Abstract
Protein tyrosine dimerization and nitration by biologically-relevant oxidants usually depend on the intermediate formation of tyrosyl radical (•Tyr). In the case of tyrosine oxidation in proteins associated to hydrophobic biocompartments, the participation of unsaturated fatty acids in the process must be considered since they typically constitute preferential targets for the initial oxidative attack. Thus, we postulate that lipid-derived radicals mediate the one-electron oxidation of tyrosine to •Tyr, which can afterwards react with another •Tyr or with nitrogen dioxide (•NO2) to yield 3,3´-dityrosine or 3-nitrotyrosine within the hydrophobic structure, respectively. To test this hypothesis, we have studied tyrosine oxidation in saturated and unsaturated fatty acid-containing phosphatidylcholine (PC) liposomes with an incorporated hydrophobic tyrosine analog BTBE (N-t- BOC L-tyrosine tert-butyl ester) and its relationship with lipid peroxidation promoted by three oxidations systems, namely peroxynitrite, hemin and 2,2´-azobis (2-amidinopropane) hydrochloride (ABAP). In all cases, significant tyrosine (BTBE) oxidation was seen in unsaturated PC liposomes, in a way that was largely decreased at low oxygen concentrations. Tyrosine oxidation levels paralleled those of lipid peroxidation (i.e. malondialdehyde and lipid hydroperoxides) and lipid-derived radicals and BTBE phenoxyl radicals were simultaneously detected by ESR-spin trapping, supporting an association between the two processes. Indeed, α-tocopherol, a known reactant with lipid peroxyl radicals (LOO•), inhibited both tyrosine oxidation and lipid peroxidation induced by all three oxidation systems. Moreover, oxidant-stimulated liposomal oxygen consumption was dose-dependently inhibited by BTBE but not by its phenylalanine analog, BPBE (N-t-BOC L-phenylalaline tert-butyl ester), providing a direct evidence for the reaction between LOO• and the phenol moiety in BTBE, with an estimated second order rate constant of 4.8 × 103 M−1s−1. In summary, the data presented herein demonstrate that LOO• mediate tyrosine oxidation processes in hydrophobic biocompartments and provide a new mechanistic insight to understand protein oxidation and nitration in lipoproteins and biomembranes.
Introduction
Tyrosine dimerization and nitration to 3,3´-dityrosine and 3-nitrotyrosine (3-nitro-Tyr), respectively, represent biologically-relevant oxidative post-translational modifications in proteins generated by the reactions with reactive oxygen and nitrogen intermediates both in vitro and in vivo. These tyrosine oxidation processes depend on the intermediate formation of tyrosyl radical (•Tyr), a transient species formed by the one-electron oxidation of tyrosine. For instance, 3,3´-dityrosine formation results from the termination reaction between two •Tyr radicals with the formation of a new C-C bond; 3,3´-dityrosine participates in protein cross-linking (1) and also serves as a marker for oxidatively damaged proteins. Indeed, elevated levels of 3,3´-dityrosine can be found as a product of aging, inflammation, exposure to UV and γ-radiation and other oxidative stress conditions (2–4). Tyrosine nitration in biological systems is also a free radical process (5) produced by nitric oxide (•NO)-derived oxidants such as peroxynitrite1 and nitrogen dioxide radical (•NO2) (5); typically, the final step in nitration involves the diffusion-controlled reaction of •Tyr with •NO2. The product of this reaction, 3-nitro-Tyr, is a footprint of nitro-oxidative damage in vivo, being revealed as a strong biomarker and predictor of disease progression in conditions such as inflammation, cardiovascular disease and neurodegeneration (6–9). Protein tyrosine nitration could result in dramatic changes in protein structure and can affect biological activity either by a loss (e.g. Mn-SOD (10–12) or by a gain of function (e.g. nerve growth factor (13) and cytochrome c (14,15); recently reviewed in (16)). As 3,3´-dityrosine and 3-nitro-Tyr formation require the intermediacy of •Tyr, both tyrosine oxidation products can be formed simultaneously in oxidizing environments where •NO and reactive oxygen intermediate radicals (e.g. superoxide radical, O2•− hydrogen peroxide, H2O2) coexist. Specifically, this chemistry can be performed by peroxynitrite, a powerful oxidant and cytotoxic species formed in vivo by the diffusion-controlled reaction between •NO and O2•− (5, 17, 19).
Peroxynitrite does not directly react with tyrosine (20) but promotes tyrosine dimerization and nitration due to the reactions of peroxynitrite-derived species such hydroxyl radical (•OH), carbonate radical (CO3•−), •NO2 or high oxidation state of redox-active metal centers (Me (n+1)+ =O, where Me is Fe, Cu or Mn) (recently reviewed in (21)) that can oxidize tyrosine to yield •Tyr; which then combines with another •Tyr or •NO2 to yield 3,3´-dityrosine or 3-nitro-Tyr, respectively (22). For free tyrosine or tyrosine analogs in aqueous solution the 3,3´-dityrosine/3-nitro-Tyr ratio after peroxynitrite exposure typically range in values of 1/20–1/25; however, this ratio may change if peroxynitrite is added as a single bolus or by slow infusion and depends on the tyrosine and peroxynitrite concentration and may become smaller in proteins, reflecting the relative ease of the nitration reaction respect to the dimerization reaction, due to diffusional and steric limitations. In addition, other tyrosine oxidation products from peroxynitrite can be formed, including the hydroxylated derivative 3,4-dihydroxyphenylalanine (DOPA) (23). Of note, 3-nitro-Tyr was initially considered a specific marker of peroxynitrite; however, there is now agreement that tyrosine nitration can also occur biologically by peroxynitrite-independent mechanisms which include hydrogen peroxide (H2O2)-dependent nitrite oxidation catalyzed by heme (24) and hemoperoxidases (e.g. myeloperoxidase (25), eosinophil peroxidase (26), reviewed in (5)), pathways that also lead to the formation of 3,3´-dityrosine.
Protein tyrosine dimerization and nitration sites and yields depend on the protein structure, oxidation mechanism and the environment where the protein tyrosine residues are located (22, 27). In this last regard, most of the mechanistic studies of oxidation for free and protein tyrosines have been performed in aqueous solution (10, 11, 28). However, many protein tyrosine residues shown to be dimerized and nitrated either in vitro and in vivo are associated to non–polar compartments, such as red cell membrane proteins (29–31), mitochondrial membrane proteins (32–34), sarcoplasmic reticulum Ca2+ ATPase, microsomal glutathione S-transferase (35), apolipoproteins A and B (3, 25, 36). Within these proteins, in some cases oxidized tyrosines have been shown in cytosolic or extracellular domains (e.g. Tyr 192, apoA-I (36)), but in other cases in domains closely related to lipids (e.g. Tyr 39 in α-synuclein (37)). Also, reversible interactions of phospholipids with proteins can modulate tyrosine oxidation yields and sites, as observed for the cases of apoA-I (36) α-synuclein (38) and matrix metalloproteinase MMP-13 during wound repair (39).
Physico-chemical factors controlling tyrosine oxidation in hydrophobic biocompartments such as biomembranes and lipoproteins differ from those in aqueous solution. For example, hydrophobic phases contain a high concentration of unsaturated fatty acids and exclude key antioxidant molecules that are potent inhibitors of tyrosine oxidation in aqueous phases such as glutathione (22). In addition, there is a differential distribution of oxidizing species in the lipid vs aqueous phase: while •NO2 can readily diffuse, concentrate and react in the hydrophobic compartment (40), CO3•− and hemeproteins have limited action due to the restricted permeation and steric restrictions, respectively. Another important aspect to consider, is the restricted lateral diffusion of •Tyr in the organized structure of membranes which limits the dimerization process and results in smaller 3,3´-dityrosine/3-nitro-Tyr ratios than those observed in aqueous phases (~1/100 –1/400) (41).
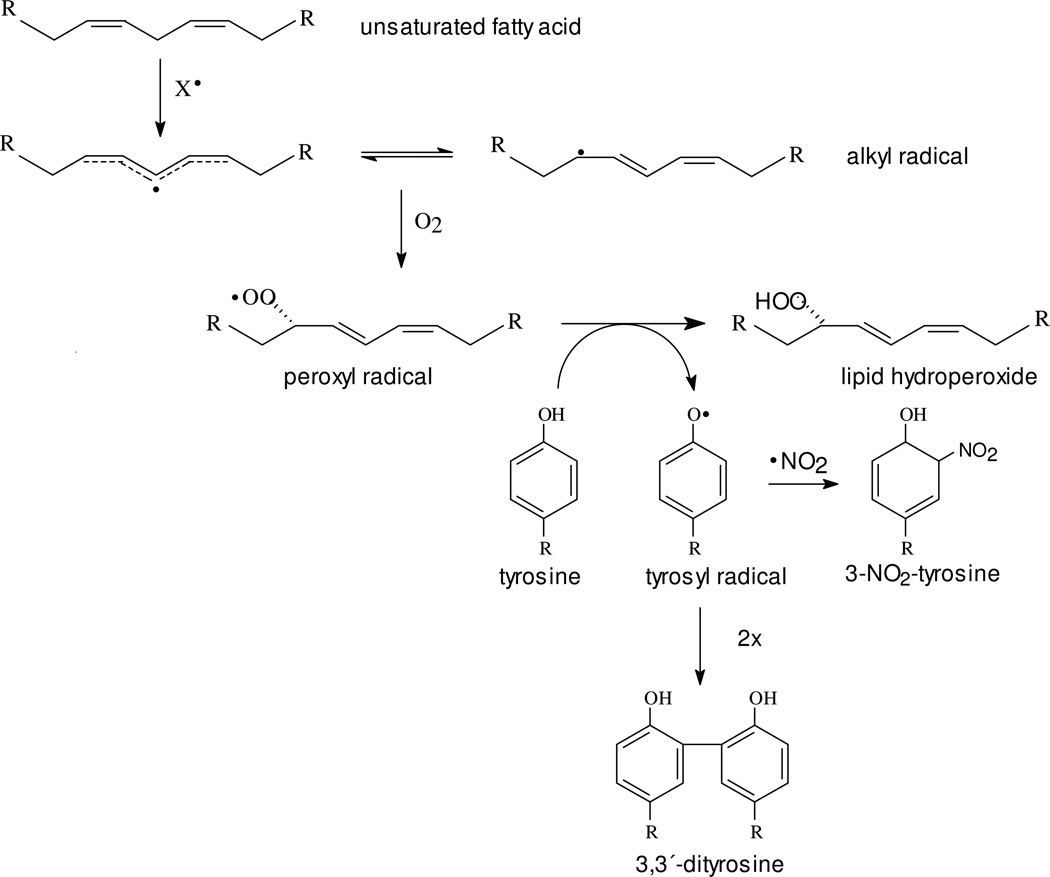
The need to further investigate tyrosine oxidation mechanisms in hydrophobic environments has led to the development of probes such as hydrophobic tyrosine analogs (41, 42) and tyrosine-containing transmembrane peptides (42). In this regard, N-t-BOC L-tyrosine tert butyl ester (BTBE) is a stable tyrosine analog that we have previously used to study peroxynitrite and MPO-mediated tyrosine oxidation in lipid phases including liposomes and biomembranes (41–43). BTBE can be efficiently incorporated (>98%) into phosphatidylcholine (PC) liposomes and the formation of BTBE-derived phenoxyl radicals and oxidation products (i.e. 3-nitro-BTBE, 3,3´-di-BTBE and 3-hydroxy-BTBE) evaluated (41–43). Previous observations (41, 43) and kinetic considerations prompted us to investigate in depth how the unsaturated fatty acids present in high concentration in membranes and other hydrophobic compartments may influence peroxynitrite-mediated tyrosine oxidation mechanisms and yields. Indeed, peroxynitrite is a known inductor of lipid peroxidation via free radical reactions (44) initiated after the homolysis of ONOOH to •OH and •NO2 (21); unsaturated fatty acids readily react with •OH (k = 1 × 1010 M−1s−1) (45) and to a lesser extent with •NO2 (k =2 ×105 M−1s−1 for linoleate at pH 9.4) (46, 47), both of which promote hydrogen abstraction from a bis-allylic hydrogen to initiate a chain reaction. Therefore, at a first glance an increased level of unsaturation in phosphatidylcholine (PC) liposomes could result on a decrease of tyrosine oxidation yields. However, peroxynitrite-dependent BTBE nitration and dimerization yields were still high in PC liposomes containing a large polyunsaturated fatty acid content (41). These data indicate that a simple competition kinetic model does not apply and suggest that lipid-derived radicals mediate tyrosine oxidation within the membrane. Indeed, lipid peroxidation is an oxygen-dependent process that results in the formation of peroxyl (LOO•) (Eqs. 1–3), and secondarily, alkoxyl radicals (LO•) (48–50).
| (1) |
| (2) |
| (3) |
These lipid-derived radicals are reactive species that can in turn oxidize biological targets (RH) including protein side chains (51). According to the one-electron redox potential of alkoxyl (E° LO•/LOH = 1.76 V) and peroxyl radicals (E° LOO•/LOOH = 1.02 V), is thermodynamically possible for both species to oxidize tyrosine to the phenoxyl radical (E° Tyr• / Tyr H = 0.88 V) while the alkyl radicals (E° L• / LH = 0.6 V) could not be able to perform that oxidation. Herein, we postulate that LOO• are capable of oxidizing tyrosine to •Tyr, therefore fueling the tyrosine oxidation pathway in hydrophobic biocompartments. To test this hypothesis, we have studied BTBE dimerization and nitration in PC liposomes of different unsaturation degree promoted by three different oxidation systems, namely peroxynitrite, hemin and the peroxyl radical donor 2,2´-azobis (2-amidinopropane) hydrochloride (ABAP) and their relationship with the lipid peroxidation process.
Experimental Procedures
Chemicals
Diethylentriaminepentaacetic acid (dtpa), manganese dioxide, sodium bicarbonate, potassium phosphate, L-tyrosine, 3-nitrotyrosine, α-tochopherol, 2-metyl-nitroso-propane (MNP), 1,1,3,3 tetra methoxypropane and hemin were purchased from SIGMA. N-t-BOC L-tyrosine tert butyl ester (BTBE), 3-nitro- N-t-BOC L-tyrosine tert butyl ester (3-nitro-BTBE) and 3,3´ di-N-t-BOC L-tyrosine tert butyl ester (3,3´-di-BTBE) were prepared and handled as previously (42). N-t-BOC L-phenylalanine tert butyl ester (BPBE) was synthesized for the first time using an identical procedure as the one previously described for BTBE but starting from commercially available (SIGMA) L-phenylalanine-t-butylester (42). Stock BTBE solutions (1 M) were prepared in methanol immediately before use. 3,3′-dityrosine was synthesized by incubating 0.5 mM L-tyrosine, 0.2 mg/ml (4.5 µM) horseradish peroxidase, and 500 µM H2O2 in 50 mM phosphate buffer (pH 7.4) for 20 min at 25°C. The resulting mixture was centrifuged through a Centricon tube (molecular weight cutoff 5,000 Da) to remove the enzyme and the concentration of 3,3´-dityrosine obtained determined spectrophotometrically at 315 nm using an extinction coefficient of 5,700 M−1 cm−1 (pH 7.4) and 8,380 M−1 cm−1 (pH 9.9) (52). 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLPC), egg yolk and soybean phosphatidylcholine (EYPC and SBPC) were from Avanti Polar Lipids. Organic solvents for synthesis of standards and chromatography were from Baker or Mallinckrodt. All other compounds were reagent grade.
Stock hemin solution was freshly prepared in 0.1 N NaOH and kept in the dark at 4° C until use. ABAP (2,2´-Azobis (2-amidinopropane) hydrochloride) was purchased from Wako Chemicals USA. A freshly stock solution (100 mM) was prepared in water and incubations were performed at 37°C for the indicated times.
Argon and nitrogen gas were purchased in AGA Chemical Co. (Uruguay).
All solutions were prepared with highly pure deionized nanopure water to minimize trace metal contamination.
Peroxynitrite synthesis and quantitation
Peroxynitrite was synthesized in a quenched-flow reactor from sodium nitrite (NaNO2) and hydrogen peroxide (H2O2) under acidic conditions as described previously (53). The H2O2 remaining from the synthesis was eliminated by treating the stock solutions of peroxynitrite with granular manganese dioxide, and the alkaline peroxynitrite stock solution was kept at −20°C until use. Peroxynitrite concentrations were determined spectrophotometrically at 302 nm (ε= 1670 M−1 cm−1) (44, 54). The nitrite concentration in the preparations was typically lower than 20% respect to peroxynitrite. Control of nitrite levels revealed to be critical for obtaining reproducible data as, if present in excess, it can react with •OH and other oxidants and yield •NO2 (41). In control experiments, peroxynitrite was allowed to decompose to nitrate in 100 mM phosphate buffer, pH 7.4 before use, i.e., “reverse order addition” of peroxynitrite.
BTBE incorporation into liposomes and oxidizing systems
BTBE incorporation into liposomes was carried out as in (41, 42) with minor modifications. Briefly, a methanolic solution of BTBE (0.35 mM) was added to 35 mM PC lipids dissolved in chloroform. Under these conditions more than 98% BTBE was incorporated (42). The mixture was then dried under a stream of nitrogen gas. Previous studies from our group (41, 42) already indicated that BTBE incorporation yields and formation of oxidation products from peroxynitrite were comparable in unilamellar and multilamellar liposomes and therefore not dependent on the membrane morphology. Therefore, due to the more simple preparation procedure, reported experiments were carried out mainly with multilamellar liposomes. Multilamellar liposomes were formed by thoroughly mixing the dried lipid with 100 mM sodium phosphate buffer (pH 7.4) plus 0.1 mM dtpa. For experiments with α-tocopherol-containing liposomes, α-tocopherol (in ethanol) was added at the desired concentration to the lipid solution in chloroform with or without BTBE and liposomes prepared thereafter as indicated above. Liposomes (30 mM PC and 0.3 mM BTBE) were exposed to peroxynitrite, hemin or ABAP under different conditions throughout the work. BTBE and BTBE-derived products (e.g. 3-nitro-BTBE and 3,3´-di-BTBE) were extracted with chloroform, methanol and 5 M NaCl as reported previously (1:2:4:0.4, sample : methanol : chloroform : NaCl v/v with recovery efficiencies for all compounds > 95% (42)). Samples were then dried and stored at −20°C. Immediately before HPLC separation, samples were resuspended in 100 µl of a mixture containing 85% methanol and 15% KPi (15 mM) pH 3. Experiments with liposomes were performed at 25°C, a temperature above the transition phase temperatures of the different liposomes and at 37°C when ABAP was used as an oxidant.
Oxidation systems
BTBE-containing PC liposomes were oxidized either by the addition of peroxynitrite, hemin or the organic peroxyl radical donor ABAP. Peroxynitrite was added as a single bolus under vigorous vortexing (t1/2 2.5 s at 25°C (55)) or by slow infusion using a motor-driven syringe system (Kd Scientific) under continuous stirring. Due to the addition of alkaline peroxynitrite solutions, the final pH was also checked at the end of the incubation to make sure that there were no significant variations (<0.1 pH units). Hemin was added directly to the PC liposomes; EYPC and SBPC liposomes always contain a basal level of pre-formed lipid hydroperoxides that serve as the redox substrate for hemin. Alternatively, tert-butyl hydroperoxide was used as hemin reactant in DLPC liposomes. Finally, in the case of ABAP, samples were incubated at 37°C during 2–3 hs to achieve a final ABAP concentration of 0–40 mM. ABAP-dependent oxygen consumption was measured using a high resolution oxymeter (Oroboros 2K) yielding a flux of 0.3 µM/min of peroxyl radicals.
Experiments under low oxygen tension (ca. 5 µM) were carried out, by extensively purging samples under argon for 30 minutes.
Lipid peroxidation analysis
Malondialdehyde (MDA), a byproduct of lipid peroxidation, was measured as a thiobarbituric acid reactive substance at 532 nm (ε= 150,000 M−1 cm−1), as previously described (44). Calibration curves and assessment of malondialdehyde content were performed with known amounts of MDA obtained from the acid hydrolysis of 1,1,3,3 tetramethoxypropane in 20 % acetic acid pH 3.5. To prevent further peroxidation of lipids during assay procedures, 0.05% (w/v) of butylated hydroxytoluene (BHT) was added to the TBARS reagent. Lipid hydroperoxide formation was evaluated by the FOX assay (56). Briefly, 50 µl of the liposome sample was added to 950 µl of FOX reagent consisting in 100 mM xylenol orange, 250 mM Fe2+ (ferrous ammonium sulfate), 25 mM H2SO4, and 4 mM BHT in 90% (v/v) methanol. Reaction mixtures were incubated 1h at room temperature and absorbance was measured at 560 nm. Concentration of lipid hydroperoxides was estimated with the apparent extinction coefficient of 43,000 M −1 cm −1 (56).
Oxygen consumption during lipid peroxidation processes was measured by high resolution oxymetry using an Oxygraph 2K (Oroboros Instruments, Austria).
HPLC analysis
BTBE, 3-nitro-BTBE and 3,3´-di-BTBE were separated on a Agilent 1200 system equipped with UV-Vis and fluorescence detectors by reverse-phase HPLC using a Agilent Eclipse XDB-C18 5 µm column (150 mm length, 4.6 mm ID). Mobile phase A consisted in 15 mM phosphate buffer pH 3 and mobile phase B consisted in methanol. Chromatographic conditions were: flow 1ml / min; 75 % mobile phase B for 25 minutes, followed by a linear increase to 100% mobile phase B for 10 minutes. UV-Vis settings were for BTBE (280 nm, ε=1200 M−1cm−1) and for 3-nitro-BTBE (360 nm, ε=1500 M−1cm−1). 3,3´-di-BTBE was detected fluorimetrically at λex = 294 nm, λem = 401 nm. Authentic 3-nitro-BTBE and 3,3´-di-BTBE were used as standards.
3-Nitrotyrosine and 3,3´-dityrosine were separated by reverse-phase HPLC, using a Partisil ODS-3 10 µm (250 mm length, 4.6 mm ID) C18 column as previously reported with minor modifications (41). Briefly, separation of tyrosine oxidation products was performed by isocratic RP-HPLC. Chromatographic conditions were: flow 1ml / min; 97% mobile phase A (KPi 15mM pH 3) and 3% mobile phase B (methanol) for 30 minutes. 3-Nitrotyrosine was measured by UV detection (280 nm and 360 nm) and 3,3´-dityrosine was measured fluorometrically (λex = 280 nm and λem = 400 nm). Authentic 3,3´-dityrosine and 3-nitro-Tyr were used as standards.
Artifactual BTBE or tyrosine nitration during the chromatographic separation procedure due to nitrite-dependent nitration at acidic pH were ruled out by appropriate controls using pre-decomposed peroxynitrite.
ESR-Spin Trapping measurements
ESR spectra were recorded at room temperature on a Bruker EMX spectrometer operating at 9.8 GHz. Typical spectrometer parameters were as follow: sweep width, 100 G; center field, 3505 G; time constant, 20.48 ms; scan time, 42 s; modulation amplitude, 1.0 G; modulation frequency, 100 kHz; receiver gain, 1×106; microwave power, 20 mW. Samples were subsequently transferred to a 50 µL capillary tube for ESR measurements.
MNP (20 mM) was used as spin trap; its photolysis may occur during the experiments and leads to the formation of a three line signal corresponding to the di-tert-butyl nitroxide radical, but it has a different aN of 17.1 G. In order to minimize this, all liposomes preparations and solutions were covered with aluminum foil and spectra were recorded in the dark and therefore the signal due to photolysis was not seen under our experimental conditions.
General experimental conditions
Experiments were typically carried out in the presence of BTBE (0.3 mM) in PC liposomes (30 mM) in 100 mM sodium phosphate plus 0.1 mM dtpa, (pH 7.4) and 25°C unless otherwise stated.
Data analysis
All experiments reported herein were repeated a minimum of three times. Results are expressed as mean values with the corresponding standard deviations. Graphics and data analysis were performed using Origin 8.0.
RESULTS
Participation of lipid-derived radicals on BTBE oxidation
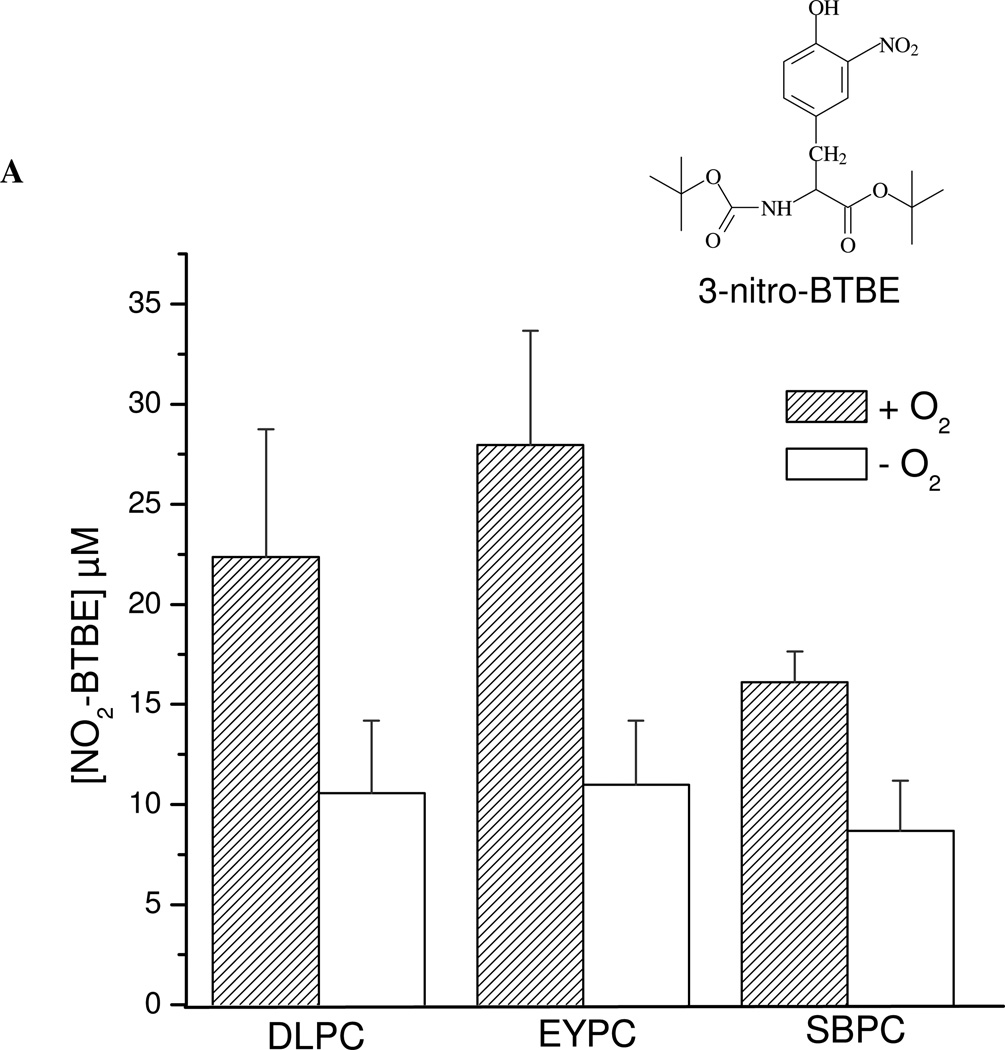
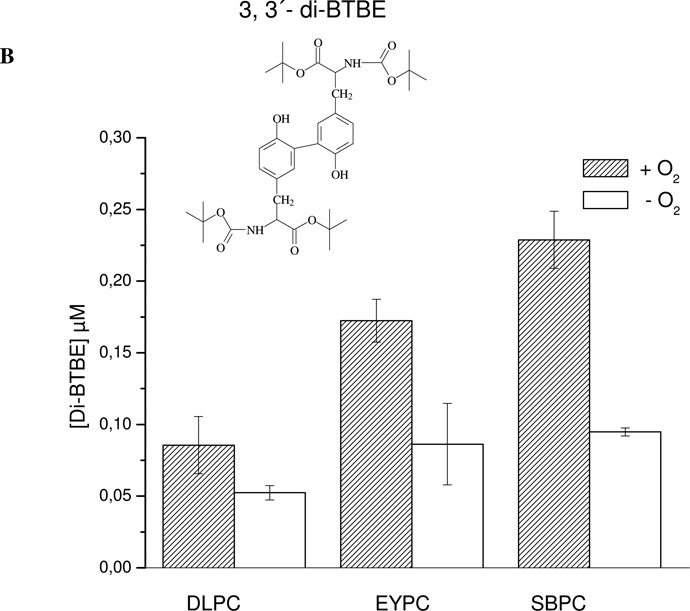
When BTBE-containing DLPC liposomes were treated with peroxynitrite (1 mM), 3-nitro-BTBE and 3,3´-di-BTBE were formed in yields of up to 2.5% and 0.01 % respect to initial peroxynitrite concentration respectively (Fig. 1A and B), in agreement with previous results (41, 57). Significant levels of both BTBE oxidation products were also formed in EYPC (Fig. 1) and SBPC, which contain a significant proportion of unsaturated fatty acids, ~ 24 and 57 % respectively. Thus, in spite of the fact that for EYPC and SBPC (30 mM) unsaturated fatty acids correspond to ~ 15 and 34 mM, respectively, a much larger concentration than that of BTBE (0.3 mM), the BTBE oxidation process was still operative.
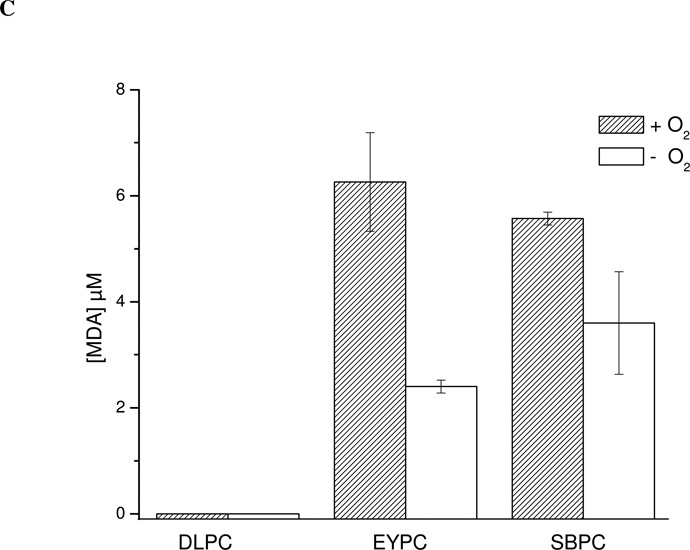
Figure 1. Effect of oxygen on peroxynitrite-dependent BTBE oxidation.
BTBE (0.3 mM) was incorporated to the different liposomes, DLPC, EYPC and SBPC (30 mM) and exposed to peroxynitrite (1 mM) in the presence of oxygen (200 µM) or under low oxygen tensions (~ 5 µM). Samples were analyzed for (A) 3-nitro-BTBE, (B) 3,3´-di-BTBE and (C) MDA content. Basal MDA levels in EYPC, SBPC and DLPC were 4.88 ±1,09; 4.97 ±0.72 and 0, respectively. Structures of 3-NO2- N-t-BOC L-tyrosine tert butyl ester (3-nitro-BTBE) and 3,3´-di N-t-BOC L-tyrosine tert butyl ester (3,3´-di-BTBE) are indicated in the corresponding panels.
To assess the participation of lipid-derived radicals in BTBE oxidation, experiments were also performed under low oxygen tensions (Fig. 1) which should result in an inhibition of LOO• formation (Eq. 2) in both saturated2 (58) and unsaturated fatty acids. Indeed, under these conditions, BTBE nitration and dimerization yields were substantially decreased either in saturated and unsaturated fatty acid-containing liposomes (Fig. 1A and B). In parallel, we evaluated lipid peroxidation in the samples by quantitating MDA, a well-known breakdown product of peroxidized lipids. Peroxynitrite caused MDA formation in BTBE-containing EYPC and SBPC liposomes (Fig. 1C), in agreement with our previous observations (41); importantly, MDA levels were reduced under low oxygen concentration, revealing the inhibition of the lipid peroxidation process. As expected, no MDA was detected in DLPC samples. Thus, in unsaturated fatty acid-containing liposomes (EYPC and SBPC), BTBE oxidation and lipid peroxidation occurred simultaneously and were both inhibited at low oxygen levels. Lipid hydroperoxide levels were significantly higher (over one order of magnitude) than those obtained for BTBE oxidation products, in agreement with the idea that lipid peroxidation is the main process within the membrane. On the other hand, when tyrosine (0.3 mM) was exposed to peroxynitrite (1 mM) in 100 mM phosphate buffer (pH 7.3) 0.1 mM DTPA, oxygen did not influence tyrosine nitration and dimerization yields (~ 6 % and 0.25 % ) yields respect peroxynitrite, respectively) in aqueous phase (not shown). Overall, the data point to the formation of LOO• as key intermediates in the BTBE oxidation process.
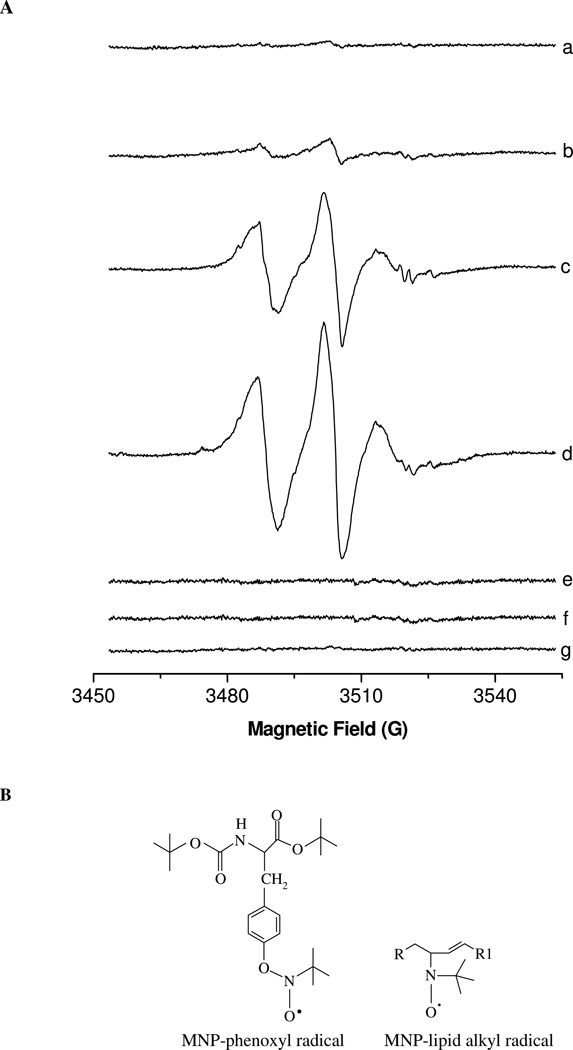
Electron spin resonance (ESR)-spin trapping was used to detect the lipid-derived and BTBE phenoxyl radicals formed during peroxynitrite exposures using MNP as described previously (41, 59). In DPLC liposomes no ESR signal was obtained in the absence of BTBE when exposed to peroxynitrite (Fig. 2, line a). On the other hand, BTBE-containing DLPC liposomes treated with peroxynitrite resulted in an anisotropic three line signal, confirming the formation of a partially immobilized phenoxyl radical in the interior of the membrane (Fig. 2, line b) and in agreement with our previous work (41, 60). When peroxynitrite-treated BTBE containing DLPC liposomes were dissolved in ethanol (60), a sharper and clear three line signal was obtained which, despite of the low signal-to-noise ratio, allowed the determination of a hyperfine constant of 13.8 G; this is consistent with a MNP adduct with the one-electron oxidation of BTBE (61). In EYPC liposomes, peroxynitrite caused the formation of a MNP adduct in the absence of BTBE, compatible with the formation of MNP-lipid alkyl (carbon-centered adduct) radical adducts (Fig.2 line c) with an estimated hyperfine splitting constant aN~15 G (59). When BTBE was incorporated to liposomes, the signal was even larger (Fig. 2 line d), supporting the coexistence of lipid-derived and BTBE phenoxyl radicals, and confirming the temporal association between the lipid peroxidation and BTBE oxidation processes. No ESR spectrum was obtained in DLPC or EYPC liposomes when treated with decomposed peroxynitrite (Fig. 2 lines e and f) or if MNP was added after peroxynitrite (Fig. 2 line g) or if peroxynitrite was added to MNP only (without liposomes, not shown).
Figure 2. Electron spin resonance- spin trapping of BTBE phenoxyl and lipid peroxyl radical.
(A) Reaction mixtures consisting on BTBE (2.5 mM) incorporated into 45 mM DLPC liposomes in phosphate buffer 100 mM (pH 7.4) containing dtpa (0.1 mM) were treated with 20 mM 2-methyl-2-nitrosopropane (MNP) spin trap and rapidly mixed with 5 mM peroxynitrite. Samples were subsequently transferred to a 50-µl capillary tube for EPR measurements. (a) DLPC liposomes plus peroxynitrite; (b) BTBE-containing DLPC liposomes plus peroxynitrite; (c) EYPC liposomes plus peroxynitrite; (d) BTBE-containing EYPC liposomes plus peroxynitrite; (e) Same as B with reverse order addition of peroxynitrite (f) Same as D with reverse order addition of peroxynitrite; (g) Peroxynitrite only. (B) The structures of the MNP-phenoxyl and MNP-lipid alkyl radical adducts are shown and correspond to the signals obtained in lines b and c of panel A, respectively. Both spin adducts are present in line d.
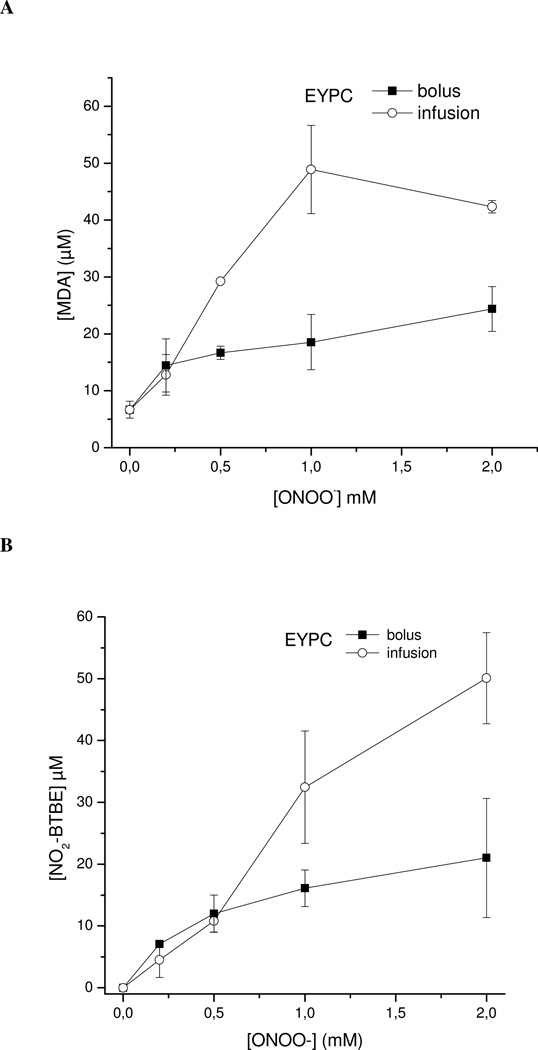
Slow infusion vs bolus addition of peroxynitrite in lipid peroxidation and BTBE oxidation
Peroxynitrite has a short half-life in 100 mM phosphate buffer, pH 7.4 and 25° C (t1/2 2.5 s) due to the proton-catalyzed homolysis to •OH and •NO2 in 30 % yields (55). Thus, addition of peroxynitrite to reaction mixtures as a single bolus may result in high initial concentration of radicals and the extent of oxidation processes in target molecules not necessarily reflect the expected outcome in more biologically-relevant conditions where peroxynitrite is formed as a continuous flow (62, 63). This consideration becomes particularly important in lipid peroxidation processes which depend on propagation reactions where an initial large flux of radicals will prematurely terminate the process. Thus, experiments were performed to study lipid peroxidation and BTBE oxidation yields with peroxynitrite addition as a single bolus or by slow infusion for up to 30 min (1 µM/min). Peroxynitrite addition to BTBE-containing EYPC liposomes resulted in dose-dependent formation of MDA (Fig. 3 panel A); MDA yields were increased two to three fold when peroxynitrite was added as a slow infusion. Similarly, BTBE nitration and dimerization (Fig. 3, panels B, C and D) yields in both EYPC and DLPC by peroxynitrite infusion were up to three-fold higher that with bolus addition. Moreover, BTBE oxidation (Fig. 3, panel E) and MDA formation (not shown) yields in EYPC were significantly reduced under low oxygen tensions when peroxynitrite was added as a slow infusion. The data further support an association between the lipid peroxidation and BTBE oxidation processes.
Figure 3. Peroxynitrite-mediated oxidations: slow infusion versus bolus addition.
BTBE (0.3 mM) was incorporated to EYPC and DLPC liposomes (30 mM) and exposed to peroxynitrite either as a single bolus (■) or by slow infusion (○) in order to achieve final concentrations of (0.2–2 mM). Samples were analyzed for 3-nitro-BTBE, 3,3´-di-BTBE and MDA content. EYPC: (A) MDA (B) 3-nitro-BTBE (C) 3,3´-di-BTBE; DLPC: (D) 3-nitro-BTBE and 3,3´-di-BTBE (inset); (E) BTBE (0.3 mM) containing liposomes were exposed to slow infusion of peroxynitrite (1 mM) in the presence and absence of oxygen and 3-nitro-BTBE and 3,3´-di-BTBE was measured as previously.
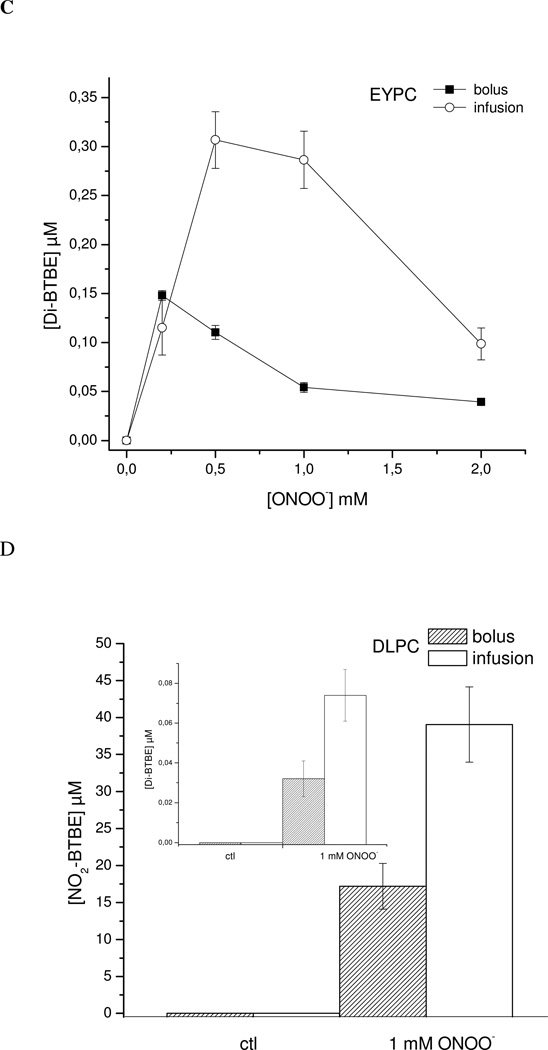
Effect of phospholipid unsaturation degree in peroxynitrite-mediated BTBE oxidation
BTBE oxidation was studied as a function of fatty acid unsaturation by using liposomes containing variable mixtures of DLPC and PLPC. Nitration and dimerization yields were significant through all the phospholipid unsaturation range (0–100 %). A tendency towards increased BTBE oxidation yields was observed at around 40 % PLPC content, while a slight decrease was observed at 100 % PLPC. Thus, in spite of a largely variable content of unsaturated fatty acids in the liposomal mixtures (from 0 to 30 mM linolenic acid), BTBE oxidation was consistently present. Thus, the data support that a simple kinetic competition model, where unsaturated fatty acids simply outcompete BTBE for peroxynitrite-derived radicals does not apply, and that secondary reactions of lipid-derived radicals with BTBE must contribute to nitration and dimerization reactions (Fig. 4).
Figure 4. Lipid unsaturation degree and BTBE oxidation.
BTBE nitration (■) and dimerization (○) were studied as a function of fatty acid unsaturation by using mixtures of DLPC and PLPC (0–100% PLPC) liposomes containing 0.3 mM of BTBE and treated with peroxynitrite (1 mM).
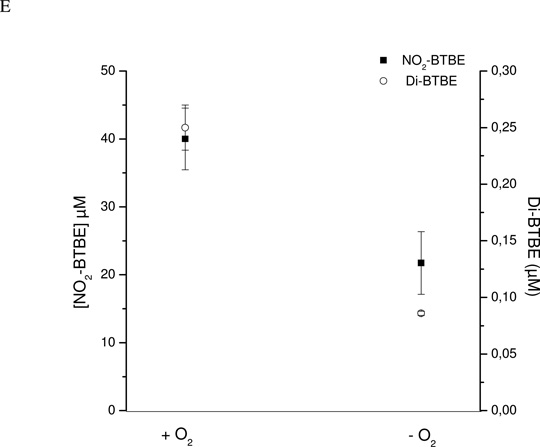
Hemin and ABAP-induced lipid peroxidation
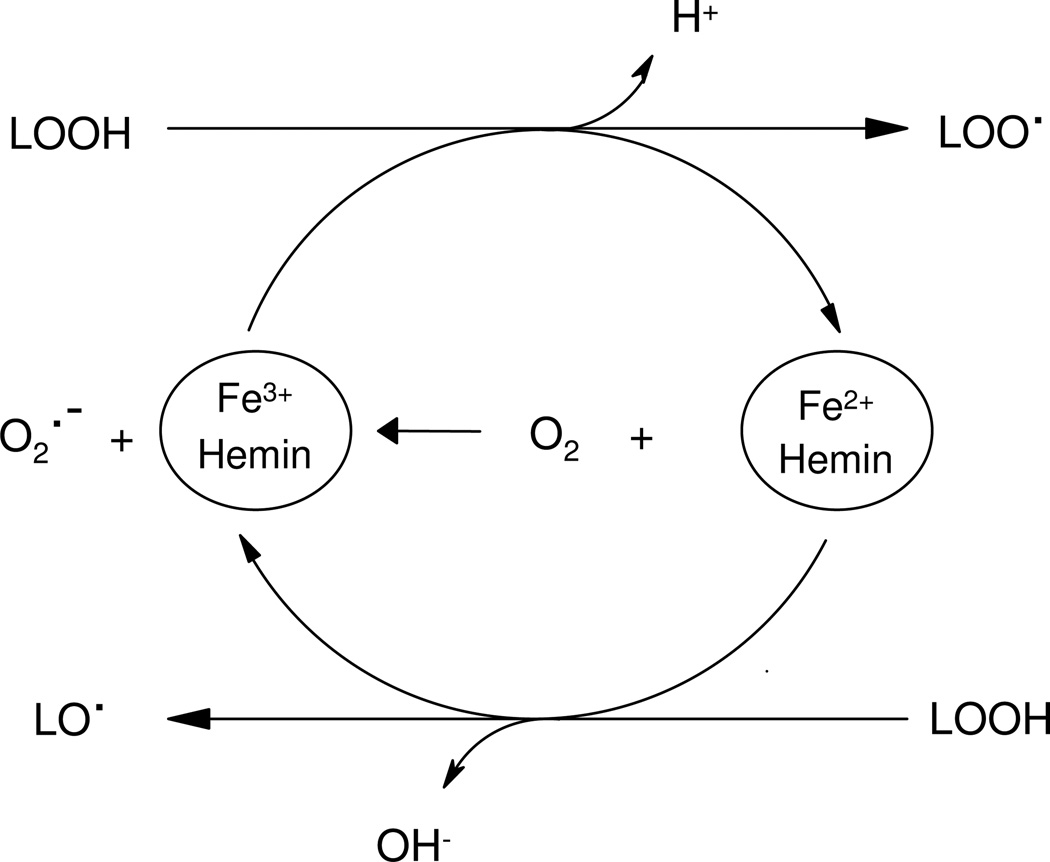
To further demonstrate that lipid peroxidation processes are related with BTBE oxidation, we exposed BTBE-containing liposomes to two other oxidation systems, namely hemin and ABAP, both of which lead to the formation of LOO• in unsaturated fatty acid-containing liposomes. Free hemin readily interacts with membranes (64) and initiates lipid peroxidation in unsaturated liposomes by reaction with LOOH always present in variable extents in EYPC and SBPC (typical hydroperoxide content in well-stored preparations represent in our 30 mM liposome samples~ 45–80 µM, 0.075–0.13 % of total unsaturated fatty acid as determined by the FOX assay). Hemin (Fe3+) promotes the one-electron oxidation of LOOH to yield LOO• and hemin in the Fe2+ redox state (Scheme 1). In turn, the reduced hemin can generate LO• by reduction of LOOH.
Scheme 1. Lipid hydroperoxide reactions with hemin and lipid-derived radicals formation.
Lipid hydroperoxide (LOOH) can react with hemin either in Fe3+ or Fe2+ redox states to yield LOO• or LO•, respectively. Secondarily, hemin in the reduced state (Fe2+) can yield O2.- that can further participate in redox reactions.
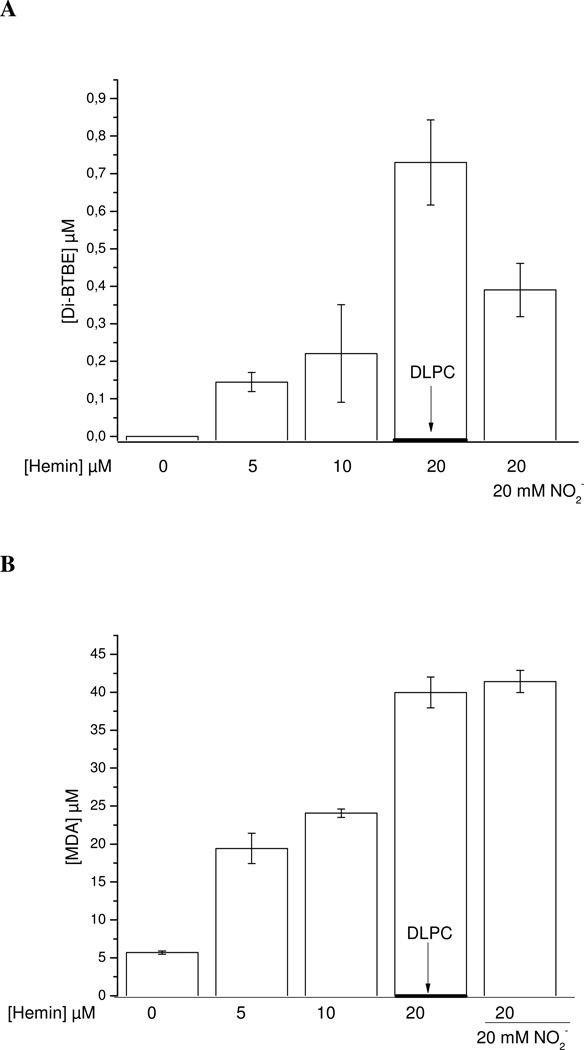
Secondary reactions in the system generate variable amounts of, O2 •− , H2O2 and heme-Fe4+=O that can amplify the process (64). In EYPC liposomes hemin induced 3,3´-di-BTBE and MDA formation in a dose-dependent manner (Fig. 5). On the other hand, in DLPC liposomes (saturated), where hemin can not cause lipid peroxidation, no formation of 3,3´-di-BTBE was detected. Thus, the data imply the participation of LOO• radicals formed from the reaction of hemin with lipid hydroperoxides (Scheme 1) in the BTBE dimerization process.
Figure 5. Hemin-induced lipid peroxidation and BTBE oxidation.
BTBE (0.3 mM)-containing DLPC and EYPC liposomes (30 mM) were exposed to hemin (5–20 µM) in sodium phosphate 100 mM pH 7.3 plus 0.1 mM DTPA. Samples were analyzed for (A) 3,3´-di-BTBE and (B) MDA content. The arrow indicates the values corresponding to DLPC liposomes which were zero for both measurements under all reaction conditions.
Secondly, we performed experiments using the organic peroxyl radical donor, ABAP, which generates a flux of peroxyl radicals by thermolysis.
| (4) |
| (5) |
| (6) |
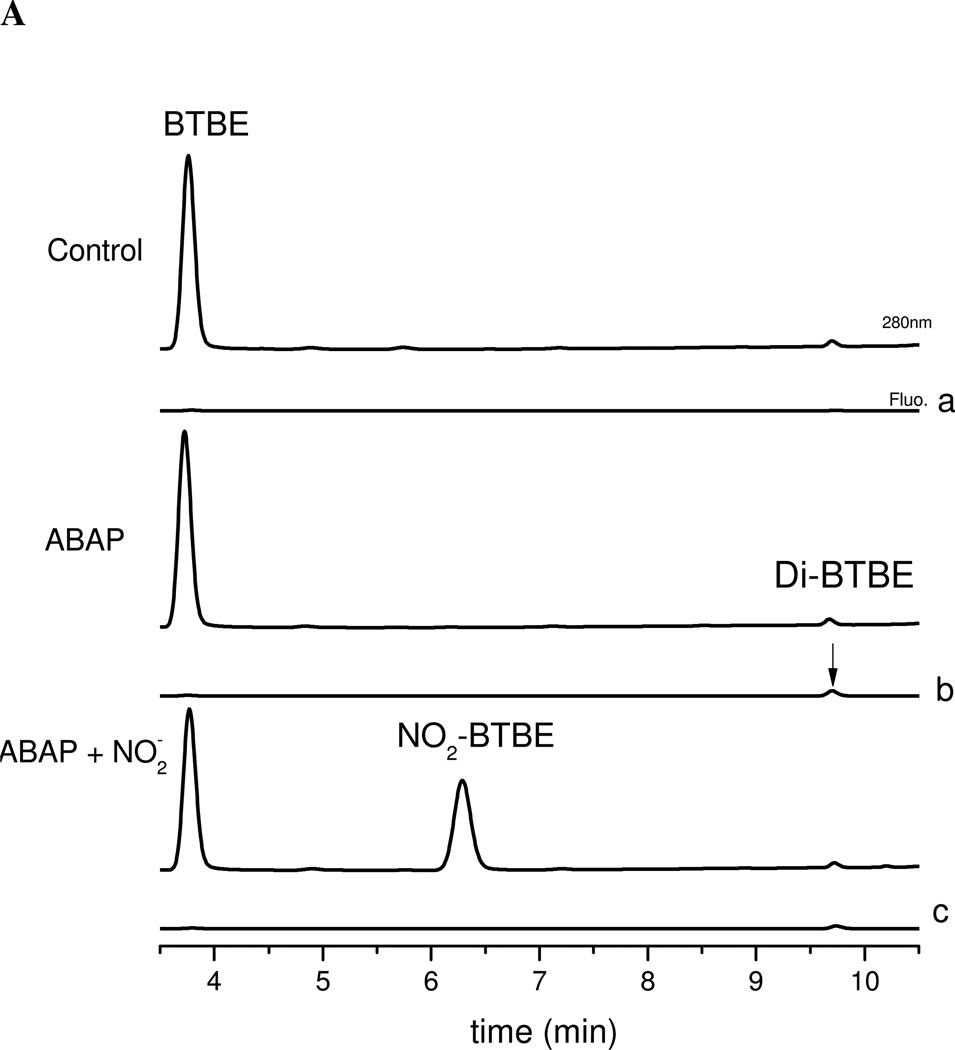
ABAP initiates lipid peroxidation in unsaturated fatty acid-containing liposomes such as EYPC, by the reaction of the ABAP-derived peroxyl radicals (AOO•) with an unsaturated alkyl chain to yield the alkyl radical (L•) which then (Eqs. 4–6), after oxygen addition forms lipid peroxyl radical. The addition of ABAP to BTBE-containing liposomes resulted in the formation of 3,3´-di-BTBE both in DLPC and EYPC liposomes (Table 1Fig. 6A), but MDA formation was observed in EYPC liposomes only. Thus, either ABAP-derived (in the DLPC experiment) or lipid-derived (in the EYPC one) peroxyl radicals react with BTBE to yield BTBE-derived phenoxyl radical and subsequently 3,3´-di-BTBE. Interestingly, samples treated with ABAP plus nitrite resulted in the formation of both 3,3´-di-BTBE and 3-NO2-BTBE supporting that ABAP-derived peroxyl radicals can also oxidize nitrite to •NO2 (Tables 1 and 2) (65). Low oxygen levels decreased BTBE oxidation yields induced by ABAP plus nitrite, both in DLPC and EYPC liposomes confirming the role of LOO• in the process. Control experiments with nitrite only showed no formation of BTBE oxidation products (Table 1).
Table 1. ABAP-mediated BTBE oxidation.
BTBE (0.3 mM) was incorporated to DLPC and EYPC liposomes (30 mM) and the mixture incubated during 2h at 37°C with ABAP (10 mM, 0.3 µM /min flux of peroxyl radicals) in the presence and absence of nitrite (NO2−) (20 mM) and 3-nitro-BTBE and 3,3´-di-BTBE content was measured. The same experiment was performed in the presence and low oxygen tension (~ 5 µM).
| Condition | EYPC |
DLPC |
||
|---|---|---|---|---|
| NO2-BTBE (µM) | Di-BTBE (µM) | NO2-BTBE (µM) | Di-BTBE (µM) | |
| Control | 0 | 0 | 0 | 0 |
| ABAP | 0 | 0.011 ± 0.001 | 0 | 0.79 ± 0.164 |
| ABAP + NO2− | 2.5 ± 0.76 | 0.043 ± 0.008 | 16.15 ± 1.39 | 0.537 ± 0.029 |
| ABAP − O2 | 0 | 0 | 0 | 0.32± 0.065 |
| ABAP + NO2− - O2 | 1.46 ± 0.8 | ND* | 9.43 ± 2 | ND |
| NO2− | 0 | 0 | 0 | 0 |
ND: Not Determined
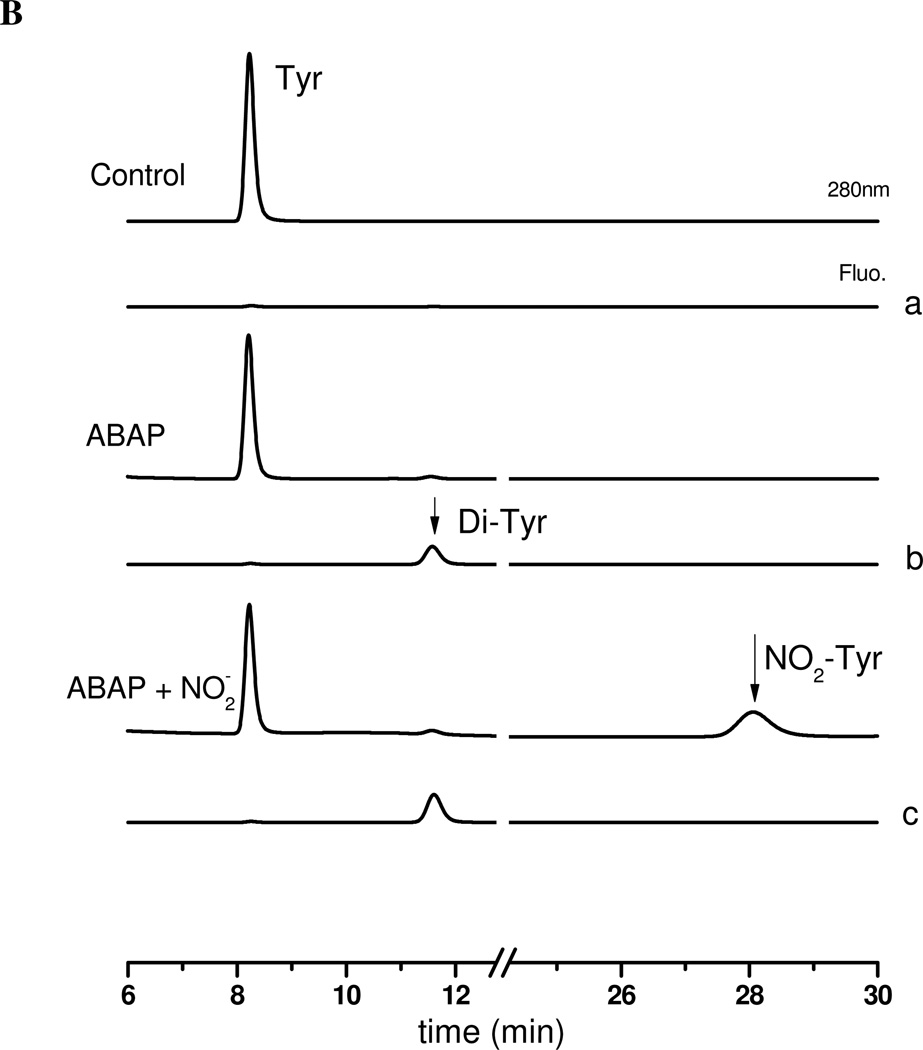
Figure 6. ABAP-induced BTBE and tyrosine oxidation.
Panel A: (a) BTBE (0.3 mM)-containing DLPC liposomes in sodium phosphate100 mM (pH 7.3) plus 0.1 mM DTPA were exposed to (b) ABAP (20 mM) in the absence and (c) presence of nitrite (40 mM) and 3-nitro-BTBE and 3,3´-di-BTBE were measured. Panel B: (a) Tyrosine (0.3 mM) in sodium phosphate 100 mM (pH 7.3) plus 0.1 mM DTPA was exposed to (b) ABAP (20 mM) in the absence and (c) presence of nitrite (40 mM) and 3-nitrotyrosine and 3,3´-dityrosine were measured.
Table 2. Main reactions involved in tyrosine oxidation in hydrophobic environments and the connection to lipid peroxidation.
| Reaction | k (M−1s−1) | Reference |
|---|---|---|
| a L + •OH → L• + OH− | 1 × 1010 | (45) |
| L + •NO2 → L• + NO2− | 2 × 105 | (46) |
| Tyr + •OH → •Tyr + OH− | 1.24 × 1010 | (90) |
| Tyr + •OH → •TyrOH b + OH− | 6.5 × 108 | (90) |
| Tyr + •NO2 → •Tyr + NO2− | 3.2 × 105 | (46) |
| •Tyr + •NO2 → 3-nitro-Tyr | 3 × 109 | (46) |
| 2 •Tyr → 3-3´-diTyr | 2.25 × 108 | (91) |
| c 2.25 × 106 | (41) | |
| L• + O2 → LOO• | 3 × 108 | (92) |
| LOO• + L → LOOH + •L | 37 | (68) |
| 2 LOO• → LOOH + O2 | 107 | (92) |
| LOO• + L• → LOOL | 5 × 107 | (92) |
| 2 L•→ LL | 5 × 108 | (92) |
| LOO• + NO2− → LOOH + •NO2 | 4.5 × 106 | (65)d |
| LOO• + Tyr → LOOH + •Tyr | 4.8×103 | This work |
| eα-Tocopherol + LOO•→ α-Tocopheryl• + LOOH | 5 × 105 | (67) |
| α-Tocopherol + •NO2 → α-Tocopheryl• + NO2− | 1 × 105 | (67) |
| α-Tocopherol + •OH → α-Tocopheryl• + OH− | 3.8 × 109 | (66) |
Reactions involving lipids refer to those containing unsaturated fatty acid unless otherwise indicated.
lipids composed of saturated fatty acid are also oxidized by hydroxyl radicals at similar rates (58).
this radical rapidly dehydrates and evolves to tyrosyl radical under the experimental conditions employed herein.
value for tyrosine dimerization in membranes, expected to occur at least 100-times slower than in aqueous phase.
this rate constant has been reported for acetylperoxyl radical reduction by nitrite.
the radical scavenging mechanisms of α-tocopherol are indicated.
To specifically address the reaction of organic peroxyl radicals with tyrosine, we studied the effect of ABAP on free tyrosine oxidation in aqueous phase (Fig. 6B). Tyrosine exposure to ABAP-derived peroxyl radicals resulted in the formation of 3,3´di-tyrosine (Fig. 6B line b); when nitrite was added to the incubation mixture, both tyrosine oxidation products, 3-nitrotyrosine and 3,3´-dityrosine were formed (Fig. 6B, line c). Tyrosine nitration and dimerization yields were lower with free tyrosine in aqueous solution than those observed for BTBE in liposomes which suggest that tyrosine oxidation by peroxyl radicals more easily occurs in non-polar environments.
Effect of α-tocopherol on BTBE oxidation and lipid peroxidation
α–tocopherol is a well known lipophilic chain breaking antioxidant that reacts with LOO• with a rate constant of 5 × 105 M−1 s−1 (66) to yield lipid hydroperoxides and the stable α–tocopheroxyl radical (Eq. 7):
| (7) |
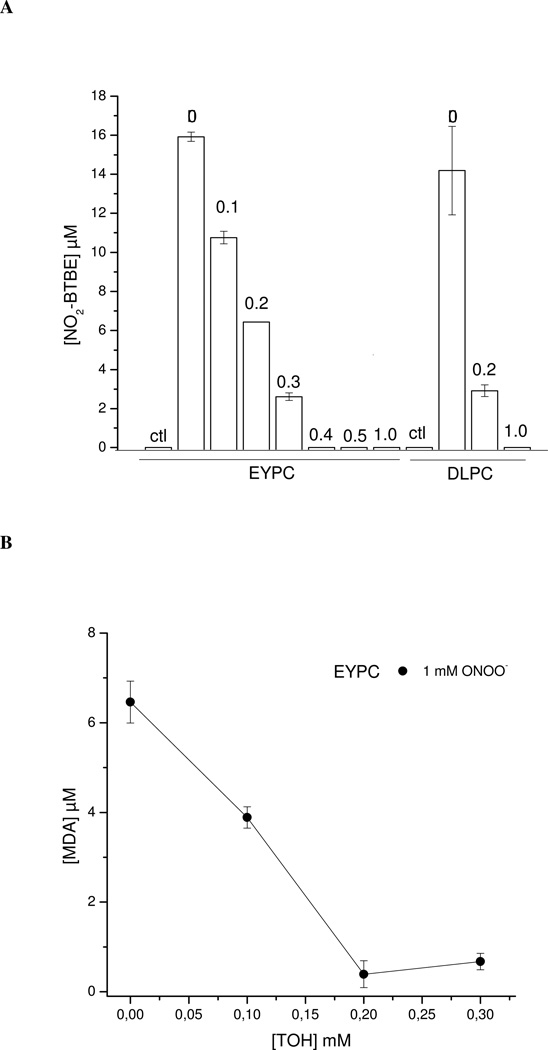
α-Tocopherol incorporated into EYPC liposomes inhibited peroxynitrite-mediated BTBE nitration (Fig. 7A), dimerization (not shown) and lipid peroxidation (Fig. 7B) in a dose- dependent manner. Interestingly, α-tocopherol also inhibited BTBE oxidation in DLPC liposomes, where no lipid peroxidation occurs. The effect of α-tocopherol is explained by the reaction between α-tocopherol with LOO•. In the case of DLPC, the reaction of α-tocopherol with •NO2 (k = 1×105 M−1 s−1 (67)) also becomes relevant to explain the inhibition of BTBE oxidation.
Figure 7. Effect of α-tocopherol on lipid peroxidation and BTBE oxidation.
The indicated concentrations of α-tocopherol (0.1–1 mM) were pre-incorporated to different EYPC and DLPC preparations (30 mM) and treated with peroxynitrite (1 mM); Samples were analyzed for (A) 3-nitro-BTBE and (B) MDA content.
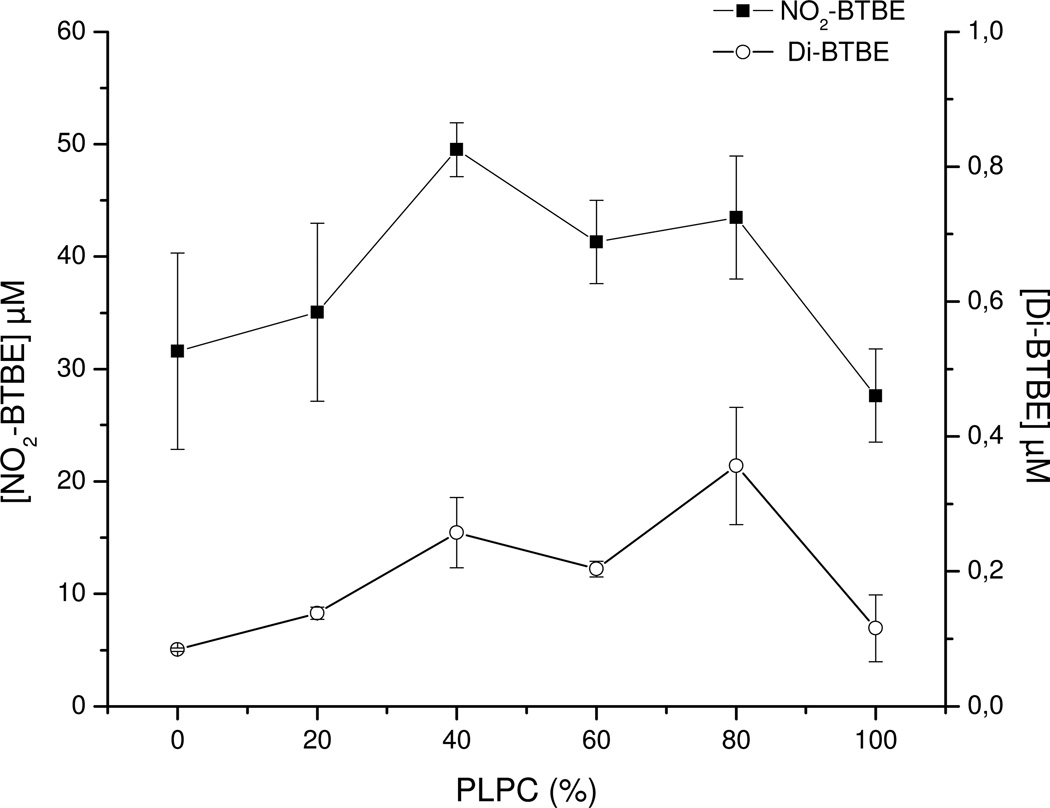
Oxygen consumption studies and kinetic determination of lipid peroxyl radical reaction with BTBE
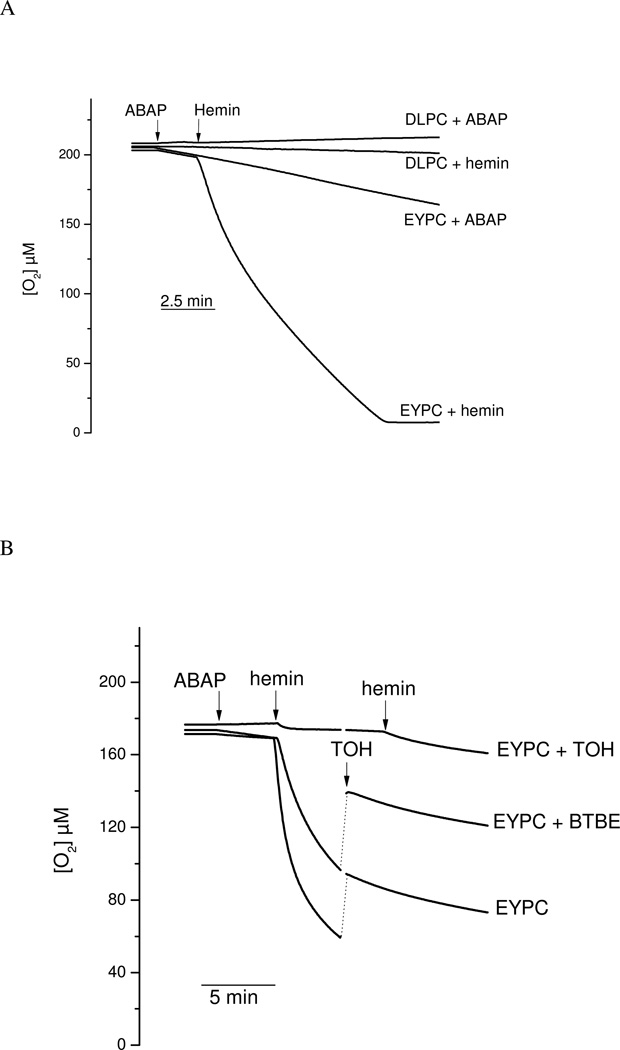
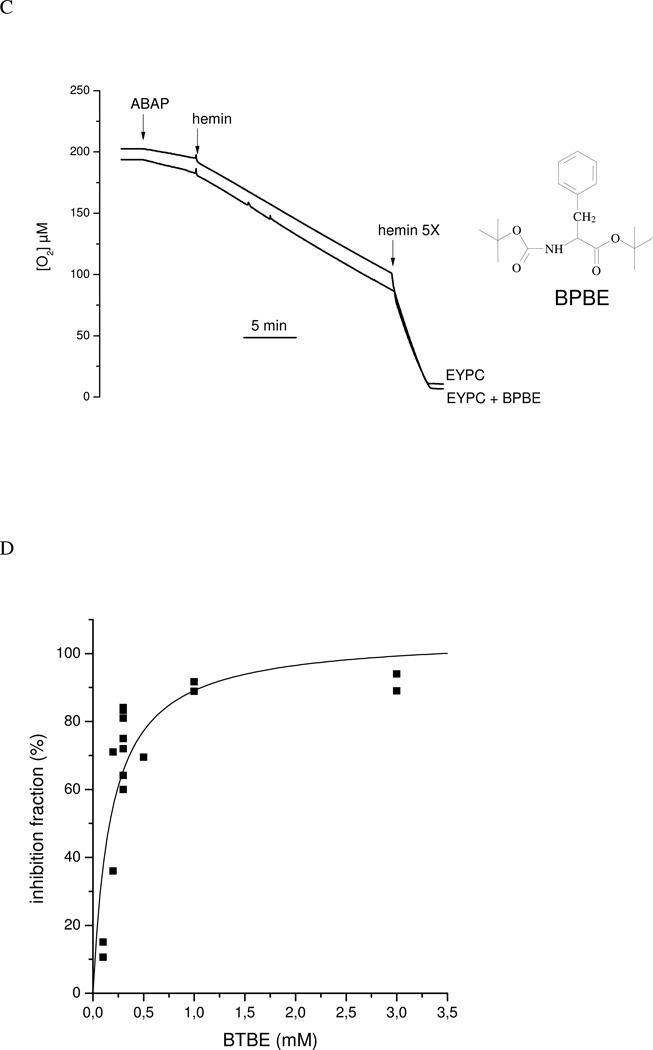
Experiments were performed to measure the oxygen consumption associated to lipid peroxidation initiated by hemin and ABAP and the potential chain breaking reaction of BTBE. If lipid peroxyl radicals react to a significant extent with BTBE, then, the hydrophobic tyrosine analog should inhibit oxygen consumption. In EYPC (but not in DPLC) liposomes (in the absence of BTBE) a basal decrease in oxygen concentration is shown. Oxygen consumption is observed upon the addition of either ABAP or hemin (Fig. 8A), in agreement with the initiation and propagation phases of lipid peroxidation. In contrast, no further oxygen consumption was observed in DLPC liposomes after ABAP or hemin addition (Fig. 8A). The incorporation of α-tocopherol to EYPC liposomes resulted in a significant decrease in oxidant-induced oxygen consumption rates (Fig. 8B). The extent of inhibition in oxygen consumption by α-tocopherol is compatible with the previously reported rate constants of LOO• with adjacent alkyl chains (k = 36 M−1s−1) (68) and with α-tocopherol (k = 5 × 105 M−1s−1 (66). The inhibitory action of α-tocopherol was also observed if added exogenously during the time course of the oxygen consumption studies, but was less potent than when pre-incorporated, due the sub-optimal penetration in the preformed liposome structure within the time frame of the experiment. Remarkably, the incorporation of BTBE to the EYPC liposomes resulted in a dose-dependent inhibition of both hemin and ABAP-induced oxygen consumption rates, which is consistent with the reaction of BTBE with lipid- derived radicals (Fig. 8B). To discard subtle structural effects in the liposomal membranes due to BTBE incorporation, experiments were performed using a phenylalanine hydrophobic analog (BTPE) pre-incorporated to the liposomes (Fig. 8C). In this case, oxygen consumption rates were identical to the one observed in control EYPC (without BTBE), which is in agreement with a direct participation of the phenolic-OH moiety in the BTBE in reaction leading to inhibition of lipid peroxidation-dependent oxygen consumption, which is not present in the phenylalanine derivative (Fig. 8C).
Figure 8. Oxygen consumption studies.
Oxygen consumption was measured in a 2K Oxygraph to assess ABAP (10 mM) and hemin (1 µM)-induced lipid peroxidation in in sodium phosphate 100 mM (pH 7.3) plus 0.1 mM DTPA. (A) EYPC and DLPC liposomes (6.25 mM). (B) BTBE (0.3 mM), or α-tocopherol (0.3 mM) containing EYPC liposomes. The arrow indicates the exogenous addition of 0.25 mM of α-tocopherol; the transient increase in oxygen levels observed in this record is due to to oxygen dissolved in the volume of reagent added (C) BPBE (0.3mM) and control EYPC liposomes. (D) EYPC liposomes in the absence and presence of different concentrations of BTBE (0.1 –3 mM) were exposed to ABAP (10 mM) and hemin (1 µM) and oxygen consumption inhibition was evaluated as a function of BTBE concentration. (E) EYPC liposomes with or without BTBE (0.3 mM) were exposed to hemin (30 min) and ABAP (2.5 h) and lipid hydroperoxide content was measured: Inset: UV-Vis spectra of same samples shown in E (a) − BTBE; (b) + BTBE; (c) + BTBE + hemin; (d) + BTBE + ABAP; (e) − BTBE + ABAP; (f) − BTBE + hemin. (F): Same samples as (E) in which MDA content was measured. The structure of N-t-BOC L-phenylalanine tert butyl ester (BPBE) is indicated in the corresponding panel.
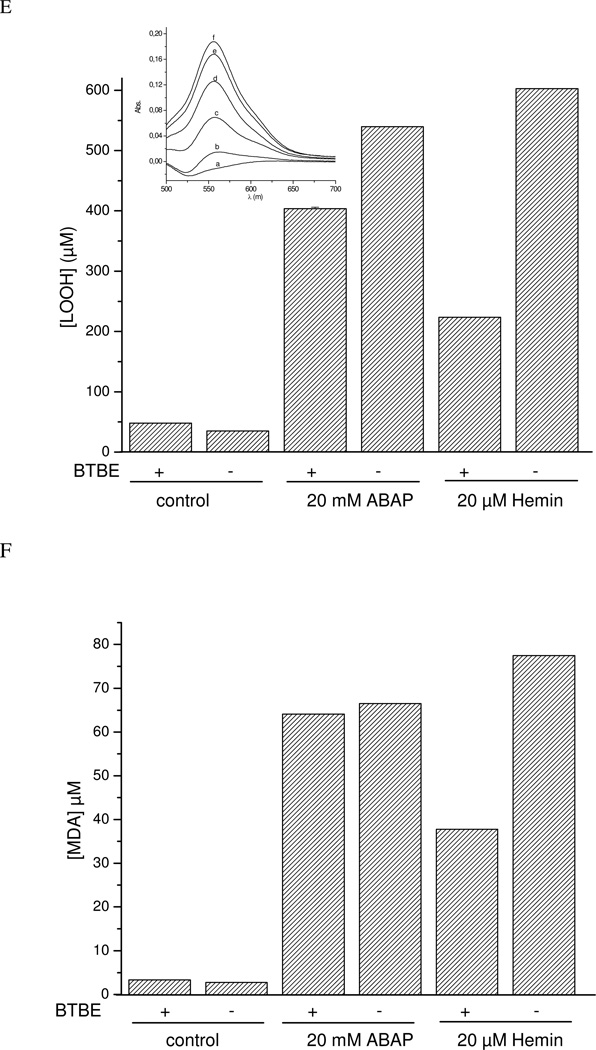
The dose-dependent inhibition of oxygen consumption by increasing concentrations of pre-incorporated BTBE in EYPC liposomes was further analyzed in order to obtain an estimated second order rate constant for the reaction of LOO• with BTBE. A plot that relates the inhibition fraction in oxygen consumption as a function of BTBE concentration is in agreement with a direct competition of BTBE for the lipid peroxyl radicals (Fig. 8D). These data allows the estimation of an apparent second order rate constant of 4.8 × 103 M−1s−1 for the following reaction:
| (8) |
This rate constant value compares well with an independent determination considering the α-tocopherol studies. From data in Fig. 8 panels B and C, one concludes that 87 % inhibition is obtained with 5 µM α-tocopherol or 1 mM BTBE. Considering the stoichiometric factor n = 2 for α-tocopherol (69), thus, 2 × ktoc [α-tocopherol] = kBTBE [BTBE], so 2 × ktoc [α-tocopherol]/ [BTBE] = kBTBE = 5 × 103 M−1s−1.
In addition, both MDA and hydroperoxide yields were decreased in the presence of BTBE, in agreement with a reaction between BTBE and lipid peroxyl radicals (Fig. 8E and F) and with the oxygen consumption data.
Discussion
Previously identified and biologically-relevant one-electron oxidants for tyrosine in aqueous environments include •OH, CO3•− and •NO2, compounds I and II of hemeperoxidases and high oxidation states of transition metal centers (5). However, in the case of tyrosine oxidation in proteins associated to hydrophobic biocompartments such as membranes or lipoproteins a) some of the oxidants may not easily reach or permeate the lipid phase and b) unsaturated fatty acids, due to its reactivity and large concentration, constitute preferential targets for the initial oxidative attack. Thus, mechanisms by which tyrosine residues become dimerized and nitrated in hydrophobic biocompartments seem to have unique characteristics from those previously described in hydrophilic phases. In this work we have explored, using a validated model system of PC liposomes containing a hydrophobic tyrosine analog (BTBE), whether lipid-derived radicals generated during lipid peroxidation processes can mediate tyrosine oxidation to tyrosyl radical, the first step in the path to 3,3´-dityrosine or 3-nitrotyrosine.
Lipid peroxidation was initiated by three independent oxidation systems, namely peroxynitrite, hemin and ABAP. Peroxynitrite promotes lipid peroxidation and tyrosine nitration in hydrophobic compartments secondary to homolysis of peroxynitrous acid (ONOOH) to •OH and •NO2 inside or at close proximity of the lipid phase (41, 44, 70). In EYPC, for instance, the large concentration ratio of unsaturated fatty acid/BTBE (50/1) and the participating rate constants (see Table 2), determine that the initial attack of peroxynitrite-derived radicals occurs at the fatty acid moieties; this initial reaction yields the alkyl (pentadienyl) radical that upon oxygen addition evolves to LOO• which propagates the process (44). Alternatively, lipid-derived radicals were generated by the reaction of hemin with pre-existing lipid hydroperoxides to directly yield LOO• or by ABAP whose peroxyl radical-derivative diffuses to the liposomes and reacts with unsaturated alkyl chains to initiate lipid peroxidation as well (71). Lipid peroxyl radicals are known to react at fast rates with some phenolic compounds, including α–tocopherol and other dietary compounds that may exert antioxidant actions in vitro and in vivo. For example, linoleate peroxyl radicals react with phenolic antioxidant compounds such as α-tocopherol, curcumin and quercetin with rate constants in the range ~106 M−1 s−1–107 M−1 s−1 (72–75) In the case of tyrosine, another phenolic compound, the reaction with LOO• is thermodynamically possible; however, tyrosine is not a particularly good hydrogen donor and therefore the reaction may be kinetically hindered. Interestingly, hydrobobic phases may increase the reactivity of phenolic compounds such as tyrosine for LOO• (see below). Thus, extensive studies were performed herein to unambiguously show the existence of the LOO• reaction with the hydrophobic tyrosine analog BTBE3.
The evidence we have gathered herein to support the participation of LOO• in the tyrosine oxidation processes promoted by the three different tested oxidation systems can be summarized as follows: a) tyrosine (BTBE) oxidation was seen in significant extents in unsaturated fatty acid-containing PC liposomes, b) tyrosine oxidation in liposomes (but not in aqueous phase) was decreased under low oxygen concentrations, c) lipid-derived and tyrosyl radicals were simultaneously detected, d) α-tocopherol strongly inhibited tyrosine oxidation, e) BTBE (but not BPTE) partially inhibited lipid peroxidation processes, f) changes in the levels of tyrosine oxidation and lipid peroxidation products followed a parallel trend, g) the data are in agreement with an overall process involving the free radical mechanisms of lipid peroxidation and tyrosine oxidation plus a “connecting reaction” represented by one-electron oxidation of tyrosine by LOO• (Eq. 7) (see also Table 2).
In regards to the reactivity of peroxyl radicals with tyrosine, the data showed that ABAP-derived peroxyl radicals were already capable of oxidizing both BTBE in DLPC (saturated) liposomes as well as free tyrosine (Table 1 and Fig. 6), but reaction yields were much larger for BTBE, compatible with the concept that the reaction of phenolic compounds with oxidizing radicals is influenced by a kinetic solvent effect (76, 77). Indeed, different phenolic compounds (e.g. quercetin, epicatechin) react with peroxyl radicals at similar rates than α-tocopherol in non-polar solvents but not in hydrogen-bonding solvents. In solvents that are strong hydrogen bond acceptors the rate constants of peroxyl radicals with phenolic compounds dramatically decline and may even become undetectable4 (78). Polar solvents a) interfere with the intramolecular stabilization (H- bonding) of the phenoxyl radical and b) generate steric hindrance for the approach of the peroxyl radical to the solvent-complexed phenol, which reduces the rate constant of H-atom abstraction. Such considerations become important in heterogenous phases of lipid bilayers, where the localization of the phenolic groups will influence their effectiveness to trap peroxyl radicals. In the case of LOO• generated in EYPC, an estimated second order rate constant for the reaction with BTBE was obtained by oxygen consumption studies, using two different approaches considering independent “primary targets”, namely unsaturated fatty acids (kLH ~ 10–50 M−1s−1 and α-tocopherol (kα-toc = 5 × 105 M−1s−1 (66)) (Fig. 8). The estimated kBTBE of 4.8 × 103 M−1 s−1 is compatible with the redox properties of tyrosine and the lack of an “induction period” in oxygen consumption studies when lipid peroxidation was initiated in BTBE-containing liposomes; this moderate rate constant (1/200 of that of α-tocopherol), is still larger than that of LOO• with LH (e.g. the reaction of linoleic acid peroxyl radical with linoleic acid, k = 36 M−1 s−1 in 70 % EtOH (68). The reactions of halogenated organic peroxyl radicals (known to be more reactive than lipid peroxyl radicals) with tyrosine have been reported at alkaline pH, where most of the tyrosine is in the phenolate form (pKTyr-OH ~ 10), with values approaching 108 M−1s−1 (79). Oxidation of the protonated form of the phenol by peroxyl radicals (as expected at neutral pH or when inside a hydrophobic structure) is significantly less favorable, in full agreement with the kBTBE value in the range of 103 M−1s−1 obtained.
In a previous work (57), the nitration of a transmembrane peptide containing tyrosine in position eight from the C-terminus (ca. Y-8 at a depth of ~ 20 Å from the surface) induced by the peroxynitrite donor 3-morpholinosydnonimine hydrochloride (SIN-1) was progressively inhibited with increasing unsaturation degree of the DLPC:PLPC mixtures. A closer look to the data indicates that the extent of inhibition was significantly less than that predicted by a simple competition of Y-8 with unsaturated fatty acids (eg. at 40% liposome unsaturation and considering the reported rate constant values for hydroxyl radical and nitrogen dioxide-dependent fatty acid and tyrosine oxidation (Table 2), over 90 % inhibition of tyrosine oxidation would be expected but only 40 % inhibition was actually observed).
The relative yields of tyrosine dimerization and oxidation are largely affected by the biomembrane structure (41–43, 80), and we have estimated a diffusion coefficient (D) for BTBE in PC liposomes as ca. 5 µm2 s−1 (41). Thus, the rate constant value is lowered 100–200 fold (k ~ 1–2 × 106 M−1 s−1) with respect to the corresponding one of tyrosyl radicals (k) 2.25 × 108 M−1 s−1). Intermolecular tyrosine dimerization will be even less likely in integral peptides and proteins as D values become >103–104 times smaller than in solution depending on protein size (eg. D ~ 0.1–0.5 µm2 s−1) (81, 82) and in line with the lack of tyrosine dimerization on transmembrane peptides treated with peroxynitrite (57). On the other hand, •NO2 concentrates to some extent in hydrophobic environments (83), and the D value (D •NO2 ) is ~ 1500 µ m2 s−1, very close to that of the aqueous phase of 4500 µm2 s−1 (84), thus nitration in membranes is not kinetically impeded, a concept that is in perfect agreement with the large 3-nitro- BTBE/3,3´diBTBE ratios found during peroxynitrite exposures.
Significant evidence supports that lipid peroxidation and tyrosine oxidation processes are associated in oxidative-stress related processes and pathophysiology (31, 43, 85, 86) for instance, membrane protein tyrosine (and BTBE) oxidation and nitration were evidenced in red blood cells exposed to peroxynitrite, in a process that was inhibited under low oxygen tensions (43). In a related way, accumulation of protein 3-nitrotyrosine and 4-hydroxynonenal protein adducts have been simultaneously detected in liver and kidney in a model of type I diabetes where peroxynitrite has been identified as a key mediator of lipid peroxidation (86). Future work should address whether both processes in vivo are mechanistically linked as suggested by our in vitro model studies. Our data also indicate that α-tocopherol can potently modulate tyrosine oxidation yields in membranes by inhibiting lipid peroxidation. In this regard, it is important to consider that the extent of inhibition is a function of α-tocopherol levels that under biologically-relevant conditions (ca. 1 molecule of α-tocopherol per 100–1000 molecules of membrane phospholipid; (87) may not be sufficient to fully prevent tyrosine oxidation events in either biomembranes (43, 86) or model membranes (this work, see Fig. 7A at 0–0.3 mM α-tocopherol for 30 mM DLPC). Thus, we predict that an increase of cell/tissue levels of α-tocopherol should result in attenuation of protein tyrosine oxidation both in biomembranes and lipoproteins.
In addition to lipid peroxidation products, other side reactions take place on lipid radical intermediates when peroxynitrite and/or •NO2 are present leading to the formation of small amounts of nitrated lipids (88) (Eq. 9)
| (9) |
Additionally, a termination reaction between •LOO and •Tyr may occur yielding Diels Alder-type structures (89) (Eq. 10):
| (10) |
Membrane phospholipids have a low D value is (DPL ca. 0.5–1 µm2 s−1; (81)), but due to their abundance the formation of tyrosyl-phospholipid adducts is at least probable and deserves investigation in further studies.
In summary, the data presented herein support that •LOO participates in tyrosine oxidation processes in hydrophobic biocompartments and provides a new mechanistic insight to understand protein nitration in lipoproteins and biomembranes (Fig. 9). Notably, cell and tissue oxygen levels become a previously unrecognized factor that will critically influence tyrosine oxidation yields via the intermediacy of lipid peroxyl radicals.
Figure 9. Proposed reaction mechanism by which lipid peroxyl radicals participate in tyrosine oxidation.
Acknowledgements
This work was supported by grants from the Howard Hughes Medical Institute and International Centre for Genetic Engineering and Biotechnology to RR, National Institutes of Health to BK and RR (2 R01Hl063119-05) and Agencia Nacional de Investigación e Innovación (ANII) / Fondo Clemente Estable to SB (FCE_362). SB was partially supported by a fellowship of ANII. RR is a Howard Hughes International Research Scholar.
Abbreviations
- BTBE
N-t-BOC L-tyrosine tert butyl ester
- BPBE
N-t-BOC L-phenylalanine tert butyl ester
- DLPC
1,2- Dilauroyl- sn-glycero-3-phosphocholine
- PLPC
1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine
- EYPC
egg chicken yolk L-α-phosphatidylcholine
- SBPC
soybean-L-α-phosphatidylcholine
- dtpa
diethylentriaminepentaacetic acid
- •NO
nitric oxide
- O2•−
Superoxide
- 3-nitro-Tyr
3-nitrotyrosine
- •Tyr
tyrosyl radical
- PC
Phosphatidylcholine
- RP-HPLC
reverse-phase high performance liquid chromatography
- ABAP
2,2´-Azobis (2-amidinopropane) hydrochloride
- MDA
Malondialdehyde
- FOX
Ferrous oxide-xylenol orange assay
- LOO•
lipid peroxyl radical
- LO•
lipid alkoxyl radical
- TBARS
thiobarbituric-acid reactive species
- ESR
electron spin resonance
- BHT
butylated hydroxytoluene and MNP (2-metyl nitrosopropane)
- BOC
butoxypyrocarbonate
Footnotes
IUPAC recommended names for peroxynitrite anion (ONOO−) and peroxynitrous acid (ONOOH) (pKa= 6.8) are oxoperoxonitrate (1-) and hydrogen oxoperoxonitrate, respectively. The term peroxynitrite is used to refer to the sum of ONOO− and ONOOH.
Saturated fatty acids (e.g. lauric acid in DLPC) will also react with •OH at fast rates (k ~ 5 × 109 M−1s−1 (58)) to yield the alkyl radical. This can evolve to lauric acid peroxyl radical, that, however, will not be capable of propagate a lipid peroxidation chain reaction in DLPC liposomes due to the lack of unsaturated fatty acids. Still, the peroxyl radical could be potentially reactive with other targets such as BTBE (vide infra).
Alkoxyl radicals (LO•) are also formed in lipid peroxidation processes via one-electron reduction of lipid hydroperoxides (48) or breakdown of tetroxide intermediates formed by the coupling of lipid two peroxyl radicals; alkoxyl radicals are far more potent oxidizing species than LOO• but mainly rearrange to epoxyallylic radicals (50) which in turn after coupling to O2 become a secondary peroxyl radical.
For instance, a decrease by a factor of 36 in the k value has been observed for the reaction of α-tocopherol with cumylperoxyl radicals when changing from hexane to tert-butyl alcohol (78).
Contributor Information
Silvina Bartesaghi, Email: sbartesa@fmed.edu.uy, Departamento de Histología y Embriología, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563; Departamento de Bioquímica, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563; Center for Free Radical and Biomedical Research, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563.
Jorge Wenzel, Email: jwenzel@fmed.edu.uy, Departamento de Bioquímica, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563; Center for Free Radical and Biomedical Research, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563.
Madia Trujillo, Email: madiat@fmed.edu.uy, Departamento de Bioquímica, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563; Center for Free Radical and Biomedical Research, Facultad de Medicina, Universidad de la República, Uruguay. Phone: +598-2-9249561, Fax: +598-2-9249563.
Marcos López, Email: marcoslopez@mcw.edu, Department of Biophysics and Free Radical Research Center, Medical College of Wisconsin, Milwaukee WI 53226, USA. Phone: + 414-456-4000, Fax: +414-456-6512.
Joy Joseph, Email: jjoseph@mcw.edu, Department of Biophysics and Free Radical Research Center, Medical College of Wisconsin, Milwaukee WI 53226, USA. Phone: + 414-456-4000, Fax: +414-456-6512.
Balaraman Kalyanaraman, Email: balarama@mcw.edu, Department of Biophysics and Free Radical Research Center, Medical College of Wisconsin, Milwaukee WI 53226, USA. Phone: + 414-456-4000, Fax: +414-456-6512.
Rafael Radi, Departamento de Bioquímica, Facultad de Medicina, Universidad de la República, Uruguay; Center for Free Radical and Biomedical Research, Facultad de Medicina, Universidad de la República, Uruguay.
References
- 1.Bodaness RS, Leclair M, Zigler JS., Jr An analysis of the H2O2-mediated crosslinking of lens crystallins catalyzed by the heme-undecapeptide from cytochrome c. Arch Biochem Biophys. 1984;231:461–469. doi: 10.1016/0003-9861(84)90409-0. [DOI] [PubMed] [Google Scholar]
- 2.Giulivi C, Traaseth NJ, Davies KJ. Tyrosine oxidation products: analysis and biological relevance. Amino Acids. 2003;25:227–232. doi: 10.1007/s00726-003-0013-0. [DOI] [PubMed] [Google Scholar]
- 3.Heinecke JW. Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology. 2002;177:11–22. doi: 10.1016/s0300-483x(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 4.Hsiai TK, Hwang J, Barr ML, Correa A, Hamilton R, Alavi M, Rouhanizadeh M, Cadenas E, Hazen SL. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radic Biol Med. 2007;42:519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ischiropoulos H. Biological Selectivity and Functional Aspects of Protein Tyrosine Nitration. Biochem. Biophys. Res. Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 7.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds MR, Berry RW, Binder LI. Nitration in neurodegeneration: deciphering the "Hows" "nYs". Biochemistry. 2007;46:7325–7336. doi: 10.1021/bi700430y. [DOI] [PubMed] [Google Scholar]
- 9.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF, Jr, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. Jama. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 10.Beckman JS, Ischiropoulos H, Zhu L, van der Woerd M, Smith C, Chen J, Harrison J, Martin JC, Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992;298:438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- 11.Quijano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J Biol Chem. 2001;276:11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- 12.Quint P, Reutzel R, Mikulski R, McKenna R, Silverman DN. Crystal structure of nitrated human manganese superoxide dismutase: mechanism of inactivation. Free Radic Biol Med. 2006;40:453–458. doi: 10.1016/j.freeradbiomed.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 13.Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batthyany C, Souza JM, Duran R, Cassina A, Cervenansky C, Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44:8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 15.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 16.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc- deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 18.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 22.Bartesaghi S, Ferrer-Sueta G, Peluffo G, Valez V, Zhang H, Kalyanaraman B, Radi R. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids. 2007;32:501–515. doi: 10.1007/s00726-006-0425-8. [DOI] [PubMed] [Google Scholar]
- 23.Santos CX, Bonini MG, Augusto O. Role of the carbonate radical anion in tyrosine nitration and hydroxylation by peroxynitrite. Arch Biochem Biophys. 2000;377:146–152. doi: 10.1006/abbi.2000.1751. [DOI] [PubMed] [Google Scholar]
- 24.Bian K, Gao Z, Weisbrodt N, Murad F. The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci U S A. 2003;100:5712–5717. doi: 10.1073/pnas.0931291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation In vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Chen Y, Hazen SL. Eosinophil peroxidase nitrates protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem. 1999;274:25933–25944. doi: 10.1074/jbc.274.36.25933. [DOI] [PubMed] [Google Scholar]
- 27.Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys. 1999;371:169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 28.Abriata LA, Cassina A, Tortora V, Marin M, Souza JM, Castro L, Vila AJ, Radi R. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J Biol Chem. 2009;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallozzi C, Di Stasi AM, Minetti M. Peroxynitrite modulates tyrosine-dependent signal transduction pathway of human erythrocyte band 3. Faseb J. 1997;11:1281–1290. doi: 10.1096/fasebj.11.14.9409547. [DOI] [PubMed] [Google Scholar]
- 30.Robaszkiewicz A, Bartosz G, Soszynski M. N-chloroamino acids cause oxidative protein modifications in the erythrocyte membrane. Mech Ageing Dev. 2008;129:572–579. doi: 10.1016/j.mad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Velsor LW, Ballinger CA, Patel J, Postlethwait EM. Influence of epithelial lining fluid lipids on NO(2)-induced membrane oxidation and nitration. Free Radic Biol Med. 2003;34:720–733. doi: 10.1016/s0891-5849(02)01370-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan P, Tatarkova Z, Racay P, Lehotsky J, Pavlikova M, Dobrota D. Oxidative modifications of cardiac mitochondria and inhibition of cytochrome c oxidase activity by 4-hydroxynonenal. Redox Rep. 2007;12:211–218. doi: 10.1179/135100007X200308. [DOI] [PubMed] [Google Scholar]
- 33.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 34.Poderoso JJ. The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide. Arch Biochem Biophys. 2009;484:214–220. doi: 10.1016/j.abb.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow DJ, Schoneich C, Cohen RA. Detection of Sequence-Specific Tyrosine Nitration of Manganese SOD and SERCA in Cardiovascular Disease and Aging. AJP-Heart. 2006;290:2220–2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 36.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, Oram JF, Heinecke JW. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Milder JB, Freed CR. Transgenic mice overexpressing tyrosine-to-cysteine mutant human alpha-synuclein: a progressive neurodegenerative model of diffuse Lewy body disease. J Biol Chem. 2008;283:9863–9870. doi: 10.1074/jbc.M710232200. [DOI] [PubMed] [Google Scholar]
- 38.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by posttranslational modification of fibrinogen by reactive nitrogen species. J Biol Chem. 2004;279:8820–8826. doi: 10.1074/jbc.M306101200. [DOI] [PubMed] [Google Scholar]
- 39.Lizarbe TR, Garcia-Rama C, Tarin C, Saura M, Calvo E, Lopez JA, Lopez-Otin C, Folgueras AR, Lamas S, Zaragoza C. Nitric oxide elicits functional MMP-13 protein-tyrosine nitration during wound repair. FASEB J. 2008;22:3207–3215. doi: 10.1096/fj.07-103804. [DOI] [PubMed] [Google Scholar]
- 40.Moller MN, Li Q, Lancaster JR, Jr, Denicola A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life. 2007;59:243–248. doi: 10.1080/15216540701311147. [DOI] [PubMed] [Google Scholar]
- 41.Bartesaghi S, Valez V, Trujillo M, Peluffo G, Romero N, Zhang H, Kalyanaraman B, Radi R. Mechanistic Studies of Peroxynitrite-Mediated Tyrosine Nitration in Membranes Using the Hydrophobic Probe N-t-BOC-L-tyrosine tert-Butyl Ester. Biochemistry. 2006;45:6813–6825. doi: 10.1021/bi060363x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Joseph J, Feix J, Hogg N, Kalyanaraman B. Nitration and oxidation of a hydrophobic tyrosine probe by peroxynitrite in membranes: comparison with nitration and oxidation of tyrosine by peroxynitrite in aqueous solution. Biochemistry. 2001;40:7675–7686. doi: 10.1021/bi002958c. [DOI] [PubMed] [Google Scholar]
- 43.Romero N, Peluffo G, Bartesaghi S, Zhang H, Joseph J, Kalyanaraman B, Radi R. Incorporation of the hydrophobic probe N-t-BOC-L-tyrosine tert-butyl ester to red blood cell membranes to study peroxynitrite-dependent reactions. Chem Res Toxicol. 2007;20:1638–1648. doi: 10.1021/tx700142a. [DOI] [PubMed] [Google Scholar]
- 44.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 45.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (.OH/.O-) in Aqueous Solution. J Phys Chem Ref Data. 1988;17 [Google Scholar]
- 46.Prutz WA, Monig H, Butler J, Land EJ. Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch Biochem Biophys. 1985;243:125–134. doi: 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- 47.Pryor WA, Lightsey JW. Mechanisms of Nitrogen Dioxide Reactions: Initiation of Lipid Peroxidation and the Production of Nitrous Acid. Science. 1981;214:435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- 48.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 49.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005;338:668–676. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox AL, Marnett LJ. Polyunsaturated fatty acid alkoxyl radicals exist as carbon-centered epoxyallylic radicals: a key step in hydroperoxide-amplified lipid peroxidation. Chem Res Toxicol. 1993;6:413–416. doi: 10.1021/tx00034a003. [DOI] [PubMed] [Google Scholar]
- 51.Spiteller G. Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic Biol Med. 2006;41:362–387. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Malencik DA, Sprouse JF, Swanson CA, Anderson SR. Dityrosine: preparation, isolation, and analysis. Anal Biochem. 1996;242:202–213. doi: 10.1006/abio.1996.0454. [DOI] [PubMed] [Google Scholar]
- 53.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 54.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 56.Jiang ZY, Woollard AC, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991;26:853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H, Bhargava K, Keszler A, Feix J, Hogg N, Joseph J, Kalyanaraman B. Transmembrane nitration of hydrophobic tyrosyl peptides. Localization, characterization, mechanism of nitration, and biological implications. J Biol Chem. 2003;278:8969–8978. doi: 10.1074/jbc.M211561200. [DOI] [PubMed] [Google Scholar]
- 58.Barber DJW, Thomas JK. Reactions of radicals with lecithin bilayers. Radiation Research. 1978;74:51–65. [Google Scholar]
- 59.Feix JB, Kalyanaraman B. Spin trapping of lipid-derived radicals in liposomes. Biochim Biophys Acta. 1989;992:230–235. doi: 10.1016/0304-4165(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 60.Bartesaghi S, Peluffo G, Zhang H, Joseph J, Kalyanaraman B, Radi R. Tyrosine nitration, dimerization, and hydroxylation by peroxynitrite in membranes as studied by the hydrophobic probe N-t-BOC-l-tyrosine tert-butyl ester. Methods Enzymol. 2008;441:217–236. doi: 10.1016/S0076-6879(08)01212-3. [DOI] [PubMed] [Google Scholar]
- 61.Mossoba M, Makino K, Riesz P. Photoionization of aromatic amino acids in aqueous solutions. A spin-trapping and electron spin resonance study. J Phys Chem. 1982;86:3478–3483. [Google Scholar]
- 62.Goldstein S, Czapski G, Lind J, Merenyi G. Tyrosine nitration by simultaneous generation of (.)NO and O-(2) under physiological conditions. How the radicals do the job. J Biol Chem. 2000;275:3031–3036. doi: 10.1074/jbc.275.5.3031. [DOI] [PubMed] [Google Scholar]
- 63.Rubbo H, Denicola A, Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch Biochem Biophys. 1994;308:96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- 64.Kalyanaraman B, Mottley C, Mason RP. A direct electron spin resonance and spin-trapping investigation of peroxyl free radical formation by hematin/hydroperoxide systems. J Biol Chem. 1983;258:3855–3858. [PubMed] [Google Scholar]
- 65.El-Agamey A. Laser flash photolysis of new water-soluble peroxyl radical precursor. Journal of Photochemistry and Photobiology A: Chemistry. 2009;203:13–17. [Google Scholar]
- 66.Niki E, Saito T, Kawakami A, Kamiya Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J Biol Chem. 1984;259:4177–4182. [PubMed] [Google Scholar]
- 67.Davies MJ, Forni LG, Willson RL. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem J. 1988;255:513–522. [PMC free article] [PubMed] [Google Scholar]
- 68.Gebicki JM, Bielski BHJ. J Am Chem Soc. 1981;103:7020–7025. [Google Scholar]
- 69.Wayner DD, Burton GW, Ingold KU, Locke S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985;187:33–37. doi: 10.1016/0014-5793(85)81208-4. [DOI] [PubMed] [Google Scholar]
- 70.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 71.Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA. Nitric oxide reaction with lipid peroxyl radicals spares alphatocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/alpha-tocopherol than alpha-tocopherol/ascorbate. J Biol Chem. 2000;275:10812–10818. doi: 10.1074/jbc.275.15.10812. [DOI] [PubMed] [Google Scholar]
- 72.Erben-Russ M, Bors W, Saran M. Reactions of linoleic acid peroxyl radicals with phenolic antioxidants: a pulse radiolysis study. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:393–412. doi: 10.1080/09553008714551871. [DOI] [PubMed] [Google Scholar]
- 73.Pedrielli P, Pedulli GF, Skibsted LH. Antioxidant mechanism of flavonoids. Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. J Agric Food Chem. 2001;49:3034–3040. doi: 10.1021/jf010017g. [DOI] [PubMed] [Google Scholar]
- 74.Priyadarsini KI. Free radical reactions of curcumin in membrane models. Free Radic Biol Med. 1997;23:838–843. doi: 10.1016/s0891-5849(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 75.Priyadarsini KI, Guha SN, Rao MN. Physico-chemical properties and antioxidant activities of methoxy phenols. Free Radic Biol Med. 1998;24:933–941. doi: 10.1016/s0891-5849(97)00382-1. [DOI] [PubMed] [Google Scholar]
- 76.Amorati R, Catarzi F, Menichetti S, Pedulli GF, Viglianisi C. Effect of ortho-SR groups on O-H bond strength and H-atom donating ability of phenols: a possible role for the Tyr-Cys link in galactose oxidase active site? J Am Chem Soc. 2008;130:237–244. doi: 10.1021/ja075554h. [DOI] [PubMed] [Google Scholar]
- 77.Foti M, Ruberto G. Kinetic solvent effects on phenolic antioxidants determined by spectrophotometric measurements. J Agric Food Chem. 2001;49:342–348. doi: 10.1021/jf0006527. [DOI] [PubMed] [Google Scholar]
- 78.Valgimigli L, Banks JT, Lusztyk J, Ingold KU. Solvent Effects on the Antioxidant Activity of Vitamin E(1) J Org Chem. 1999;64:3381–3383. doi: 10.1021/jo982360z. [DOI] [PubMed] [Google Scholar]
- 79.Kapoor SK, Gopinathan C. Reactions of halogenated organic peroxyl radicals with various purine derivatives, tyrosine and thymine: a pulse radiolysis study. International of Chemical Kinetics. 1992;24:1035–1042. [Google Scholar]
- 80.Lide DR. Handbook of Chemistry and Physics. 71st ed ed. Boca Raton, FL: 1990. [Google Scholar]
- 81.Sackmann E, Trauble H, Galla H, Overath P. Lateral Diffusion, Protein Mobility and Phase Transitions in Escherichia coli Membranes. A Spin Label Study. Biochemistry. 1973;12:5360–5369. doi: 10.1021/bi00750a020. [DOI] [PubMed] [Google Scholar]
- 82.Vanderkooi J, Fischkoff S, Chance B, Cooper RA. Fluorescent probe analysis of the lipid architecture of natural and experimental cholesterol-rich membranes. Biochemistry. 1974;13:1589–1595. doi: 10.1021/bi00705a006. [DOI] [PubMed] [Google Scholar]
- 83.Moller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR, Jr, Denicola A. Membrane "lens" effect: focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol. 2007;20:709–714. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 84.Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005;280:8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 85.Hamilton RT, Asatryan L, Nilsen JT, Isas JM, Gallaher TK, Sawamura T, Hsiai TK. LDL protein nitration: implication for LDL protein unfolding. Arch Biochem Biophys. 2008;479:1–14. doi: 10.1016/j.abb.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stadler K, Bonini MG, Dallas S, Jiang J, Radi R, Mason RP, Kadiiska MB. Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic Biol Med. 2008;45:866–874. doi: 10.1016/j.freeradbiomed.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44:739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 88.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 89.Masuda T, Bando H, Maekawa T, Takeda Y, Yamaguchi H. A Novel Radical Terminated Compound Produced in the Antioxidation Process of Curcumin Against Oxidation of a Fatty Acid Ester. Tetrahedron Lett. 2000;41:2157–2160. [Google Scholar]
- 90.Solar S, Solar W, Getoff N. Reactivity of OH with tyrosine in aqueous solution studie by pulse radiolysis. J Phys Chem. 1984;88:2091–2095. [Google Scholar]
- 91.Jin F, Leitich J, Sonntag Cv. J Chem Soc Perkin Trans. 1993;2:1583–1588. [Google Scholar]
- 92.Babbs CF, Steiner MG. Simulation of free radical reactions in biology and medicine: a new two-compartment kinetic model of intracellular lipid peroxidation. Free Radic Biol Med. 1990;8:471–485. doi: 10.1016/0891-5849(90)90060-v. [DOI] [PubMed] [Google Scholar]