Abstract
The pyrenoid is a proteinaceous structure found in the chloroplast of most unicellular algae. Various studies indicate that ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is present in the pyrenoid, although the fraction of Rubisco localized there remains controversial. Estimates of the amount of Rubisco in the pyrenoid of Chlamydomonas reinhardtii range from 5% to nearly 100%. Using immunolocalization, the amount of Rubisco localized to the pyrenoid or to the chloroplast stroma was estimated for C. reinhardtii cells grown under different conditions. It was observed that the amount of Rubisco in the pyrenoid varied with growth condition; about 40% was in the pyrenoid when the cells were grown under elevated CO2 and about 90% with ambient CO2. In addition, it is likely that pyrenoidal Rubisco is active in CO2 fixation because in vitro activity measurements showed that most of the Rubisco must be active to account for CO2-fixation rates observed in whole cells. These results are consistent with the idea that the pyrenoid is the site of CO2 fixation in C. reinhardtii and other unicellular algae containing CO2-concentrating mechanisms.
Pyrenoids are electron-dense structures that are found in the plastids of most algae. When pyrenoids are isolated, the most abundant protein present is Rubisco (Kuchitsu et al., 1991; McKay and Gibbs, 1991; Okada, 1992). However, the amount of Rubisco present in the pyrenoid is controversial. Estimates of the amount of Rubisco in the pyrenoid versus the amount in the entire chloroplast range from 60% or higher (Vladimirova et al., 1982; Lacoste-Royal and Gibbs, 1987; Morita et al., 1997) to as low as 5% (Süss et al., 1995).
The function of Rubisco in the pyrenoid is also unclear. Lacoste-Royal and Gibbs (1987) found that a higher percentage of Rubisco was localized to the pyrenoid in stationary cells rather than in actively growing cells of Chlamydomonas reinhardtii, an observation that is consistent with the hypothesis that the pyrenoid is a storage body. Süss et al. (1995) interpreted their localization data to indicate that there were two forms of Rubisco in C. reinhardtii: form I, which bound to the thylakoid membranes in the stroma, and form II, which was found in the pyrenoid. They further speculated that the pyrenoid-localized form II might function as part of the CO2-concentrating mechanism.
In photosynthetic organisms that possess a CO2-concentrating mechanism, Rubisco has a very specific localization. In higher plants with C4-type photosynthesis, Rubisco is specifically localized to chloroplasts of bundle-sheath cells (Hatch, 1992). In unicellular cyanobacteria with CO2-concentrating mechanisms, Rubisco is specifically localized to structures known as carboxysomes (Allen, 1984; Codd and Marsden, 1984). Recent studies of the cyanobacteria's CO2-concentrating mechanism indicate that HCO3− accumulated by the cell is dehydrated specifically in the carboxysome, where the resulting CO2 can be fixed before leaking out of the cell (Badger and Price, 1994). Models of this process have also been proposed (Reinhold et al., 1991).
C. reinhardtii also possesses a CO2-concentrating mechanism that is inducible (Badger et al., 1980; Aizawa and Miyachi, 1986; Moroney and Mason, 1991). When C. reinhardtii is grown under elevated CO2, the CO2-concentrating mechanism is not present, but when the alga is grown under low CO2, the CO2-concentrating mechanism is operational. With these observations in mind, we investigated the distribution of Rubisco in C. reinhardtii under a variety of growth conditions, including high- and low-CO2 concentrations. Under all growth conditions we found that a significant fraction of Rubisco was localized to the pyrenoid. In particular, under limiting CO2 more than 90% of the Rubisco was found within the pyrenoid. In addition, we found that the in vivo rates of CO2 fixation are similar to the total amount of Rubisco activity, as estimated by in vitro Rubisco assays. This means that nearly all of the Rubisco in the cell must be functional to account for the rates of CO2 fixation observed in cells. This observation implies that the Rubisco localized to the pyrenoid is active in CO2 fixation.
MATERIALS AND METHODS
Algal Cultures
The wild-type Chlamydomonas reinhardtii strain used in this study, 137 mt+, was obtained from Dr. R.K. Togasaki (Indiana University, Bloomington). Strain 18–7G (Spreitzer et al., 1985), containing a truncation of the large subunit of Rubisco, was obtained as CC-2653 from the Chlamydomonas Genetics Center (Duke University, Durham, NC). When grown photoautotrophically, strain 137 was cultured in minimal medium (Sueoka, 1960) in 2.8-L carboys, illuminated with 200 μE m−2 s−1 at room temperature, and shaken continuously. Cultures were bubbled with 5% CO2 in air (final Ci concentration = 2 mm) or in ordinary air (final Ci = 4 μm). When grown with acetate as a C source, Tris-acetate-phosphate medium was used (Sueoka, 1960). Strain 18–7G was grown with this medium in the dark.
Electron Microscopy
For transmission electron microscopy, various cell strains were prepared according to Henk et al. (1995). Equal parts of the cell suspension were mixed with equal parts of 4% OsO4, 8% formaldehyde, and 2% glutaraldehyde and fixed for 15 min. The sample was then filtered and fixed for an additional 15 min in equal parts of 4% OsO4, 8% formaldehyde, 2% glutaraldehyde, and 0.2 m sodium cacodylate buffer (pH 7.2). The final concentration of the fixative components were: 1% OsO4, 2% formaldehyde, 0.5% glutaraldehyde, and 50 mm sodium cacodylate buffer. The subsequent steps for obtaining sections and treatment of the grids were the same as described by Henk et al. (1995).
Immunolocalization
The immunocytochemical procedure was similar to the method of Schroeder et al. (1993), with some modifications. Sections were pretreated with 2% sodium-meta-periodate (Sigma) for 15 min to remove any glutaraldehyde, then blocked two times for 30 min each in 2% BSA and 0.1% Tween 20 in PBS. The sections were then incubated for 1 h with diluted primary antibody (1–50 dilutions of anti-Rubisco) or with preimmune serum diluted similarly as a control, washed with 0.5% Tween 20 in PBS four times for a total of 20 min, and blocked again in 2% BSA two times for 15 min each. To detect bound antibodies, we used Protein A conjugated with 15-nm colloidal gold particles (BB International, Cardiff, UK) and diluted 1:50 with 1% BSA for 40 min.
Evaluation of immunogold labeling was made using the computer program Image-1/AT release version 4 (Universal Imaging Co., West Chester, PA). The images from the negatives were frozen and stored in a frame buffer as an 8-bit, 256-gray-scale image. Once this was freshholded, the gold particles associated with the pyrenoid were counted, as were the particles in the same size area in the cytoplasm and thylakoid regions of the individual cells. Because the size of the pyrenoid was different from cell to cell, we calculated the number of particles in a 1-μm2 area as a means for comparing the labeling intensity for the various cell compartments of the different strains in the various experimental conditions.
Enzymes Assays
C. reinhardtii Rubisco was purified according to Spreitzer and Mets (1980). Rubisco activity in vitro was estimated by the method described by Pierce et al. (1982).
Photosynthesis Assays
The photosynthetic rate of algal cells was measured with an O2 electrode (Rank Brothers, Cambridge, UK). Algae were centrifuged at 5000 rpm for 5 min, and the pellet resuspended at 25 μg chlorophyll mL−1 in 4 mL of 25 mm Hepes-KOH (pH 7.3) and transferred to the electrode chamber. Algal cells were assayed at 300 μmol m−2 s−1 of PAR. They were allowed to consume the Ci of the buffer and the intracellular pool of Ci until no net O2 exchange was observed, which took between 3 and 10 min. NaHCO3 at the indicated concentrations was added and the rate of O2 evolution was measured over the next 30 s to 2 min. Chlorophyll concentrations were determined spectrophotometrically. The K0.5(CO2) value is the CO2 concentration required to give half-maximal rates of O2 evolution.
SDS-PAGE and Immunoblotting
SDS-PAGE was performed on 12.5% polyacrylamide gels as described by Laemmli (1970). The protocol from Bio-Rad was followed when immunoblotting. The blots were probed with antisera raised against C. reinhardtii Rubisco (HTI Bioproducts, Ramona, CA). Immunoblotting was visualized using horseradish peroxidase-conjugated secondary antibodies, as described in the protocol from Bio-Rad.
RESULTS
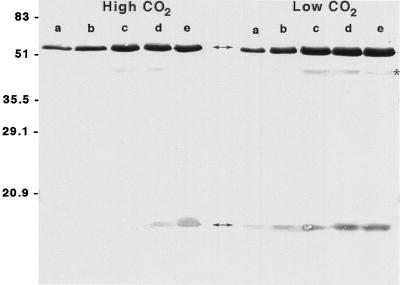
In this study an antibody raised against Rubisco isolated from C. reinhardtii was used. This antibody reacted strongly and specifically with C. reinhardtii Rubisco, as judged by immunoblots (Fig. 1). The antibody recognized the C. reinhardtii Rubisco large subunit much better than the small subunit (Fig. 1). For this reason, the presence of the small subunit precursor should not complicate the immunolocalization studies.
Figure 1.
Specificity of the C. reinhardtii anti-Rubisco antibody. Different amounts of cell homogenate from high- and low-CO2-grown cells were separated by SDS-PAGE, blotted to nitrocellulose, and probed with an anti-Rubisco antibody, as described in Methods. Lanes a, 5 μg of total protein; lanes b, 10 μg of protein; lanes c, 20 μg of protein; lanes d, 25 μg of protein; and lanes e, 30 μg of protein. The large and small subunits of Rubisco are indicated with arrows. The weak band at 44 kD (indicated with *) is a breakdown product of the large subunit (Rawat, 1994).
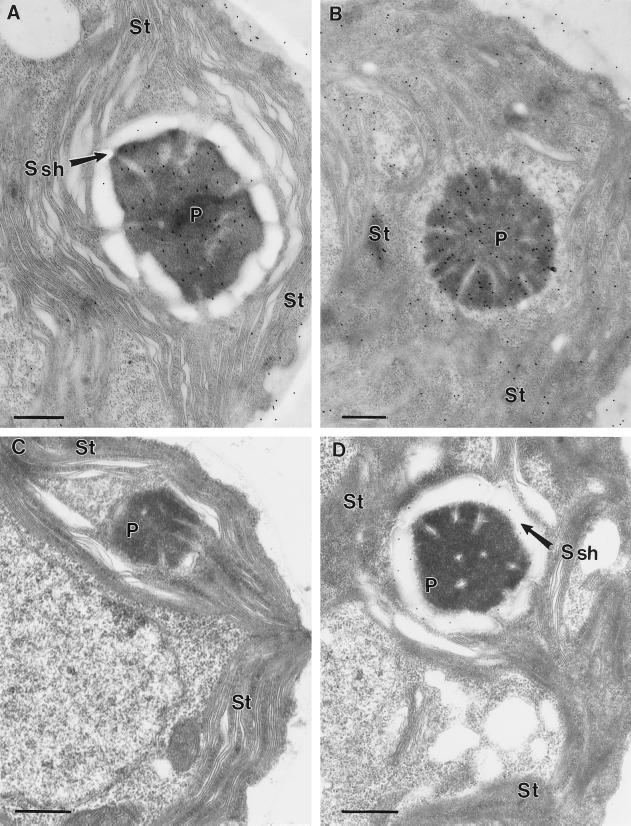
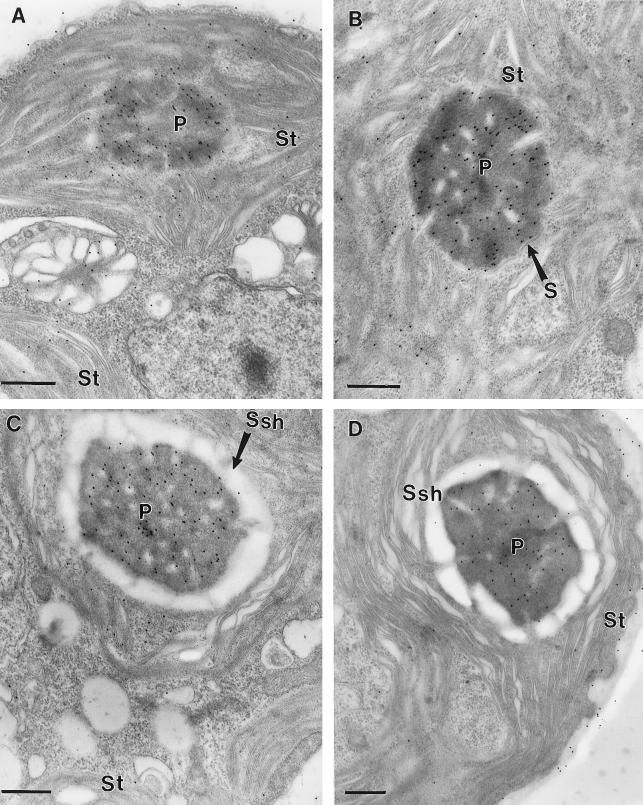
When the anti-Rubisco antibody was used in immunolocalization studies, immunogold particles were localized preferentially to the chloroplast pyrenoid (Fig. 2). Two different growth conditions were used for the initial study: growth on elevated CO2 or growth on air levels of CO2. In both cases the pyrenoid had a high concentration of immunogold particles. In contrast, the immunogold labeling density in the chloroplast stroma depended on the growth condition. Growth on low CO2 resulted in a low density of immunogold particles in the stroma (Fig. 2A), with the labeling density being similar to that seen in the cytoplasm. In contrast, cells grown on elevated CO2 had higher densities of immunogold particles in the stroma (Fig. 2B). In the case of cells grown on high CO2 the labeling of the stroma was significantly higher (P < 0.01) than the labeling of the cytoplasm. Cells grown under either growth condition showed a very low density of immunogold particles over the entire cell when probed with the preimmune serum (Fig. 2, C and D).
Figure 2.
Immunogold labeling of C. reinhardtii cells grown on high- or low-CO2 conditions. A, Low-CO2-grown wild-type cells grown on minimal medium probed with an antibody raised against C. reinhardtii Rubisco. B, High-CO2-grown cells grown on minimal medium probed with an antibody raised against C. reinhardtii Rubisco. C, High-CO2-grown wild-type cells grown on minimal medium probed with a preimmune serum. D, Low-CO2-grown cells grown on minimal medium probed with a preimmune serum. Bars indicate 0.5 μm. P, Pyrenoid; Ssh, starch sheath; and St, chloroplast stroma.
When the relative volumes of the pyrenoid and the stroma are taken into account (Lacoste-Royal and Gibbs, 1987), it is clear that almost all of the Rubisco appears to be localized to the pyrenoid in C. reinhardtii cells grown under low levels of CO2 (Table I). Similar calculations using data from the high-CO2-grown cells show that a significant fraction (approximately 60%) of the Rubisco is localized to the chloroplast stroma in those cells (Table I). These data imply that the localization of Rubisco in C. reinhardtii depends in part on the growth conditions of the organism.
Table I.
Calculation of Rubisco fraction in both the pyrenoid and stroma of the chloroplast
| Growth Condition | Immunogold Density
|
Rubisco in Pyrenoid | ||
|---|---|---|---|---|
| Pyrenoid | Stroma | Cytoplasm | ||
| particles mm−2 | % | |||
| High CO2 | 70 ± 14 | 9.0 ± 2.1 | 2.0 ± 0.9 | 40 |
| Low CO2 | 65 ± 15 | 2.5 ± 1.2 | 2.0 ± 0.8 | 89 |
To obtain the fraction of Rubisco in the pyrenoid, the average density of immunogold particles in the cytoplasm was subtracted from the average density of particles in the stroma or in the pyrenoid. The net particle density of the pyrenoid or stroma was then multiplied by the average volume of the compartment (which is 2.4 mm3 and 35.6 mm3, respectively), giving the total particles for each compartment. To calculate the fraction of Rubisco in the pyrenoid, the total number of particles in the pyrenoid was divided by the combined number of particles in the pyrenoid and in the stroma. The stroma includes the thylakoid area. The data shown are the averages ± sd of 10 samples and 40 different evaluations. Preimmune sera gave immunogold densities of less than 2 particles mm−2.
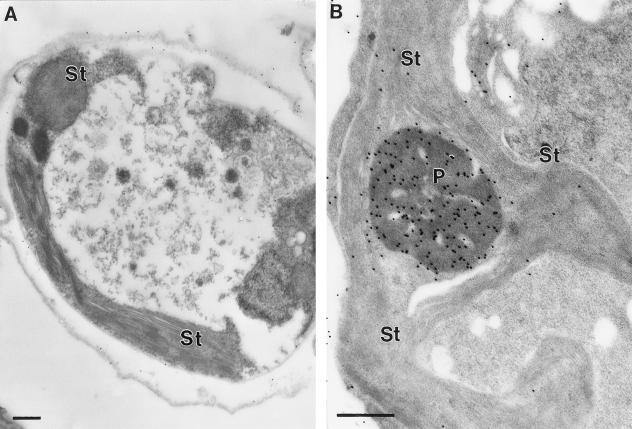
We also grew cells under different conditions to determine whether the distribution of Rubisco changes with a different C source. The growth condition tested was mixotrophic growth (Fig. 3). All of the wild-type cultures were grown at 150 μmol m−2 s−1 of PAR. In these studies the distribution of immunogold particles in acetate-grown cells indicated that a significant fraction of the Rubisco was in the stroma (Figs. 2B and 3). C. reinhardtii strain 18–7G was also tested as a negative control. This strain has a stop codon in the rbcL gene and does not have detectable Rubisco (Spreitzer et al., 1985). However, strain 18–7G can grow with acetate as a C source under low light. As shown previously, this strain also does not have a detectable pyrenoid (Rawat et al., 1996). When probed with a Rubisco antibody, strain 18–7G had a very low immunogold density over the chloroplast stroma (Fig. 3A). The low immunogold density seen in this strain is similar to the immunogold density seen in wild-type cells growing on low CO2, implying that the amount of Rubisco in the stroma of wild-type cells is very low. This result from strain 18–7G also supports the contention that the immunogold particle density in the stroma of wild-type cells grown on high CO2 or on acetate is higher than background (Table II).
Figure 3.
Immunogold labeling of C. reinhardtii strain 18–7G or wild-type cells grown on acetate. A, Strain 18–7G. B, Wild-type cells. Sections were probed with an antibody raised against C. reinhardtii Rubisco, as described in Methods. Bars indicate 0.5 μm. P, Pyrenoid; and St, chloroplast stroma. No pyrenoids were seen in strain 18–7G, in agreement with previous observations (Rawat et al., 1996).
Table II.
Calculation of the Rubisco fraction in both the pyrenoid and the stroma of wild-type cells and strain 18–7G grown on acetate
| Strain | Immunogold Density
|
Rubisco in Pyrenoid | ||
|---|---|---|---|---|
| Pyrenoid | Stroma | Cytoplasm | ||
| particles mm−2 | % | |||
| Wild type | 149 ± 63 | 6.2 ± 1.5 | 3.8 ± 1.7 | 80 |
| 18–7G | N.A. | 1.3 ± 0.9 | 1.6 ± 0.6 | N.A. |
Both cell lines were grown in moderate light (150 μmol mm−2 s−1) on acetate medium. To quantitate the number of immunogold particles in the stroma and in the pyrenoid the method described in the legend to Table I was employed. Because the pyrenoid is absent from 18–7G, the number of particles in the pyrenoid is not applicable (N.A.). Ten samples of each strain were sampled. The data shown are the averages ± sd.
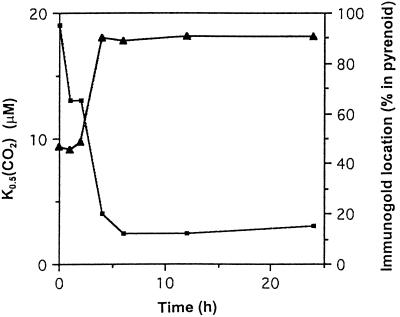
The change in distribution of Rubisco under high- and low-CO2 growth conditions follows the presence or absence of the CO2-concentrating mechanism. To further test this correlation, we determined the localization of Rubisco while cells were adapting to low-CO2 growth conditions. In this experiment cells were first grown on elevated CO2 and then switched to low CO2. Using immunogold, the distribution of Rubisco between the pyrenoid and stroma was estimated for various times after the switch in CO2 level (Figs. 4 and 5). As seen from the time course (Fig. 4), the distribution of Rubisco changes about 2 h after the change in CO2 supply. This is also the time when the affinity of the cells for CO2, as estimated by the K0.5(CO2), is rapidly changing. Representative electron micrographs showing the immunogold distributions are shown in Figure 5. The data in this experiment are consistent with that of Morita et al. (1997) and our earlier experiment (Fig. 2A), indicating that 90% or more of the Rubisco is in the pyrenoid when cells are grown on low CO2.
Figure 4.
Change in the apparent affinity of cells for Ci and the change in distribution of Rubisco after cells are switched to low CO2. At 0 h cells were switched from high- to low-CO2 conditions. The apparent affinity of the cells was determined by estimating the K0.5(CO2) of the cells (▪) as described in Methods. The percentage of Rubisco in the pyrenoid (▴) is calculated from the distribution of immunogold particles.
Figure 5.
Immunogold labeling of C. reinhardtii cells after being switched from high- to low-CO2 conditions. A, Cells just before transfer to low CO2 (0 h). B, Cells 2 h after the switch to low CO2. C, Cells 6 h after the switch to low CO2. D, Cells 12 h after the switch to low CO2. Bars indicate 0.5 μm. P, Pyrenoid; Ssh, starch sheath; and St, chloroplast stroma.
Since 90% of the Rubisco is localized to the pyrenoid, we compared the in vitro carboxylation activity of Rubisco isolated from low-CO2-grown wild-type cells with the in vivo 14CO2-fixation rate of these same cells. The in vitro Rubisco carboxylation rate measured under saturating CO2 (186 ± 50 μmol h −1 mg chlorophyll −1) closely matched the maximal in vivo rate of 14CO2 fixation (199 ± 44 μmol h −1 mg chlorophyll −1). This result supports the suggestion that the majority of Rubisco within the low-CO2-grown cell must be active to support the rates of CO2 fixation observed in whole cells. Since most of the Rubisco is localized to the pyrenoid, it follows that the Rubisco within the pyrenoid is active and that the pyrenoid is the actual location of CO2 fixation in C. reinhardtii.
DISCUSSION
One aim of this report was to resolve the present controversy concerning the localization of Rubisco in C. reinhardtii. Lacoste-Royal and Gibbs (1987) reported that between 60 and 70% of Rubisco appeared to be localized to the pyrenoid. Recently, however, Süss et al. (1995) challenged that earlier finding by reporting that only 5% of the Rubisco was localized in the pyrenoid. In addition, Morita et al. (1997) have recently reported than 99% of the Rubisco is localized to the pyrenoid, illustrating the wide range of estimates in the literature. Süss et al. (1995) used cryo-techniques to avoid spatial dislocation of soluble enzymes such as Rubisco and other C3 enzymes. They cited this as a possible difference between their results and those of Lacoste-Royal and Gibbs (1987). However, Morita et al. (1997) used similar techniques in their study. Süss et al. (1995) also argued that Lacoste-Royal and Gibbs (1987) did not take into account the relative volumes of the pyrenoid and stroma in their calculations. The data presented in this report are consistent with the data of Lacoste-Royal and Gibbs (1987) and Morita et al. (1997). The small differences between the reports of Lacoste-Royal and Gibbs (1987), Morita et al. (1997), and this present work are likely due to growth-condition differences. For example, many of the cultures used by Lacoste-Royal and Gibbs (1987) were mixotrophically grown, whereas Morita et al. (1997) grew cells phototrophically on low CO2.
Our results are in agreement with all earlier studies that showed a high level of immunogold particles in the pyrenoid when anti-Rubisco antibodies were used. However, we disagree with the conclusions of Süss et al. (1995). The primary reason for our different conclusions is the method of counting the immunogold particles. In this report and in the paper by Lacoste-Royal and Gibbs (1987), the number of immunogold particles is reported as the number of particles per unit area. Süss et al. (1995) counted the total number of particles per section in each subcellular location and did not take into account the area of each organelle in the section sampled.
According to Weibel (1979) a section is a two-dimensional projection of the volume of the organelle or tissue being examined in the electron microscope. As such, the average area of an organelle or cell observed in many sections is proportional to the volume of that object. Therefore, the quantitative method employed by Süss et al. (1995) accounts for the volume difference twice, first in the counting of the particles and a second time when they multiply their number by the relative volumes of the pyrenoid and the stroma. If the number of immunogold particles reported by Süss et al. (1995) is expressed on a particles per area basis, their data are much more in line with our results and those of Lacoste-Royal and Gibbs (1987) and Morita et al. (1997). From these studies it appears that at a minimum 60 to 70% of the Rubisco in C. reinhardtii is localized to the pyrenoid.
A second aim of this report was to determine whether Rubisco is mobilized or relocated when cells are switched from high to low CO2. It appears that growth conditions do affect the intracellular localization of Rubisco. We looked at cells grown on high CO2, low CO2, or acetate. We consistently found significant levels of Rubisco in the chloroplast stroma if the cells were grown on high CO2 or acetate. In the extreme case of strain 18–7G, where no Rubisco is produced, no pyrenoid was observed and the immunogold density in the stroma was at background levels. Our results are consistent with the pyrenoid being the primary location of Rubisco in C. reinhardtii under low-CO2 growth conditions when the components of the CO2-concentrating mechanism are being expressed. The shift in Rubisco localization correlates well with the formation of the starch sheath, which also forms shortly after cells are switched to low CO2 (Ramazanov et al., 1994). Süss et al. (1995) also reported that Rubisco was associated with thylakoid membranes in the chloroplast stroma and the pyrenoid. For the pyrenoid we found no particular association of Rubisco with the tubules that penetrate the pyrenoid matrix. The concentration of Rubisco is such that it is likely to be found throughout the matrix of the pyrenoid. Our immunolabeling of the stroma was not dense enough to determine whether there is a particular association of Rubisco with the stromal lamellae.
The finding of Rubisco in the pyrenoid has raised the question of whether pyrenoidal Rubisco is active in CO2 fixation. Clearly, if 100% of the Rubisco is localized to the pyrenoid, then that Rubisco must be active. However, even if a lower estimate of the amount of Rubisco in the pyrenoid is used, our activity measurements are consistent with the idea that pyrenoidal Rubisco is active in vivo. Rubisco activity measurements indicate that there is not an excess of Rubisco activity in the cell, and that most of the Rubisco in the cell must be active to account for the levels of CO2 fixation observed in whole cells. Because 90% of the Rubisco is localized to the pyrenoid when cells are grown on low CO2 (Figs. 2A and 4D; Table I) (Morita et al., 1997), these results are consistent with the idea that the primary location of CO2 fixation in C. reinhardtii is the pyrenoid.
A specific localization of Rubisco to the pyrenoid is compatible with the view that organisms with CO2-concentrating mechanisms package Rubisco in very specific fashions. The most analogous situation is found in cyanobacteria, in which Rubisco is found in carboxysomes (Codd and Marsden, 1984; Reinhold et al., 1991). In cyanobacteria it appears that the CO2 level is elevated within the carboxysome, thus favoring the carboxylation activity of Rubisco over the oxygenase activity. The pyrenoid may serve the same function in C. reinhardtii and other eukaryotic algae.
ACKNOWLEDGMENTS
We thank Ronald Bouchard for his help with the Image-1/AT, and Dr. William Henk and M.C. Henk for their valuable suggestions.
Abbreviations:
- Ci
inorganic carbon
- high CO2
air supplemented with CO2 so that the final CO2 concentration is 5% (v/v)
- low CO2
air containing ambient (350 ppm) CO2
Footnotes
This work was supported by the National Science Foundation (grant no. IBN-9632087).
LITERATURE CITED
- Aizawa K, Miyachi S. Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria. FEMS Micro Rev. 1986;39:215–233. [Google Scholar]
- Allen MM. Cyanobacterial cell inclusions. Annu Rev Microbiol. 1984;38:1–25. doi: 10.1146/annurev.mi.38.100184.000245. [DOI] [PubMed] [Google Scholar]
- Badger MR, Kaplan A, Berry JA. Internal inorganic carbon pool of Chlamydomonas reinhardtii. Evidence for a carbon dioxide-concentrating mechanism. Plant Physiol. 1980;66:407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD. The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:369–392. [Google Scholar]
- Codd GA, Marsden WJN. The carboxysomes (polyhedral bodies) of autotrophic prokaryotes. Biol Rev. 1984;59:389–422. [Google Scholar]
- Hatch MD. C4 Photosynthesis: An unlikely process full of surprises. Plant Cell Physiol. 1992;33:333–342. [Google Scholar]
- Henk MC, Rawat M, Hugghins SY, Lavigne LL, Ramazanov Z, Mason CB, Moroney JV. Pyrenoid morphology in Rubisco and CO2 concentrating mutants of Chlamydomonas reinhardtii. In: Mathis P, editor. Photosynthesis: from Light to Biosphere, Vol V. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 595–598. [Google Scholar]
- Kuchitsu K, Tsuzuki M, Miyachi S. Polypeptide composition of the pyrenoid and its regulation by CO2 concentration in unicellular green algae. Can J Bot. 1991;69:1062–1069. [Google Scholar]
- Lacoste-Royal G, Gibbs SP. Immunocytochemical localization of ribulose-1,5-bisphosphate carboxylase in the pyrenoid and thylakoid region of the chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1987;83:602–606. doi: 10.1104/pp.83.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKay RML, Gibbs SP. Composition and function of pyrenoids: cytochemical and immunocytochemical approaches. Can J Bot. 1991;69:1040–1052. [Google Scholar]
- Morita E, Kuroiwa H, Kuroiwa T, Nozaki H. High localization of ribulose-1,5-bisphosphate carboxylase/oxygenase in the pyrenoids of Chlamydomonas reinhardtii (Chlorophyta), as revealed by immunogold electron microscopy. J Phycol. 1997;33:68–72. [Google Scholar]
- Moroney JV, Mason CB. The role of the chloroplast in inorganic carbon acquisition by Chlamydomonas reinhardtii. Can J Bot. 1991;69:1017–1024. [Google Scholar]
- Okada M (1992) Recent studies on the composition and the activity of algal pyrenoids. In FE Round, DJ Chapman, eds, Progress in Phycological Research, Vol 8. Biopress, Ltd., Bristol, UK, pp 117–138
- Pierce JW, McCurry SD, Mulligan RM, Tolbert NE. Activation and assay of ribulose-1,5-bisphophate carboxylase/oxygenase. Methods Enzymol. 1982;89:47–55. doi: 10.1016/s0076-6879(82)89011-3. [DOI] [PubMed] [Google Scholar]
- Ramazanov Z, Rawat M, Henk MC, Mason CB, Matthews SW, Moroney JV. The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta. 1994;195:210–216. [Google Scholar]
- Rawat M (1994) The effect of carbon dioxide concentration on carbonic anhydrase and other proteins in Chlamydomonas reinhardtii. PhD thesis. Louisiana State University, Baton Rouge
- Rawat M, Henk MC, Lavigne LL, Moroney JV. Chlamydomonas reinhardtii mutants without ribulose-1,5-bisphosphate carboxylase-oxygenase lack a detectable pyrenoid. Planta. 1996;198:263–270. [Google Scholar]
- Reinhold LR, Kosloff R, Kaplan A. A model for inorganic carbon fluxes and photosynthesis in cyanobacterial carboxysomes. Can J Bot. 1991;69:984–988. [Google Scholar]
- Schroeder MR, Borkhsenious ON, Matsuoka K, Nakamura K, Raikhel NV. Colocalization of barley lectin and sporamin in vacuoles of transgenic tobacco plants. Plant Physiol. 1993;101:451–458. doi: 10.1104/pp.101.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer RJ, Goldschmidt-Clermont M, Rahire M, Rochaix JD. Nonsense mutations in the Chlamydomonas chloroplast gene that codes for the large subunit of ribulose bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci USA. 1985;82:5460–5464. doi: 10.1073/pnas.82.16.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer RJ, Mets LJ. Non-Mendelian mutation affecting ribulose-1,5-bisphosphate carboxylase structure and activity. Nature. 1980;285:114–115. [Google Scholar]
- Sueoka N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss K-H, Prokhorenko I, Adler K. In situ association of calvin cycle enzymes, ribulose-1,5-bisphosphate carboxylase/oxygenase activase, ferredoxin-NADP+ reductase, and nitrate reductase with thylakoid and pyrenoid membranes of Chlamydomonas reinhardtii chloroplasts as revealed by immunoelectron microscopy. Plant Physiol. 1995;107:1387–1397. doi: 10.1104/pp.107.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirova MG, Markelova AG, Semenenko VE. Identification of ribulose bisphosphate carboxylase location in the pyrenoids of unicellular algae by the cytoimmunofluorescent method. Phyziol Rast (Moscow) 1982;29:941–950. [Google Scholar]
- Weibel ER (1979) Practical methods for biological morphometry. In Stereological Methods, Vol 1. Academic Press, New York, pp 9–60