001 | ns | ns | ns | ns |
| TRα2 | cc=−0.329, P=0.003 | cc=−0.487, P=0.002 | cc=−0.542, P=0.009 | cc=0.248, P=0.028 | ns |
| TRβ1/2 | ns | ns | ns | cc=−0.349, P=0.002 | ns |
| TRβ1 | cc=−0.293, P=0.009 | ns | ns | cc=0.252, P=0.025 | ns |
| TRβ2 | cc=−0.314, P=0.006 | ns | ns | ns | ns |
| RXRα | ns | ns | cc=−0.248, P=0.029 | ns | ns |
| PPARγ | ns | cc=0.318, P=0.005 | cc=0.225, P=0.047 | ns | ns |
| VDR | cc=−0.278, P=0.016 | cc=0.411, P<0.01 | ns | ns | ns |
LNI, lymph node involvement; ER, oestrogen receptor; PR, progesterone receptor; TR, thyroid receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; VDR, vitamin D receptor; cc, coefficient of correlation; ns, not statistically significant. Data presented as correlation coefficient and P-values.
The results of the single TRα1/2 antibodies with a median IRS of 4 (range, 0–12) differed from the combined antibody TRα1/2. For TRα1/2, the IRS median was 0 (range, 0–6).
As for TRα1/2, the median IRS values of the individual TRβ1 and TRβ2 antibodies were higher with values of 2 and 3, respectively (range, 0–12). The TRβ1/2 results were comparable to those for TRα1/2 with a median IRS of 1 (range, 0–9).
For RXR, the median IRS value was 4 (range, 0–8) and for PPAR it was 2 (range, 0–12). VDR showed the highest value with an IRS of 8 (range, 0–12).
Correlation analysis among the histopathological parameters
Tumour size, lymph node involvement and grading were significantly correlated with each other (data not shown). ER/PR had no significant associations with tumour size, lymph node involvement or grading.
Correlation analysis of steroid family members with histopathological findings
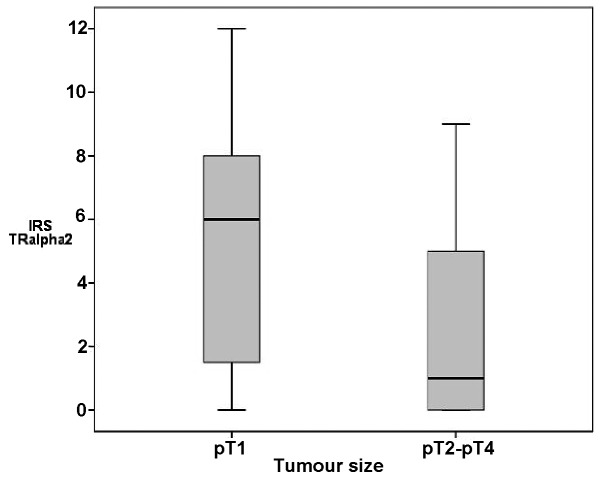
Tumour size. Tumour size was negatively correlated with TRα1 [correlation coefficient (cc)= −0.357, P=0.001], TRα2 (cc=−0.329, P=0.003), TRβ1 and TRβ2 expression (cc=−0.293, P= 0.009; cc= −0.314, P= 0.006). TRα2 levels were higher in pT1 tumours (median IRS, 6) compared with pT2-4 tumours (P=0.024; Fig. 2). RXR and PPAR were not associated with tumour size. In correlation analyses, VDR was negatively associated with tumour size (cc=−0.278, P=0.016).
Figure 2.
Box plot analysis of TRα2 and tumour size. The box plots show a higher IRS of TRα2 in small malignant breast tumours. IRS, immunoreactive score; TR, thyroid receptor.
Axillary lymph node involvement. Lymph node involvement was negatively correlated with TRα2 (cc=−0.487, P=0.002) and VDR (cc=−0.411, P<0.01). However, only PPARγ had a positive significant correlation with lymph node involvement (cc=0.318, P=0.005).
Differentiation grading. Differentiation grading was negatively correlated with TRα2 (cc=−0.542, P=0.009) and RXRγ (cc=−0.248, P=0.029). Positive correlations of grading were only observed with PPARγ (cc=0.236, P=0.038).
Furthermore, the correlation analysis of ER/PR expression (data shown in Table IV) showed positive results for TRα2 expression in the tumours (cc=0.248, P=0.028) and also for TRβ1 (cc=0.252, P=0.025). A negative correlation was found between TRβ1/2 expression and ER/PR expression (cc=−0.349, P=0.002). For RXR, PPAR and VDR, the correlation analysis showed no significant values for ER/PR expression.
Her-2/neu. As determined by retrospective analyses, most patients had a negative Her-2 status (60/82, 80%). In 7 patients it was not possible to determine Her-2 expression. No significant correlations were demonstrated with other clinicopathological parameters (Table IV).
Correlations among the members of the steroid hormone receptor family. The results of the correlations among the single functionally related steroid hormone receptors are listed in detail in Table V.
Table V.
Correlations among TR, RXR, PPAR and VDR antibodies.
| Antigen | TRα1/2 | TRα1 | TRα2 | TRβ1/2 | TRβ1 | TRβ2 | RXRα | PPARγ | VDR |
|---|---|---|---|---|---|---|---|---|---|
| TRα1/2 | - | cc=0.300 P=0.009 |
ns | ns | cc=0.247 P=0.032 |
cc=0.287 P=0.014 |
cc=0.274 P=0.018 |
ns | ns |
| TRα1 | cc=0.300 P=0.009 |
- | ns | ns | ns | cc=0.291 P=0.013 |
cc=0.399 P=0.000 |
ns | ns |
| TRα2 | ns | ns | - | ns | ns | cc=0.282 P=0.014 |
cc=0.316 P=0.006 |
ns | cc=0.433 P=0.000 |
| TRβ1/2 | ns | ns | ns | - | ns | ns | ns | ns | ns |
| TRβ1 | cc=0.247 P=0.032 |
ns | ns | ns | - | cc=0.557 P=0.000 |
ns | cc=0.270 P=0.017 |
cc=0.403 P=0.000 |
| TRβ2 | cc=0.287 P=0.014 |
cc=0.291 P=0.013 |
cc=0.282 P=0.014 |
ns | cc=0.557 P=0.000 |
- | ns | cc=0.458 P=0.000 |
cc=0.370 P=0.001 |
| RXRα | cc=0.274 P=0.018 |
cc=0.399 P=0.000 |
cc=0.316 P=0.006 |
ns | ns | ns | - | ns | ns |
| PPARγ | ns | ns | ns | ns | cc=0.270 P=0.017 |
cc=0.458 P=0.000 |
ns | - | ns |
| VDR | ns | ns | cc=0.433 P=0.000 |
ns | cc=0.403 P=0.000 |
cc=0.370 P=0.001 |
ns | ns | - |
TR, thyroid receptor; RXR, retinoid X receptor; PPAR, peroxisome proliferator-activated receptor; VDR, vitamin D receptor; cc, coefficient of correlation; ns, not statistically significant. Data presented as correlation coefficient and P-values.
Thyroid receptors. For the combined TRα1/2, a correlation was demonstrated with TRα1, TRβ1, TRβ2 and RXR. TRα1 expression was correlated with TRα1/2, TRβ2 and RXR. TRα2 showed positive correlations with TRβ2, RXR and VDR. No correlations with other steroid factors were found for TRβ1/2. TRβ1 correlated positively with TRβ2, PPAR and VDR. TRβ2 showed positive correlations with almost all receptors (TRα1/2, TRα1, TRα2, TRβ1, PPAR and VDR).
RXR. RXRα was positively correlated with TRα1, TRα2 and TRα1/2.
PPAR. PPARγ showed two correlations, with TRβ1 and TRβ2.
VDR. For VDR, significant correlations were demonstrated with TRα2, TRβ1 and TRβ2.
Discussion
The present study demonstrated significant correlations between the known histopathological parameters, including tumour size, lymph node involvement, differentiation grade, ER, PR and other members of the nuclear receptor family. Furthermore, significant correlations among different steroid receptors (excluding the combined TRβ1/2) were shown. To the best of our knowledge, this is the first study to examine the coexpression and thus the immunohistochemical correlation between members of steroid receptors in a cohort of breast cancer patients.
The rationale for this study was the known significance of immunohistochemical ER/PR expression in breast cancer and the similarity of these receptors with the surface of the other members of the nuclear receptor family. In the latter, ER/PR detection in breast cancer is associated with prognostic relevance (25), and it has long been known that overexpression is treatable with antihormonal therapy (26), regardless of the oestrogen and progesterone blood levels.
Certain authors have focused on thyroid receptors due to an assumed correlation between thyroid dysfunction and breast cancer (9,27,28). Few studies have reported clear results demonstrating associations, although in these studies, TRs and other histopathological findings were not further differentiated; for example, a negative correlation between the TR receptor level and the axillary involvement of lymph nodes (29). By contrast, Silva et al did not find clear correlations between single TRβ1 expression and other histopathological factors (30). The inconsistency between the results of different TRs may be attributable to different distributions of the TRs in the examined tissue (certain sections had mainly mixed epitopes of TRs, while other sections had mainly single TRs). Taking this into account, as was demonstrated in our study, clear associations between different TRs and histopathological findings support the assumption that the interactions identified may have inherent prognostic relevance.
As with TRs, most of the literature for RXR and PPAR does not refer to in vivo but in vitro data (31,32). In our study, the expression of RXR, which is known for its antitumour effects, was negatively correlated with differentiation grade (33).
For PPARγ, an inverse association with tumour size was found (34). In contrast to our previous findings (35), which demonstrated a correlation between PPAR and positive lymph node involvement, discrepant results have also been reported (34). These current conflicting results need to be resolved in larger trials. Hence, drawing clinical conclusions from these findings is considered premature at this time.
In a previous study (36), an immunohistochemical expression of VDR in most of the tumour cells was shown. Nonetheless, data based on the correlation between VDR and ER/PR were inconsistent and contradictory (36–39). Furthermore, the presence of ER/PR and VDR was only partially correlated with other clinical features of tumour stage (36).
We cannot underline the finding of a clear association of VDR and ER/PR but, in contrast to previous findings, our data demonstrated a correlation between VDR, tumour size and lymph node involvement. A single study (40) demonstrated a role for vitamin D and its receptor in breast cancer in humans. As previous data have shown (41,42), an improved outcome was achieved in patients with high VDR-IRS than in patients with low IRS. Taken together, the current findings support the assumption that VDR is a factor with prognostic relevance in breast cancer.
The immunohistochemical association of these receptors supports the knowledge of interactions at the molecular level (43). Hence, these results await confirmation in larger trials. Unfortunately, HER2/neu status was not routinely determined in the cohort investigated at the time of initial diagnosis. Given the high prognostic value of HER2/neu status, it was of significant interest whether this prognosticator also interacts with other receptors. Although selection bias cannot be excluded and the number of patients was small, results may have significance for malignant breast tumour diseases and may be of interest for future innovative therapeutic approaches.
We have demonstrated significant correlations for all the major isoforms of TRs, and furthermore, between RXR, PPAR and VDR. Significantly, and in contrast to TRβ2, TRα2 demonstrated significant correlations with each of the known independent histopathological markers in breast cancer. It was unusual that, with larger tumour size, higher differentiation grade and axillary lymph node involvement, TRα2 became negative, but with high ER/PR values, TR increased. This may lead to the assumption that high expression, particularly of TRα2, is associated with a better prognosis at higher values of ER/PR and therefore protects breast cancer cells from de-differentiation. Furthermore, TRβ2, RXR and VDR were significantly correlated, the latter two of which are known to be of prognostic importance in breast cancer.
Acknowledgments
We thank the patients for providing samples for this study. We acknowledge the excellent technical assistance of M. Rübekeil, S. Hofmann, S. Kunze and C.H. Kuhn.
References
- 1.Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- 2.Schräder M, Nayeri S, Kahlen JP, Müller KM, Carlberg C. Natural vitamin D3 response elements formed by inverted palindromes: polarity-directed ligand sensitivity of vitamin D3 receptor-retinoid X receptor heterodimer-mediated transactivation. Mol Cell Biol. 1995;15:1154–1161. doi: 10.1128/mcb.15.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segars JH, Marks MS, Hirschfeld S, Driggers PH, Martinez E, Grippo JF, Wahli W, Ozato K. Inhibition of estrogen-responsive gene activation by the retinoid X receptor beta: evidence for multiple inhibitory pathways. Mol Cell Biol. 1993;4:2258–2268. doi: 10.1128/mcb.13.4.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka T, Danchek BL, Trifiletti LC, Birnkrant RE, Taylor BJ, Garfield SH, Thorgeirsson U, De Luca LM. Altered localization of retinoid X receptor α coincides with loss of retinoid responsiveness in human breast cancer MDA-MB-231 cells. Mol Cell Biol. 2004;24:3972–3982. doi: 10.1128/MCB.24.9.3972-3982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroplasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 6.Smallridge RC, Latham KR. Nuclear thyroid hormone receptor in human breast tumours. Clin Res. 1980;28:421. doi: 10.1210/jcem-51-1-106. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich M, Axt-Fliedner R, Villena-Heinsen C, Tilgen W, Schmidt W, Reichrath J. Analysis of vitamin D-receptor (VDR) and retionoid X-receptor α in breast cancer. Histochem J. 2002;34:35–40. doi: 10.1023/a:1021343825552. [DOI] [PubMed] [Google Scholar]
- 8.Conde I, Lobo MVT, Zamora J, Pérez J, González FJ, Alba E, Fraile B, Paniagua R, Arenas MI. Human pregnane X receptor is espressed in breast carcinomas, potential heterodimers formation between hPXR and RXR-α. BMC Cancer. 2008;8:174. doi: 10.1186/1471-2407-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Lin Q, Yoon HG, Huang ZQ, Strahl BD, Allis CD, Wong J. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol Cell Biol. 2002;22:5688–5697. doi: 10.1128/MCB.22.16.5688-5697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlstedt-Duke J, Strömstedt PE, Persson B, Cederlund E, Gustafsson JA, Jörnvall H. Identification of hormone-interacting amino acid residues within the steroid-binding domain of the glucocorticoid receptor in relation to other steroid hormone receptors. J Biol Chem. 1988;263:6842–6846. [PubMed] [Google Scholar]
- 11.Carlstedt-Duke J. Cellular estrogen activity: implications for pulsed estrogen therapy. Maturitas. 2001;38(Suppl 1):S7–S13. doi: 10.1016/s0378-5122(01)00199-2. [DOI] [PubMed] [Google Scholar]
- 12.Ling Y, Xu X, Hao J, Ling X, Du X, Liu X, Zhao X. Aberrant methylation of the TRB gene in tissue and plasma of breast cancer patients. Cancer Genet Cytogenet. 2010;196:140–145. doi: 10.1016/j.cancergencyto.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM. Terminal differentiation of human breast cancer through PPARγ. Mol Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 14.Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB. A new ligand for the peroxisome proliferator activated receptor gamma (PPARgamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671–5673. [PubMed] [Google Scholar]
- 15.Elstner E, Williamson EA, Zang C, Fritz J, Heber D, Fenner M, Possinger K, Koeffler HP. Novel therapeutic approach: ligands for PPARγ and retinoid receptors induce apoptosis in bcl-2-positive human breast cancer cells. Breast Cancer Res Treat. 2002;74:155–165. doi: 10.1023/a:1016114026769. [DOI] [PubMed] [Google Scholar]
- 16.Kilgore MW, Tate PL, Rai S, Sengoku E, Price TM. MCF-7 and T47 D human breast cancer cells contain a functional peroxisomal response. Mol Cell Endocrinol. 1997;129:229–235. doi: 10.1016/s0303-7207(97)04057-4. [DOI] [PubMed] [Google Scholar]
- 17.Zinser GM, Welsh JE. Accelerated mammary gland development during pregnancy and delayed post-lactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18:2208–2223. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 18.Gombart AF, Luong QT, Koeffler HP. Vitamin D compounds: activity against microbes and cancer. Anticancer Res. 2006;26(4A):2531–2542. [PubMed] [Google Scholar]
- 19.Dian D, Janni W, Kuhn C, Mayr D, Karsten U, Mylonas I, Friese K, Jeschke U. Evaluation of a novel anti-mucin 1 (MUC1) antibody (PankoMab) as a potential diagnostic tool in human ductal breast cancer; comparison with two established antibodies. Onkologie. 2009;32:238–244. doi: 10.1159/000209280. [DOI] [PubMed] [Google Scholar]
- 20.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. 3rd edition. World Health Organization; Geneva: 2000. [Google Scholar]
- 21.Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993;46:189–190. doi: 10.1136/jcp.46.2.189-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. (In German) [PubMed] [Google Scholar]
- 23.Jeschke U, Bischof A, Speer R. Development of monoclonal and polyclonal antibodies and an ELISA for the determination of glycodelin in human serum, amniotic fluid and cystic fluid of benign and malignant ovarian tumours. Anticancer Res. 2005;25:1581–1589. [PubMed] [Google Scholar]
- 24.Mylonas I, Makovitzky J, Jeschke U, Briese V, Friese K, Gerber B. Expression of Her2/neu, steroid receptors (ER and PR), Ki67 and p53 in invasive mammary ductal carcinoma associated with ductal carcinoma in situ (DCIS) versus invasive breast cancer alone. Anticancer Res. 2005;25:1719–1723. [PubMed] [Google Scholar]
- 25.Knight WA, Livingston RB, Gregory EJ, McGuire WL. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977;37:4669–4671. [PubMed] [Google Scholar]
- 26.Osborne CK, McGuire WL. The use of steroid hormone receptors in the treatment of human breast cancer: a review. Bull Cancer. 1979;66:203–210. [PubMed] [Google Scholar]
- 27.Smyth PPA. The thyroid and breast cancer: a significant association? Ann Med. 1997;29:189–191. doi: 10.3109/07853899708999335. [DOI] [PubMed] [Google Scholar]
- 28.Ditsch N, Liebhardt S, von Koch F, Lenhard M, Vogeser M, Spitzweg C, Gallwas J, Toth B. Thyroid function in breast cancer patients. Anticancer Res. 2010;30:1713–1717. [PubMed] [Google Scholar]
- 29.Lemaire M, Baugnet-Mahieu L. Nuclear thyroid hormone receptors in human cancer tissues. Anticancer Res. 1986;6:695–700. [PubMed] [Google Scholar]
- 30.Silva JM, Domínguez G, González-Sancho JM, García JM, Silva J, García-Andrade C, Navarro A, Muñoz A, Bonilla F. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene. 2002;21:4307–4316. doi: 10.1038/sj.onc.1205534. [DOI] [PubMed] [Google Scholar]
- 31.Wu K, Zhang Y, Xu XC, Hill J, Celestino J, Kim HT. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumours in transgenic mice. Cancer Res. 2002;62:6376–6380. [PubMed] [Google Scholar]
- 32.Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 33.Bonofiglio D, Cione E, Qi H, Pingitore A, Perri M, Catalano S, Vizza D, Panno ML, Genchi G, Fuqua SA, Ando S. Combined low doses of PPARgamma and RXR ligands trigger an intrinsic apoptic pathway in human breast cancer cells. Am J Pathol. 2009;175:1270–1280. doi: 10.2353/ajpath.2009.081078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, Hayashi S, Miki Y, Nakamura Y, Moriya T, Sugawara A, Ishida T, Ohuchi N, Sasano H. Peroxisome proliferator-activated receptor gamma in human breast carcinoma: a modulator of estrogenic actions. Endocr Relat Cancer. 2006;13:233–250. doi: 10.1677/erc.1.01075. [DOI] [PubMed] [Google Scholar]
- 35.Ditsch N, Vrekoussis T, Lenhard M, Rühl I, Gallwas J, Weissenbacher T, Friese K, Mayr D, Makrigiannakis A, Jeschke U. Retinoid X receptor alpha (RXRα) and peroxisome proliferator-activated recptor gamma (PPARγ) expression in breast cancer: an immunohistochemical study. In vivo. 2012;26:87–92. [PubMed] [Google Scholar]
- 36.Berger U, McClelland RA, Wilson P, Greene GL, Haussler MR, Pike JW, Colston K, Easton D, Coombes RC. Immunocytochemical determination of estrogen receptor, progesterone receptor, and 1,25-dihydroxyvitamin D3 receptor in breast cancer and relationship to prognosis. Cancer Res. 1991;51:239–244. [PubMed] [Google Scholar]
- 37.Freake HC, Abeyasekera G, Iwasaki J, Marcocci C, MacIntyre I, McClelland RA, Skilton RA, Easton DF, Coombes RC. Measurement of 1,25-dihydroxyvitamin D3 receptors in breast cancer and their relationship to biochemical and clinical indices. Cancer Res. 1984;44:1677–1681. [PubMed] [Google Scholar]
- 38.Mason BH, Holdaway IM, Mullins PR, Yee LH, Kay RG. Progesterone and estrogen receptors as prognostic variables in breast cancer. Cancer Res. 1983;43:2985–2990. [PubMed] [Google Scholar]
- 39.Howat JM, Harris M, Swindell R, Barnes DM. The effect of oestrogen and progesterone receptors on recurrence and survival in patients with carcinoma of the breast. Br J Cancer. 1985;51:263–270. doi: 10.1038/bjc.1985.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–132. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 41.Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, Coombes RC. Immunocytochemical detection of 1,25-dihydroxyvitamin D3 receptor in breast cancer. Cancer Res. 1987;47:6793–6799. [PubMed] [Google Scholar]
- 42.Ditsch N, Toth B, Mayr D, Lenhard M, Gallwas J, Weissenbacher T, Dannecker C, Friese K, Jeschke U. The association between vitamin D receptor and prolonged overall survival in breast cancer. J Histochem Cytochem. 2012;60:121–129. doi: 10.1369/0022155411429155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Martinez R, Zambrano A, Castillo AI, Aranda A. Vitamin D-dependent recruitment of corepressors to vitamin D/retinoid X receptor heterodimers. Mol Cell Biol. 2007;28:3817–3829. doi: 10.1128/MCB.01909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]