Abstract
Ovarian carcinoma has been difficult to diagnose at an early stage. Recently, it has been recognized that the measurement of blood N-ERC/mesothelin levels aids early detection in and postoperative therapeutic monitoring of patients with mesothelioma, who have been exposed to asbestos. ERC/mesothelin has also been reported to be expressed in ovarian carcinoma. We determined serum N-ERC/mesothelin levels in patients with ovarian carcinoma using an enzyme-linked immunosorbent assay (ELISA). In addition, we immunohistochemically evaluated surgically resected specimens for C-ERC/mesothelin expression. As a result, of the 32 patients with ovarian tumors (18 carcinoma, 2 borderline tumors), one patient with serous adenocarcinoma showed increased N-ERC/ mesothelin levels. Immunohistochemically, of the 20 ovarian tumor (carcinoma and borderline tumor) specimens evaluated for serum N-ERC/mesothelin, 9 (45.0%) were positive for C-ERC/mesothelin. The C-ERC/mesothelin-positive specimens were found to be serous and clear cell adenocarcinomas. If serum N-ERC/mesothelin, which is considered useful for early detection in and therapeutic monitoring of patients with mesothelioma, may also be used for ovarian carcinoma monitoring, it may be a valuable serum tumor marker for the early detection of ovarian carcinoma.
Keywords: ovarian carcinoma, serous adenocarcinoma, mesothelin, biomarker, ELISA
Introduction
ERC/mesothelin is a glycoprotein which is expressed on the surface of mesothelial cells covering body cavities such as the pleura, pericardium and peritoneum. This protein is expressed on various malignant tumors, including mesothelioma and pulmonary, pancreatic and ovarian carcinomas. Recently, it has been recognized that the measurement of blood N-ERC/mesothelin levels aids early detection in and postoperative therapeutic monitoring of patients with mesothelioma, who have been exposed to asbestos (1–3).
The early detection of mesothelioma by diagnostic imaging is considered to be difficult; however, if N-ERC/mesothelin is used as a tumor marker for mesothelioma, patient prognosis may be improved. ERC/mesothelin has also been reported to be expressed in ovarian carcinoma (4), however, similar to mesothelioma, its early detection is also considered to be difficult. Therefore, it is believed that if blood N-ERC/mesothelin levels in patients with ovarian carcinoma increase, it may be used as a tumor marker for early detection in and therapeutic monitoring of patients with ovarian carcinoma, similar to mesothelioma.
ERC/mesothelin is a homolog of the mesothelin/MPF gene, which is preferentially expressed in hereditary rat renal carcinoma, and its products are secreted in the blood (5). ERC/mesothelin, a 71-kDa precursor protein, is also expressed on the surface of human cells and is cleaved by catabolic enzymes. While the 40-kDa C-terminal fragment remains bound to the cell membrane as it contains the GPI anchor region, the 31-kDa N-terminal fragment is extracellularly secreted as a soluble protein (6). The C-terminal fragment or C-ERC/mesothelin has been reported to be used as a tumor marker for mesothelioma and ovarian carcinoma (7). Blood samples from patients with mesothelioma have been examined for the N-terminal fragment or N-ERC/mesothelin by enzyme-linked immunosorbent assay (ELISA). In the present study, we determined serum N-ERC/mesothelin levels in patients with ovarian carcinoma using sandwich ELISA. In addition, we immunohistochemically evaluated surgically resected specimens for C-ERC/mesothelin expression on the tumor cell membranes.
Subjects and methods
Human subjects
Our study for the tumor marker of ovarian carcinoma was approved by the Institutional Review Broad of Juntendo Urayasu Hospital and the hospital of Juntendo University School of Medicine, Immuno-Biological Laboratories. Patients signed an informed consent form.
Serum samples
Peripheral blood was obtained from patients who were evaluated for possible participation in this study. Serum from 32 patients with ovarian tumors were evaluated in this study. The median age of the patients with ovarian tumors was 51.2 years (range, 20–75). Serum samples from the 32 patients with ovarian tumors (18 carcinoma, 2 borderline tumors, 4 Krukenberg tumors, 3 sex-cord stromal tumors and 5 benign tumors) were evaluated in the present study. Sera from healthy volunteers were also obtained. Tissue sections were obtained from archival paraffin-embedded tumor blocks from surgically resected specimens to evaluate the ovarian tumors.
Immunohistochemistry
Tissue sections obtained from archival paraffin-embedded tumor blocks from surgically resected specimens were evaluated for C-ERC/mesothelin expression. Tissue sections 4-μm thick were prepared from formalin-fixed, paraffin-embedded specimens. The detection of ERC/mesothelin was performed using the anti-mesothelin monoclonal antibodies (mAbs) 22A31 or 5B2 (1,8). Following deparaffinization, the tissue sections were heated in 10 mM citrate buffer for antigen retrieval. Immunohistochemical detection was performed with the Ventana automated staining system. C-ERC/mesothelin positivity was identified by brownish staining of the tumor cell membranes and graded as positive if ≥30% of the accessible tumor cells were labeled.
Sandwich ELISA
Serum N-ERC/mesothelin was determined using the sandwich ELISA system. The details of sandwich ELISA have been previously described (1). The 7E7, PoAb282 and 16K16 mAbs were used as the antibody. 7E7 mAb is the common capture antibody and PoAb282 and 16K16 mAb were used as the capture antibody. The epitope of PoAb282 is in the C-terminal side [N(7-4)] and 16K16 mAb is in the N-terminal side [N(7-16)].
Results
Serum N-ERC/mesothelin levels
We measured serum N-ERC/mesothelin levels in 32 patients with ovarian tumors. Of these, 20 patients had primary ovarian carcinoma and borderline tumors; 8 were at stage I, 1 was at stage II and 11 were at either stage III or IV. Serum samples were collected prior to surgery or chemotherapy from 11 patients and during chemotherapy against carcinomatous pleural effusion or ascites following surgery from 9. The cut-off levels of N(7-4) and N(7-16) at 2.78 and 5.6 ng/ml, respectively, in the patients with mesothelioma have been previously reported (2). Of the 20 patients with ovarian tumors, one in the preoperative group showed increased N-ERC/mesothelin levels [N-ERC/mesothelin: N(7-4) = 4.76 ng/ml, N(7-16) = 18.67 ng/ml; Table I]. The median serum levels of the 4 Krukenberg tumor patients, 3 sex-cord stromal tumor patients and 5 benign tumor patients were N(7-4) = 2.12, 1.6 and 1.9 ng/ml and N(7-16) = 4.2, 1.5 and 2.0 ng/ml, respectively.
Table I.
ERC/mesothelin levels of ovarian carcinoma and ERC/mesothelin expression observed by immunohistochemical staining.a
| Histological type | N(7-4) ng/ml | N(7-16) ng/ml | CA125(0–35.0) | 5B2 | 22A31 |
|---|---|---|---|---|---|
| Clear adenocarcinoma | 1.06 | 1.26 | 124.70 | + | + |
| Endometrioid adenocarcinoma | 1.06 | 1.62 | - | − | − |
| Serous adenocarcinoma | 7.16 | 8.06 | 793.20 | + | + |
| Clear adenocarcinoma | 1.54 | 2.11 | 104.70 | − | − |
| Serous adenocarcinoma | 4.33 | 3.89 | 4.10 | − | − |
| Clear adenocarcinoma | 2.69 | 1.79 | 33.20 | − | − |
| Endometrioid adenocarcinoma | 1.91 | 2.69 | 6.40 | − | − |
| Clear adenocarcinoma | 1.00 | 0.56 | 14.40 | − | − |
| Serous adenocarcinoma | 2.66 | 1.84 | 33.00 | + | + |
| Mucinous adenocarcinoma | 1.21 | 2.76 | 22.60 | − | − |
| Endometrioid adenocarcinoma | 1.55 | 2.39 | 264.5 | − | − |
| Serous borderline tumor | 1.99 | 2.23 | 58.6 | + | + |
| Clear adenocarcinoma | 2.22 | 4.02 | 19 | + | + |
| Serous adenocarcinoma | 2.37 | 2.09 | 91.8 | + | + |
| Serous adenocarcinoma | 4.76 | 18.67 | 577.3 | + | + |
| Clear adenocarcinoma | 3.83 | 3.88 | 641.8 | + | + |
| Serous adenocarcinoma | 1.32 | 1.56 | 565.3 | + | + |
| Mucinous borderline tumor | 2.54 | 3.32 | 5.1 | − | − |
| Serous adenocarcinoma | 1.07 | 1.67 | 206.7 | − | − |
| Endometrioid adenocarcinoma | 1.31 | 2.50 | 80.2 | − | − |
One patient with serous adenocarcinoma had particularly increased ERC/mesothelin levels compared to the other patients (underlined).
ERC/mesothelin expression observed by immunohistochemical staining
Of the 20 ovarian tumor (carcinoma and borderline tumor) specimens evaluated for serum N-ERC/mesothelin, 9 (45.0%) were positive for C-ERC/mesothelin while the remaining 11 (55.0%) were negative. Of the 18 ovarian carcinoma specimens, 8 (44.4%) were positive for C-ERC/mesothelin (Fig. 1). Of the 2 borderline tumor specimens, the serous borderline tumor was positive for C-ERC/mesothelin on the luminal side of the cell membrane of the tumor while the mucinous borderline tumor was negative (Fig. 2). When cases positive for C-ERC/mesothelin were sorted by tissue type, 5 of the 7 serous adenocarcinoma cases (71.4%) and 3 of the 6 clear cell adenocarcinoma cases (50.0%) were positive, whereas 1 mucinous adenocarcinoma and 4 endometrioid adenocarcinoma cases were negative. Thus, when sorted by tissue type, ovarian carcinomas that were immunohistochemically C-ERC/mesothelin-positive were found to be serous and clear cell adenocarcinomas. The tissue specimen of one patient with serous adenocarcinoma, who had increased serum N-ERC/mesothelin levels, was also positive for C-ERC/mesothelin. Of the 10 cases that were immunohistochemically C-ERC/mesothelin-positive, 1 (10%) had increased serum N-ERC/mesothelin levels. The cell membranes of all the positive cases were stained and no difference was found in stainability between those with increased serum N-ERC/mesothelin levels and those with constant levels.
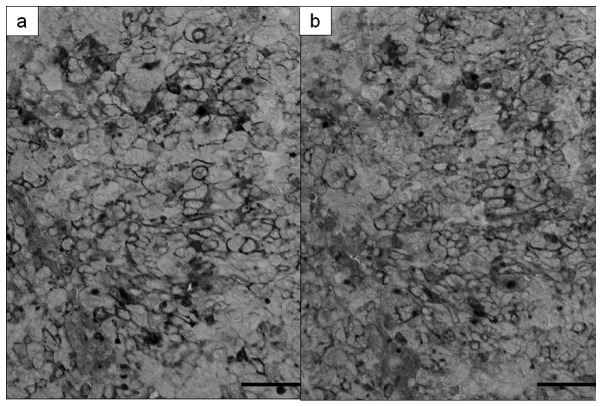
Figure 1.
A case of serous adenocarcinoma with increased serum ERC/ mesothelin levels. ERC/mesothelin was expressed on the tumor cell membranes. Detection with (a) 5B2 and (b) 22A31 anti-mesothelin monoclonal antibodies. Magnification ×200; bar, 50 μm.
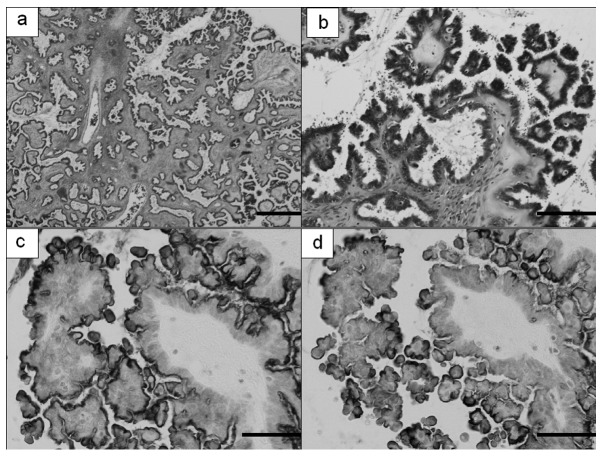
Figure 2.
Microscopic findings of serous borderline tumor. (a and b) Hematoxylin and eosin stain, (a) magnification, ×20; bar, 500 μm; (b) magnification, ×200; bar, 50 μm. Detection with (c) 5B2 (magnification, ×200; bar, 50 μm) and (d) 22A31 (magnification, ×200; bar, 50 μm) anti-mesothelin monoclonal antibodies.
A case of serous adenocarcinoma in which serum N-ERC/mesothelin levels increased
The patient with ovarian carcinoma whose serum N-ERC/mesothelin levels increased presented with a sense of abdominal fullness. An exploratory laparotomy was performed due to the presence of a pelvic tumor and a large amount of ascites. On surgery, it was found that the tumor had spread in and around both ovaries in the pelvic cavity, resulting in the adhesion of the bilateral ovaries to the surrounding organs. The bilateral ovarian tumor and greater omentum were extirpated. The resected bilateral ovaries had collapsed, and hence the nature of the tumor was unclear, but it was solid and partly cystic (Fig. 3). Histologically, atypia and mitotic figures were predominant and neoplastic cells containing large nuclei were found to be mixed with multi-nucleated giant cells. Some neoplastic cells were found to be growing solidly, forming cysts in places, while others were found to be growing in partial papillary and tubular patterns. Immunohistochemical staining revealed that the neoplastic cells were AE1/AE3-positive and vimentin/calretinin-negative. Subsequently, the patient was diagnosed with serous adenocarcinoma. Similar invasion by adenocarcinoma cells was also observed in the greater omentum. After the ovarian tumor was removed, 6 cycles of chemotherapy were administered for residual lesions in the pelvic and peritoneal cavities.
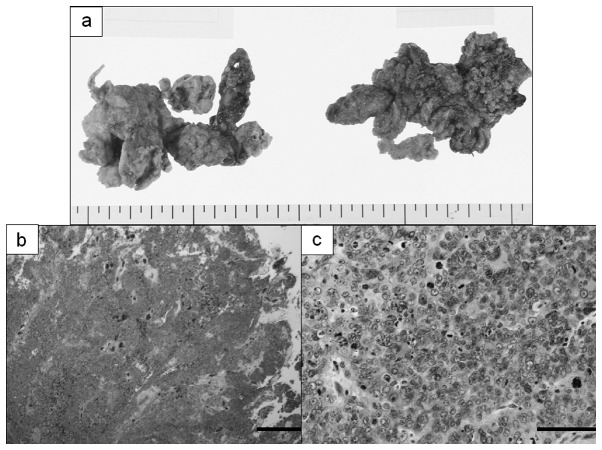
Figure 3.
Resected bilateral ovaries of a serous adenocarcinoma case in which serum N-ERC/mesothelin levels increased. (a) Macroscopically the nature of the tumor was unclear, but it was solid and partly cystic. (b) Hematoxylin and eosin stain (magnification, ×20; bar, 500 μm). (c) Atypia and mitotic figures were predominant (magnification, ×200; bar, 50 μm).
After surgery and chemotherapy, imaging demonstrated that the tumor had disappeared and serum N-ERC/mesothelin levels decreased [N(7-4) = 2.00 ng/ml, N(7-16) = 2.50 ng/ml and CA125 = 5.2 U/ml; Table II]. Subsequently, the wait and see approach was adopted and blood tests performed 6 months after the end of chemotherapy revealed increased serum N-ERC/mesothelin levels and CA125 [N(7-4) = 3.33 ng/ml, N(7-16) = 9.95 ng/ml and CA125 = 108.5 U/ml]. Furthermore, imaging revealed an enlarged tumor in the peritoneal cavity (Fig. 4). It was determined to be a recurrence and chemotherapy was initiated again. After 4 cycles of chemotherapy, serum N-ERC/mesothelin and CA125 levels increased further [N(7-4) = 5.00 ng/ml, N(7-16) = 26.80 ng/ml and CA125 = 303.7 U/ml] and imaging revealed an enlarged tumor. These results were consistent with the clinical course.
Table II.
Change in serum ERC/mesothelin levels in serous adenocarcinoma.
| Marker | Prior to surgery or chemotherapy | Following surgery or chemotherapy | 6 months after chemotherapy | Following 4 cycles of chemotherapy |
|---|---|---|---|---|
| N(7-4), ng/ml | 4.76 | 2.00 | 3.33 | 5.00 |
| N(7-16), ng/ml | 18.67 | 2.50 | 9.95 | 26.80 |
| CA125, U/ml | 577.3 | 5.2 | 108.5 | 303.7 |
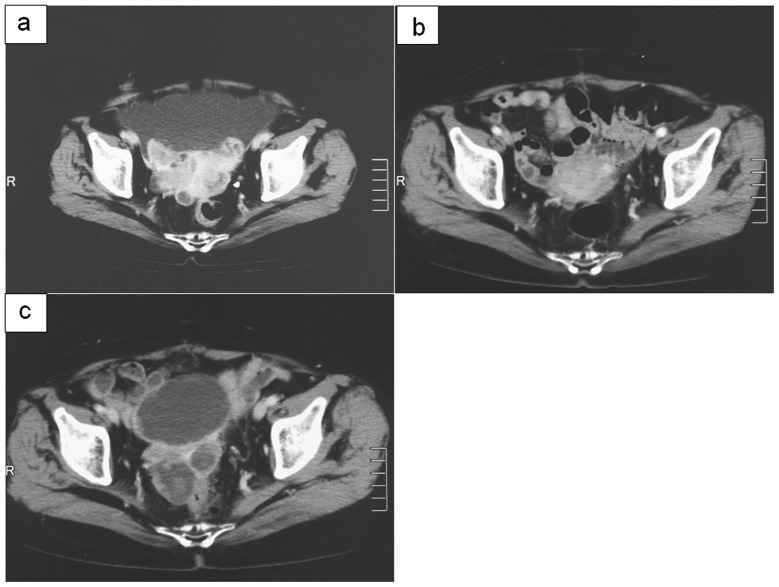
Figure 4.
Abdominal enhanced CT of serous adenocarcinoma (a) prior to surgery or chemotherapy; (b) following surgery or chemotherapy; (c) with increased serum CA125 and ERC/mesothelin levels.
Discussion
In clinical practice, measuring tumor markers is an important method to detect tumors and monitor therapeutic effects. For ovarian carcinoma, CA125, CA19-9 and CEA are the most commonly used markers. However, these tumor markers have low specificity and their levels may increase due to adenocarcinomas in other organs or ascites. ERC/mesothelin, a protein that is expressed by mesothelial cells, has been found not only in mesothelioma but also in ovarian carcinoma (4). We examined the serum of patients with ovarian carcinoma and measured N-ERC/mesothelin expression in neoplastic cells by ELISA. Of the 32 patients with ovarian tumor, one showed increased serum ERC/mesothelin levels. In a study conducted by Hassan et al, an increase in serum C-ERC/mesothelin levels was observed in 67% of patients with ovarian carcinoma (7), markedly higher than the percentage obtained in our study. We determined serum N-ERC/mesothelin levels using ELISA. Further studies are required to investigate the difference between these positive rates.
In the present study, the serous adenocarcinoma which was associated with increased serum N-ERC/mesothelin levels severely adhered to the periovular organs and its shape was ill-defined. Histologically, the tumor had spread from the ovaries and the neoplastic cells grew solidly in a papillary pattern. It is often necessary to differentiate advanced ovarian carcinoma from Fallopian tube and peritoneal carcinomas. Although the carcinoma in question, with macroscopically ill-defined ovary shapes, had to be differentiated from the other carcinomas, the tumor was most predominant in the ovaries and neoplastic cells were present in the ovarian parenchyma. Immunohistochemically, the tumor was AE1/AE3-positive and vimentin/calretinin-negative. Cytologically, no definite difference from other serous adenocarcinomas was observed. Immunohistochemical staining for C-ERC/mesothelin in various ovarian tumors resulted in the neoplastic cell membranes being stained in every C-ERC/mesothelin-positive case. Every ovarian carcinoma with a positive result was either serous or clear cell adenocarcinoma. Serous adenocarcinoma was once considered to be a de novo carcinoma developing directly from the surface epithelium or inclusion cysts. However, the disease is currently considered to be of 2 types, one being adenocarcinoma with low-grade atypia in which a serous borderline tumor transforms as a result of malignant progression, and the other being adenocarcinoma with high-grade atypia emerging from de novo carcinogenesis. In addition, it has been suggested that certain cases belonging to the latter type involve adenocarcinoma developing in the distal Fallopian tube and spreading to the ovaries and pelvic cavity (9). As ERC/mesothelin is a protein which is expressed on mesothelial cells, it may be surmised that C-ERC/mesothelin emerging directly from the surface epithelia of the ovaries has its origin in serous adenocarcinoma. We found that C-ERC/mesothelin in serous borderline tumors was expressed not circumferentially, but on the luminal side of the neoplastic cell membranes. The mucinous tumor, despite being another variety of borderline tumor, was negative for C-ERC/mesothelin.
It has been suggested that ovarian endometriosis is the origin of clear cell adenocarcinomas since one is frequently concomitant with the other (10). However, it should be noted that we found C-ERC/mesothelin expressed in clear cell adenocarcinoma, but not in endometrioid adenocarcinoma, while ovarian endometriosis is suspected to be the origin of both. As we used a cut-off value of C-ERC/mesothelin expression of at least 30% of tumor cells to define tumor ERC/mesothelin positivity, some of the ERC/mesothelin-negative tumors could have weak or partial ERC/mesothelin expression (7). Studies have been conducted on serum ERC/mesothelin levels in patients with ovarian carcinoma (4,7,11), but none have sorted ovarian carcinomas by tissue type. Our finding that C-ERC/mesothelin was expressed in 2 different tissue types of carcinoma, the causes of which also appeared to differ, is valuable in clarifying the histogenesis of ovarian carcinoma. Further investigation into the various tissue types of ovarian carcinoma is needed to understand the etiological differences between the types and their correlation with ERC/mesothelin expression.
We found no clear difference in stainability between serous adenocarcinoma cases with increased serum N-ERC/mesothelin levels and those with constant levels. The difference in serum N-ERC/mesothelin levels, despite their identical C-ERC/mesothelin expression on the cell surfaces, may be attributed to the size of the proteins degraded by protease in serum or the presence and size of the lesions of the peritoneal cavity. The 8 other ovarian carcinomas had extraovarian lesions but did not increase the serum N-ERC/mesothelin levels. We measured the N(7-4) and N(7-16) levels for N-ERC/mesothelin. In serous adenocarcinoma N(7-16) levels increased more markedly than N(7-4) levels. We used a cut-off value established by serum N-ERC/mesothelin levels in healthy individuals and patients with mesothelioma, but the validity of using the same value for patients with ovarian carcinoma is not certain. Further studies with larger sample sizes are required.
It has been reported that blood N-ERC/mesothelin levels are useful for the early detection of mesothelioma and postoperative monitoring for its recurrence (3).
In a case of ovarian serous adenocarcinoma, we found increased serum N-ERC/mesothelin levels and changes corresponding to therapeutic effects. If serum N-ERC/mesothelin, which is considered useful for early detection in and therapeutic monitoring of patients with mesothelioma, may also be used for ovarian carcinoma monitoring, it may be a valuable serum tumor marker for the early detection of ovarian carcinoma.
Acknowledgments
We thank Masaaki Abe, Naoko Aoki and Tetsuya Okazaki for assistance with the management of this study.
References
- 1.Shiomi K, Miyamoto H, Segawa T, et al. Novel ELISA system for detection of N-ERC/mesothelin in the sera of mesothelioma patients. Cancer Sci. 2006;97:928–932. doi: 10.1111/j.1349-7006.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiomi K, Hagiwara Y, Sonoue K, et al. Sensitive and specific new enzyme-linked immunosorbent assay for N-ERC/mesothelin increases its potential as a useful serum tumor marker for mesothelioma. Clin Cancer Res. 2008;14:1431–1437. doi: 10.1158/1078-0432.CCR-07-1613. [DOI] [PubMed] [Google Scholar]
- 3.Imashimizu K, Shiomi K, Maeda M, et al. Feasibility of large-scale screening using N-ERC/mesothelin levels in the blood for the early diagnosis of malignant mesothelioma. Exp Ther Med. 2011;2:409–411. doi: 10.3892/etm.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho M, Hassan R, Zhang J, Wang Q, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 5.Hino O, Kobayashi E, Nishizawa M, et al. Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol. 1995;121:602–605. doi: 10.1007/BF01197777. [DOI] [PubMed] [Google Scholar]
- 6.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 7.Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa K, Segawa T, Hagiwara Y, Maeda M, Abe M, Hino O. Establishment of novel mAb to human ERC/mesothelin useful for study and diagnosis of ERC/mesothelin-expressing cancers. Pathol Int. 2009;59:161–166. doi: 10.1111/j.1440-1827.2009.02344.x. [DOI] [PubMed] [Google Scholar]
- 9.Kurman RJ, Shin IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scully RE, Barlow JF. “Mesonephroma” of ovary. Tumor of Müllerian nature related to the endometrioid carcinoma. Cancer. 1967;20:1405–1417. doi: 10.1002/1097-0142(196709)20:9<1405::aid-cncr2820200907>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom I, Raycraft J, Kanan S, Sardesai NY, Verch T, Yang Y, Hellstrom KE. Mesothelin variant 1 is released from tumor cells as a diagnostic marker. Cancer Epidemiol Biomarker Prev. 2006;15:1014–1020. doi: 10.1158/1055-9965.EPI-05-0334. [DOI] [PubMed] [Google Scholar]