Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the liver. Since postoperative recurrence and intrahepatic metastases occur frequently, the postoperative 5-year survival rate is low. To investigate the molecular mechanisms of HCC progression, mRNA as well as microRNA (miRNA) expression levels have been profiled in various studies. However, no previous study has comprehensively compared the expression of miRNAs in HCC patients with various clinical features using the tumor and surrounding non-tumor tissues and normal liver samples. In this study, we profiled the expression of miRNAs in tumor and non-tumor tissues from 40 HCC patients with heterogeneous pathogenesis and 6 surrounding non-tumor tissues from patients with metastatic liver cancer. To identify miRNAs specific to each disease state, we comprehensively compared the expression of miRNAs in various combinations. The results indicate that the expression of many known as well as novel miRNAs was altered in patients with the hepatitis C virus infection compared with those with the hepatitis B virus and without any virus infection. The following miRNAs were downregulated in the tumor and non-tumor tissues, and thus could serve as novel biomarkers for chronic liver diseases: miR-18b*, miR-296-5p, miR-557, miR-581, miR-625*, miR-1228, miR-1249 and miR-2116*. Similarly, miR-129*, miR-146b-3p and miR-448 are novel candidates for HCC biomarkers regardless of virus infection.
Keywords: hepatocellular carcinoma, microRNA, expression profiling, microarray
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the liver and the third most common cause of mortality from cancer in eastern Asia. More than 85% of HCC is caused by the hepatitis B virus (HBV) or C virus (HCV) infection (1–3). Other causes are exposure to aflatoxin B1 (4), vinyl chloride (5) and tobacco (6), as well as chronic ethanol ingestion (7). Many of these factors are known causes of chronic hepatitis (CH) and liver cirrhosis, which represent a pre-neoplastic condition of HCC. HCC is often far advanced and may have multiple lesions at the time of diagnosis. Curative resection cannot be expected in cases with extrahepatic metastases. In cases without extrahepatic metastases, curative resection could potentially be performed; however, postoperative recurrence and intrahepatic metastases occur frequently, and the postoperative 5-year survival rate is reported to be 30–40% (2).
microRNAs (miRNAs) are short (19–25 nucleotides) noncoding single-stranded RNA molecules, which are cleaved from 70–100 nucleotide miRNA precursors. miRNAs regulate gene expression either at the transcriptional or translational level, based on specific binding to the complementary sequence in the coding or noncoding region of mRNA transcripts. Recent findings, based on microarray analysis of global miRNA expression profiles in cancer tissues, have revealed that miRNA profiles discriminate malignancies of the breast (8), lung (8,9), pancreas (8,10) and liver (11–17) from their counterparts.
Expression profiling of miRNA in HCC was first reported by Murakami et al (11). They comprehensively analyzed miRNA expression in HCC and non-tumor tissues and compared expression patterns in tumor and non-tumor tissues, in three differentiation levels and also between chronic hepatitis and liver cirrhosis (11). Since then, various groups have profiled miRNA expression in HCC and surrounding non-tumor tissues (12–17). Laderio et al performed miRNA profiling in HCC patients with various clinical features including normal liver samples (14). However, in their comparison, not all of the HCC samples were accompanied by their surrounding non-tumor tissues. Ura et al compared miRNA expression between HBV- and HCV-related HCC (16). Although they used normal liver tissue samples, all patients analyzed had the virus infection. In this way, there is no study which comprehensively compared the expression of miRNAs in HCC patients with various clinical features using tumor and surrounding non-tumor tissues. In this study, we profiled miRNA expressions in tumor and non-tumor tissues from 40 HCC patients with heterogeneous pathogenesis. We also investigated 6 surrounding non-tumor tissues from patients with metastatic liver cancer. These were used as normal liver samples. To identify miRNAs specific to each disease state, we compared the expression of miRNAs in various combinations.
Materials and methods
Clinical specimens
Operative specimens of primary HCC and metastatic liver cancer tissues were obtained with informed consent from 40 and 6 patients, respectively, at the Department of Hepato-Biliary-Pancreatic Surgery at Tokyo Medical and Dental University Hospital between November 2005 and May 2008 (18). This research project was approved by the local ethics committee and all samples were obtained with the informed consent of the patients. The clinical findings were reviewed and analyzed from the patients’ medical records, and are shown in Table I. Of the 40 patients with HCC, 12 were infected with HBV, 12 with HCV, and 16 were not infected with HBV or HCV. In addition, 6 normal liver tissue samples obtained during surgery for metastatic liver cancer originating in the colon were used as control samples. All specimens were immediately frozen in liquid nitrogen and then stored at −80°C for RNA analysis.
Table I.
Clinical characteristics of 40 HCC patients and 6 patients with metastatic liver cancer.
| Patient | Age | Gender | Cancer type | Virus |
|---|---|---|---|---|
| L18 | 70 | M | HCC | HBV |
| L23 | 57 | M | HCC | HBV |
| L34 | 57 | M | HCC | HBV |
| L64 | 48 | F | HCC | HBV |
| L76 | 70 | F | HCC | HBV |
| L84 | 76 | F | HCC | HBV |
| L85 | 51 | M | HCC | HBV |
| L87 | 46 | M | HCC | HBV |
| L116 | 68 | M | HCC | HBV |
| L127 | 40 | M | HCC | HBV |
| L144 | 78 | M | HCC | HBV |
| L154 | 66 | F | HCC | HBV |
| L42 | 66 | M | HCC | HCV |
| L50 | 55 | F | HCC | HCV |
| L63 | 68 | M | HCC | HCV |
| L71 | 75 | M | HCC | HCV |
| L72 | 53 | M | HCC | HCV |
| L77 | 73 | M | HCC | HCV |
| L119 | 63 | F | HCC | HCV |
| L120 | 71 | F | HCC | HCV |
| L124 | 43 | F | HCC | HCV |
| L130 | 63 | M | HCC | HCV |
| L132 | 67 | M | HCC | HCV |
| L155 | 77 | M | HCC | HCV |
| L57 | 58 | M | HCC | - |
| L58 | 73 | M | HCC | - |
| L73 | 85 | M | HCC | - |
| L88 | 72 | M | HCC | - |
| L90 | 65 | M | HCC | - |
| L97 | 80 | M | HCC | - |
| L98 | 75 | M | HCC | - |
| L100 | 77 | M | HCC | - |
| L102 | 69 | M | HCC | - |
| L104 | 64 | F | HCC | - |
| L112 | 78 | M | HCC | - |
| L117 | 47 | M | HCC | - |
| L131 | 76 | M | HCC | - |
| L139 | 73 | M | HCC | - |
| L160 | 56 | M | HCC | - |
| L165 | 50 | F | HCC | - |
| C62 | 56 | M | Metastatic liver cancer | |
| C81 | 70 | M | Metastatic liver cancer | |
| C114 | 54 | M | Metastatic liver cancer | |
| C126 | 59 | M | Metastatic liver cancer | |
| C132 | 65 | F | Metastatic liver cancer | |
| C145 | 76 | M | Metastatic liver cancer |
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus.
RNA isolation
Small RNA with a miRNA-rich fraction was extracted from tissue specimens using the miRNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNAs were quantified with NanoDrop ND-1000. The integrity of the obtained RNA was assessed using Agilent Bioanalyzer RNA 6000 Nano Assay (Agilent Technologies, Palo Alto, CA, USA). All samples had an RNA integrity number (RIN) ≥4.0. The extracted RNAs were then analyzed by miRNA microarray using 3D-Gene (Toray Industries, Tokyo, Japan). The microarrays were scanned using GenePix 4000B.
Statistical analysis
The obtained microarray datasets were background corrected and used for statistical analyses using R statistical software. The Wilcoxon rank-sum test for paired data was performed to estimate the significance of miRNA gene expression differences between tumor and non-tumor tissues in 1) the 12 patients with HBV infection, 2) the 12 patients with HCV infection, 3) the 16 patients without virus infection, 4) the 24 patients with HBV or HCV infection, and 5) all 40 patients (see Fig. 1). In addition, an unpaired version of the Wilcoxon rank-sum test was used to compare the expression of miRNAs in the 6 normal liver samples with those in 6) the tumor and 7) non-tumor tissues in the 12 patients with HBV infection, 8) the tumor and 9) non-tumor tissues in the 12 patients with HCV infection, 10) the tumor and 11) non-tumor tissues in the 16 patients without virus infection, and 12) the tumor and 13) non-tumor tissues in all 40 patients (Figs. 2 and 3). MiRNAs with a fold change >1.5 and a p-value <0.05 were deemed as differentially expressed.
Figure 1.
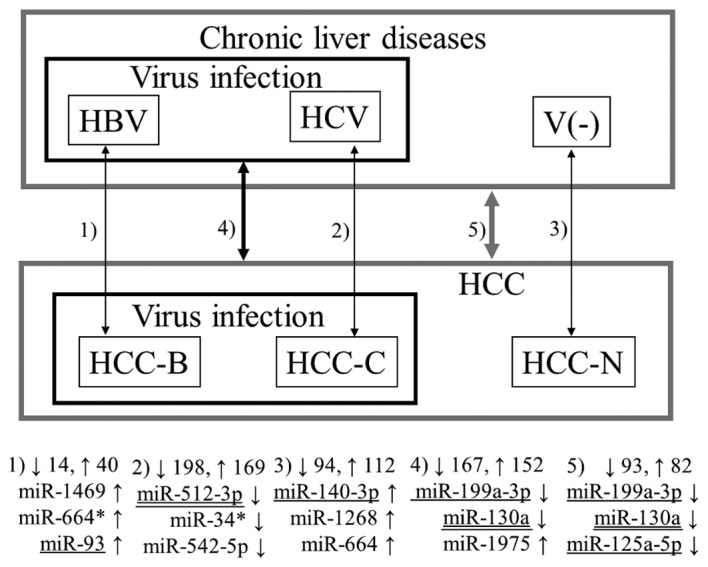
Representative miRNAs detected in pairwise comparisons between tumor and non-tumor tissues. The upward and downward arrows indicate up- and downregulation, respectively, of miRNA in the tumor tissues.
Figure 2.
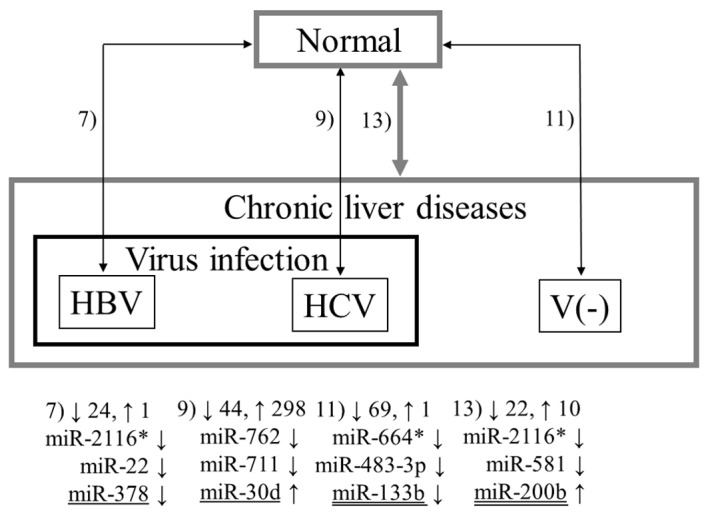
Representative miRNAs detected in comparisons of the normal liver samples with non-tumor tissues. The upward and downward arrows indicate up- and downregulation, respectively, of miRNA in the non-tumor tissues. Combinations 7, 9, 11 and 13 are explained in Materials and methods.
Figure 3.
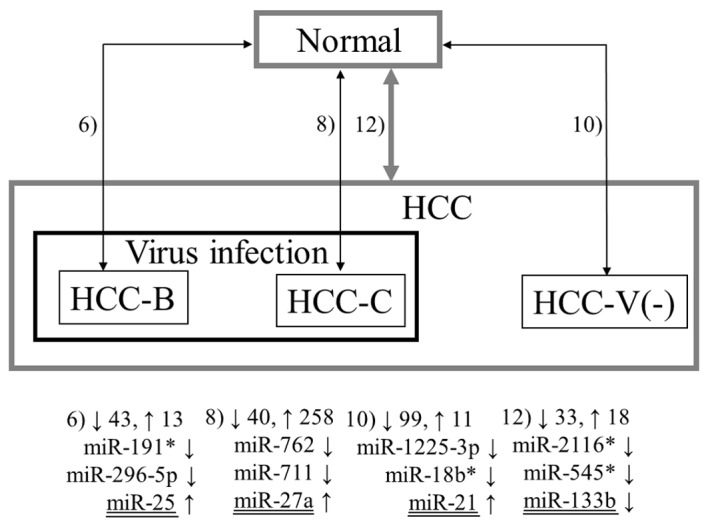
Representative miRNAs detected in comparisons of the normal liver samples with tumor tissues. The upward and downward arrows indicate up- and downregulation, respectively, of miRNA in the tumor tissues. Combinations 6, 8, 10 and 12 are explained in Materials and methods.
Many miRNAs have been reported to be involved in cancer. In addition to the previously mentioned miRNA profiling studies (11–17), numerous reviews and papers (19–56) were found through a literature survey on miRNA and cancer. It should be noted that we deemed a miRNA to be previously reported only when the exact name of the miRNA was found in a study. In what follows, a miRNA whose association with cancer has been previously reported is called as a previously reported miRNA. Such miRNAs may be classified into two types depending on whether they show the same or opposite tendencies to the previous reports. For certain miRNAs, it is difficult to determine whether their expressions were reportedly up- or downregulated due to inconsistent reports and lack of available information on the change direction. In the latter case, such miRNAs were described simply as cancer markers.
Results
Table II indicates the differentially expressed miRNAs between the tumor tissues in all HCC patients and the 6 normal liver samples (Combination 12). Thirty miRNAs were downregulated in the tumor tissues while 18 were upregulated. The double-underlined miRNAs have been previously reported to be involved in HCC while the miRNAs with a single underline have a reported association with other types of cancer. Most of the 18 upregulated miRNAs were known to be involved in HCC and other types of cancer. The expression of miR-15b is reported to be upregulated in HCC (23). Overexpression of miR-18a contributes to HCC cell proliferation in females through the targeting of the estrogen receptor (21). miR-21 is known to be highly overexpressed in HCC, and its inhibition in cultured HCC cells increases the expression of PTEN, a direct target of miR-21, as well as tumor cell proliferation, migration and invasion (20). The overexpression of miR-96 is reported in HBV-related HCC (14,21). miR-222 is a HCC biomarker which is irrespective of viral association (21). miR-224 is overexpressed in HCC when compared to a benign hepatocellular tumor, hepatocellular adenoma (14), and is involved in the inhibition of apoptosis in HCC cells (21). In contrast to the upregulated miRNAs, only two downregulated miRNAs are previously reported. miR-101 is involved in the inhibition of apoptosis and its downregulation contributes to the spread of HCC (21).
Table II.
Differentially expressed miRNAs among the tumor tissues in all HCC patients and the 6 normal liver samples.
| miRNA | Fold change | P-value |
|---|---|---|
| m-2116* | 0.048 | <0.001 |
| m-545* | 0.077 | 0.012 |
| m-581 | 0.101 | 0.004 |
| m-2114 | 0.104 | 0.012 |
| m-129* | 0.127 | 0.012 |
| m-296-5p | 0.161 | <0.001 |
| m-1267 | 0.168 | 0.008 |
| m-191* | 0.178 | 0.001 |
| m-222* | 0.188 | 0.025 |
| m-1249 | 0.197 | <0.001 |
| m-18b* | 0.208 | <0.001 |
| m-92a-2* | 0.233 | 0.040 |
| m-625* | 0.233 | 0.001 |
| m-934 | 0.237 | 0.002 |
| m-1225-5p | 0.239 | 0.026 |
| m-1913 | 0.284 | 0.001 |
| m-1238 | 0.286 | 0.002 |
| m-557 | 0.288 | 0.001 |
| m-940 | 0.292 | 0.003 |
| m-1228 | 0.293 | 0.002 |
| m-133b | 0.315 | 0.010 |
| let-7f-1* | 0.324 | 0.002 |
| m-1225-3p | 0.364 | 0.028 |
| m-802 | 0.369 | 0.030 |
| m-1224-3p | 0.377 | 0.012 |
| m-144 | 0.382 | 0.006 |
| m-101 | 0.427 | 0.034 |
| m-486-5p | 0.551 | 0.038 |
| m-29c | 0.573 | 0.047 |
| m-422a | 0.576 | 0.004 |
| m-1979 | 2.71 | 0.009 |
| m-140-3p | 2.83 | 0.023 |
| let-7i | 3.59 | 0.017 |
| m-151-3p | 3.78 | 0.005 |
| m-130b | 3.80 | 0.031 |
| m-425 | 4.86 | 0.033 |
| m-25 | 6.55 | <0.001 |
| m-21 | 7.44 | 0.005 |
| m-18a | 9.05 | 0.024 |
| m-221 | 10.9 | 0.004 |
| m-452 | 11.0 | 0.017 |
| m-222 | 14.2 | 0.026 |
| m-15b | 18.3 | 0.016 |
| m-224 | 82.4 | 0.006 |
| m-34b* | 134 | 0.032 |
| m-374b | 148 | 0.039 |
| m-96 | 181 | 0.032 |
| m-216a | 263 | 0.039 |
miRNA, microRNA; HCC, hepatocellular carcinoma.
Figs. 1–3 summarize the results from all 13 combinations described in Materials and methods. The numbers 1–13 denote the aforementioned combinations. The numbers beside the upwards and downwards-pointing arrows indicate the numbers of up- and downregulated miRNAs, respectively. Three representative miRNAs in each combination are also shown. Here, ‘representative’ means the miRNAs with the first and second lowest p-values and the previously reported miRNA with the lowest p-value among the previously reported ones (underlined in the figures in the same way as in Table II). If the previously reported miRNA was the same as either of the first two, the miRNA with the third lowest p-value is shown in the figure. In all five combinations comparing tumor and non-tumor tissues, previously reported miRNAs demonstrated the lowest p-values (Fig. 1). Notably, the three representative miRNAs for HCC with HBV infection (Combination 1) and HCC without virus infection (Combination 3) do not include any miRNA with previous association with HCC. Conversely, many novel miRNAs as well as previously reported miRNAs were detected by the unpaired Wilcoxon rank-sum test (Figs. 2 and 3).
If a miRNA is deemed to be upregulated in the tumor tissue when comparing tumor and non-tumor tissue, and also downregulated in the non-tumor tissues when comparing normal liver samples and non-tumor tissues, these results indicate that the miRNA was actually downregulated in the non-tumor tissue and thus it was detected as upregulated in the tumor when comparing the tumor and non-tumor tissues. To investigate such detailed changes, we compared lists of the differentially expressed miRNAs from the 13 combinations. Table III shows the results. For example, Normal↑ denotes that these miRNAs were downregulated in the tumor and non-tumor tissues compared with the normal liver. Similarly, Tumor↑ denotes that these miRNAs were upregulated in the tumor tissues only and their expression was not altered in the non-tumor tissues. HCV infection↑ indicates upregulation in the tumor and non-tumor tissues in patients with HCV infection while HCV↑ represents upregulation in the non-tumor tissue of the patients with HCV. V(−) denotes patients without virus infection. The expression levels of many miRNAs were altered in HCV-positive patients whereas a relatively small number of miRNAs were detected in patients with HBV and without virus infection.
Table III.
Differentially expressed miRNAs detected in various conditions.
| Expression change | Total detected | Detected miRNAs |
|---|---|---|
| Normal↑ | 8 | miR-18b*, miR-296-5p, miR-557, miR-581, miR-625*, miR-1228, miR-1249, miR-2116* |
| Tumor↑ | 1 | miR-183 |
| Tumor↓ | 5 | miR-29c, miR-129*, miR-146b-3p, miR-448, miR-486-5p |
| HCV infection↑ | 125 | let-7a, let-7c, miR-22, miR-23a, miR-93, miR-100, miR-106b, miR-126, miR-135a, miR-210 |
| HCV infection↓ | 7 | miR-143, miR-548q, miR-670, miR-711, miR-718, miR-759, miR-762 |
| HCC-C↑ | 68 | miR-31, miR-96, miR-103-2*, miR-130b*, miR-132, miR-134, miR-138, miR-184, miR-301a, miR-372 |
| HCC-C↑, V(-)_T↓ | 24 | let-7a2, miR-21*, miR-25*, miR-33a*, miR-92b*, miR-127-3p, miR-149, miR-330-5p, miR-338-3p, miR-517* |
| HCV↑ | 94 | miR-10a, miR-101, miR-130a, miR-146a, miR-155, miR-181b, miR-195, miR-197, miR-200a, miR-200b |
| HCV↑, V(-)_N↓ | 10 | miR-30b*, miR-187*, miR-188-5p, miR-193b, miR-199a-5p, miR-542-5p, miR-574-5p, miR-658, miR-720, miR-1287 |
| HCV↓ | 7 | let-7b*, miR-139-3p, miR-220c, miR-616*, miR-708, miR-767-3p, miR-1911 |
| HBV infection↑ | 1 | miR-422a |
| HCC-B↑ | 2 | miR-605, miR-1909 |
| V(-)↑ | 1 | miR-217 |
| V(-)↓ | 5 | miR-92a-2*, miR-361-3p, miR-513a-5p, miR-1234, miR-1914* |
| V(-)_T↑ | 1 | miR-216a |
| V(-)_T↓ | 10 | miR-133a, miR-199b-5p, miR-338-5p, miR-491-3p, miR-509-3-5p, miR-543, miR-627, miR-887, miR-921, miR-1247 |
| V(-)_N↓ | 6 | miR-664, miR-671-3p, miR-1246, miR-1268, miR-1280, miR-1978 |
Normal↑ denotes downregulation in the tumor and non-tumor tissues when compared with normal liver. Tumor↑ denotes upregulation in the tumor tissues only and no alteration in the non-tumor tissues. HCV infection↑ denotes upregulation in the tumor and non-tumor tissues in patients with HCV infection while HCV↑ denotes upregulation in the non-tumor tissue of such patients. V(-) denotes patients without virus infection. HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus. The double-underlined miRNAs have been previously reported to be involved in HCC while the miRNAs with a single underline have a reported association with other types of cancer.
Discussion
In this study, miRNA expression was profiled in tumor and surrounding non-tumor tissues from 40 HCC patients with various clinical features. We first performed pairwise comparisons of miRNA expression between the tumor and non-tumor tissues. We also profiled miRNA expression in non-tumor tissue samples from 6 patients with metastatic liver cancer. These non-tumor samples were considered as normal liver and compared with the results from each of the tumor and non-tumor tissues in patients with HBV and HCV or without infection. These comprehensive comparisons provided valuable insight into the alteration of miRNAs in the tumor and non-tumor tissues. For example, when a ratio of 1.5 is obtained from a comparison of the miRNA expression level in tumor and non-tumor tissues, it cannot be proven whether the miRNA expression was increased in the tumor tissue or decreased in the non-tumor tissue, since both cases will produce the same results. Employing normal liver samples enables this distinction.
As shown in Fig. 2, miR-30d, miR-124, miR-200b and miR-378 demonstrated significant changes in expression in the non-tumor tissues in HCC patients compared with normal liver samples in Combinations 7, 9, 11 and 13. In all combinations except 9, only a few previously detected miRNAs were observed: let-7e, let-7f, miR-98 and miR-144. Notably, none of them demonstrated the same tendency observed in previous studies. In Combination 9, more than 80 of the 342 miRNAs detected were previously reported, and 21 and 24 of them demonstrated the same and opposite tendencies observed in previous reports, respectively. These results suggest that these miRNAs may play a role in the very early stages of carcinogenesis in HCC and some may have different roles in HCC and chronic liver diseases.
As shown in Table III, miR-2116* was significantly downregulated in HCC as well as the surrounding non-tumor tissues compared with the normal liver samples in all possible combinations; thus, miR-2116* may have some influence upon carcinogenesis of HCC. Although this miRNA has no previously reported correlation with cancer, its predicted target genes involve genes associated with cancer. The top four predicted targets with the strongest prediction scores (57) were ZDHHC11, MTCH2, DIRC2 and PEA15 (as of January 2012 according to www.microrna.org). The gene copy number of ZDHHC11 was altered in nearly half of the patients with non-small cell lung cancer (58) and bladder cancer (59). MTCH2 is a gene exhibiting highly restricted levels of gene expression variation in tumor tissues compared to non-malignant tissues (60). Bodmer et al identified DIRC2 as a familial renal cell carcinoma-associated gene (61). The expression level of PEA15 was used for grading the malignancy of astrocytic tumors (62). These facts indicate that the predicted targets play roles in cancer. The expression of miR-2116* was altered in the non-tumor tissues when compared to the normal liver samples, and accordingly, as discussed earlier, this miRNA may also play a role in the early stages of carcinogenesis. Other miRNAs which were consistently downregulated in the tumor and non-tumor tissues are miR-18b*, miR-296-5p, miR-557, miR-581, miR-625*, miR-1228 and miR-1249. Similar to miR-2116*, the predicted target genes of miR-1249 involved numerous genes whose association with cancer has been reported.
The results strongly suggest that the above miRNAs are novel biomarker candidates for chronic liver diseases. Some are novel biomarker candidates for HCC irrespective of virus infection and HCC with HCV, as shown in Table III. In this way, through miRNA expression profiling, we identified various pathogenesis-related miRNAs which may be used as biomarkers for a specific disease state, although further investigation is required.
Acknowledgments
This study was funded by the Scientific Research Grant (No. 20510184), Science and Technology Promotion Adjustment Expenses (No. 08005234), and the Integrated Database Project, from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Blum H. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391–7400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramp M. HBV + HCV = HCC? Gut. 1999;45:168–169. doi: 10.1136/gut.45.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soini Y, Chia SC, Bennett WP, et al. An aflatoxin-associated mutational hotspot at codon 249 in the p53 tumor suppressor gene occurs in hepatocellular carcinomas from Mexico. Carcinogenesis. 1996;17:1007–1012. doi: 10.1093/carcin/17.5.1007. [DOI] [PubMed] [Google Scholar]
- 5.Boffetta P, Matisane L, Mundt KA, Dell LD. Meta-analysis of studies of occupational exposure to vinyl chloride in relation to cancer mortality. Scand J Work Environ Health. 2003;29:220–229. doi: 10.5271/sjweh.725. [DOI] [PubMed] [Google Scholar]
- 6.Tsukuma H, Hiyama T, Oshima A, et al. A case-control study of hepatocellular carcinoma in Osaka, Japan. Int J Cancer. 1995;45:231–236. doi: 10.1002/ijc.2910450205. [DOI] [PubMed] [Google Scholar]
- 7.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–333. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 8.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanaihara N, Caplen N, Bowman E, et al. Unique micro RNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami Y, Yasuda T, Saigo K, et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 12.Budhu A, Jia HL, Forgues M, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 13.Varnhort H, Drebber U, Schulze F, et al. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology. 2008;47:1223–1232. doi: 10.1002/hep.22158. [DOI] [PubMed] [Google Scholar]
- 14.Laderio Y, Couchy G, Balabaud C, et al. MicroRNA profiling in hepatocellular tumor is associated with clinical features and oncogene/tumor suppressors gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Xie L, He X, et al. Diagnostic and prognositic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 16.Ura S, Honda M, Yamashita T, et al. Differential microRNA expression between hapatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009;49:1098–1112. doi: 10.1002/hep.22749. [DOI] [PubMed] [Google Scholar]
- 17.Toffanin S, Hoshida Y, Lachenmayer A, et al. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastoenterology. 2011;140:1618–1628. doi: 10.1053/j.gastro.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasen M, Mizushima H, Mogushi K, et al. Expression of Aurora B and alterative variant forms in hepatocellular carcinoma and adjacent tissue. Cancer Sci. 2009;100:472–480. doi: 10.1111/j.1349-7006.2008.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramantieri L, Fornari F, Callegari E, et al. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12:2189–2204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aravalli RN, Steer CJ, Cressman NK. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 21.Ji J, Wang XW. New kids on the block. Cancer Biol Therapy. 2009;8:1683–1690. doi: 10.4161/cbt.8.18.8898. [DOI] [PubMed] [Google Scholar]
- 22.Liang L, Wong CM, Ying Q, et al. MicroRNA-125b suppressed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B. Hepatology. 2010;52:1731–1740. doi: 10.1002/hep.23904. [DOI] [PubMed] [Google Scholar]
- 23.Osaki M, Takeshita F, Ochiya T. MicroRNAs as biomarkers and therapeutic drugs in human cancer. Biomarkers. 2008;13:658–670. doi: 10.1080/13547500802646572. [DOI] [PubMed] [Google Scholar]
- 24.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 micro RNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 25.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Crawford M, Brawner E, Battle K, et al. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem Biophys Res Commun. 2008;373:607–612. doi: 10.1016/j.bbrc.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 28.Hayashita Y, Osada H, Tatematsu Y, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 29.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garofalo M, Quintavalle C, Di Leva G, et al. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845–3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- 31.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Hu Z, Chen J, Tian T, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu SL, Chen HY, Chang GC, et al. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Geogoulias V, Lianidou ES. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008;54:1696–1704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 35.Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan SH, Wu CW, Li AF, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008;28:907–911. [PubMed] [Google Scholar]
- 37.Schepeler T, Reinert JT, Ostenfeld MS, et al. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 38.Childs G, Fazzari M, Kung G, et al. Low-level expression of microRNAs let-7d and miR-205 are prognostic markers of head and neck squamous cell carcinoma. Am J Pathol. 2009;174:736–745. doi: 10.2353/ajpath.2009.080731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roldo C, Missiaglia E, Hagan JP, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 40.Marcucci G, Radmacher MD, Maharry K, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 41.Calin GA, Ferracin M, Cimmino A, et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 42.Lu L, Katsaros D, de la Longrais IA, Sochirca O, Yu H. Hypermethylation of let-7a-3 in epithelial ovarian cancer is associated with low insulin-like growth factor-II expression and favorable prognosis. Cancer Res. 2007;67:10117–10122. doi: 10.1158/0008-5472.CAN-07-2544. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y, Chen Z, Zhang L, et al. Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res. 2008;68:26–33. doi: 10.1158/0008-5472.CAN-06-4418. [DOI] [PubMed] [Google Scholar]
- 44.Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in material plasma. Clin Chem. 2008;54:482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 45.Gilad S, Meiri E, Yogev Y, et al. Serum microRNAs are promising biomarkers. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchel PS, Parkin RA, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrie CH, Cooper Cd, Ballabio E, Chi J, Tramonti D, Hatton CS. Aberrant expression of microRNA biosynthetic pathways components is a common feature of haematological malignancy. Br J Hematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2009.07642.x. [DOI] [PubMed] [Google Scholar]
- 48.Diaz R, Silva J, Garcia JM, et al. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008;47:794–802. doi: 10.1002/gcc.20580. [DOI] [PubMed] [Google Scholar]
- 49.Schee K, Fodstad O, Flatmark K. MicroRNAs as biomarkers in colorectal cancer. Am J Patho. 2010;177:1592–1599. doi: 10.2353/ajpath.2010.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu WKK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJY. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29:5761–5671. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- 51.Zavadil J, Ye H, Liu Z, et al. Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS One. 2010;5:e12362. doi: 10.1371/journal.pone.0012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iorio MV, Piovan C, Croce CM. Interplay between microRNAs and the epigenetic machinery: an intricate network. Boichim Biophys Acta. 2010;1799:694–701. doi: 10.1016/j.bbagrm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Valeri N, Vannini I, Fanini F, Calore F, Adair B, Fabbri M. Epigenetics, miRNAs, and human cancer: a new chapter in human gene regulation. Mamm Genome. 2009;20:573–580. doi: 10.1007/s00335-009-9206-5. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer A, Jung M, Kristiansen G, et al. MicroRNAs and cancer: current state and future perspectives in urologic oncology. Urologic Oncol. 2010;28:4–13. doi: 10.1016/j.urolonc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Betel D, Koppal A, Angius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang JU, Koo SH, Kwon KC, Park JW, Kim JM. Gain at chromosomal region 5p15.33, containing TERT, is the most frequent genetic event in early stages of non-small cell lung cancer. Cancer Genet Cytogenet. 2008;82:1–11. doi: 10.1016/j.cancergencyto.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto Y, Chochi Y, Matuyama H, et al. Gain of 5p15.33 is associated with progression of bladder cancer. Oncology. 2007;72:132–138. doi: 10.1159/000111132. [DOI] [PubMed] [Google Scholar]
- 60.Yu K, Ganesan K, Tan LK, et al. A precisely regulated gene expression cassette potentially modulates metastasis and survival in multiple solid cancers. PLoS Genet. 2008;4:e1000129. doi: 10.1371/journal.pgen.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bodmer D, Eleveld M, Kater-Baats E, et al. Disruption of a novel MFS transporter gene, DIRC2, by a familial renal cell carcinoma-associated t(2;3)(q35;q21) Hum Mol Genet. 2002;11:641–649. doi: 10.1093/hmg/11.6.641. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe Y, Yamasaki F, Kajiwara Y, et al. Expression of phosphoprotein enriched in astrocytes 15 kDa (PEA-15) in astrocytic tumors: a novel approach of correlating malignancy grade and prognosis. J Neurooncol. 2010;100:449–457. doi: 10.1007/s11060-010-0201-1. [DOI] [PubMed] [Google Scholar]


