Abstract
We aimed to investigate the correlations between lymphangiogenesis, cyclooxygenase (COX)-2 and vascular endothelial growth factor-C (VEGF-C) in non-Hodgkin’s lymphoma (NHL) and their associations with the clinical parameters of patients. A total of 75 patients diagnosed with NHL were enrolled in this study. Of the 75 patients, 14 (18.7%) had low-grade and 61 (81.3%) had aggressive lymphoma. We examined the immunohistochemical expression of COX-2 and VEGF-C and estimated lymphangiogenesis by counting lymphatic vessels expressing lymph vessel endothelial hyaluronan receptor-1 (LYVE-1). Our results showed that lymphatic vessel density (LVD) was positive in 59.0% of the cases with aggressive morphology, while the LVD positive rate of indolent lymphoma was only 28.6% (P=0.039). Both COX-2 and VEGF-C were correlated with lymphangiogenesis (P=0.030 and 0.000, respectively). The expression levels of COX-2 and VEGF-C were significantly correlated (P=0.015). LVD, COX-2 and VEGF-C were not correlated with the gender, age, lactate dehydrogenase (LDH) levels, β2 microglobulin (β2M) levels, extranodal involvement, disease stage, B symptoms or international prognostic index of the patients. In conclusion, lymphangiogenesis was correlated with aggressive histology in NHL. COX-2 and VEGF-C are inducers of lymphangiogenesis and their expression levels were correlated in NHL.
Keywords: lymphangiogenesis, cyclooxygenase-2, vascular endothelial growth factor-C, non-Hodgkin’s lymphoma
Introduction
Lymphangiogenesis, the formation of new lymphatic vessels, plays a critical role in the systemic metastasis of malignant tumors. It is a complicated process tightly regulated by a variety of direct and indirect growth factors (1,2). With the discovery of antibodies against lymphatic endothelial cells in the past decade, lymphangiogenesis has attracted increasing interest and become a new area of cancer metastasis research. Lymphangiogenesis has been demonstrated to be associated with lymph node metastasis and negative prognosis in various types of cancer (3,4). Lymphoma cells invade the lymph nodes through the lymphatic vessels. However, few studies have been performed on lymphangiogenesis and related factors in lymphoma.
Vascular endothelial growth factor-C (VEGF-C) is a potent inducer of lymphangiogenesis. It is essential in the formation of the first lymphatic vessel during embryonic development (5) and in mediating cancer metastasis through lymphatic vessels (6). VEGF-C promotes lymphangiogenesis by binding to VEGFR-3, which is expressed on the lymphatic endothelial cells of adults (6). Cyclooxygenase (COX) plays an important role in the conversion of arachidonic acid to prostaglandin. It has two isoforms, COX-1 and COX-2. COX-1 maintains the homeostatic level of prostaglandin (7) and COX-2 is induced by mitogenic or inflammatory stimuli, including cytokines, growth factors and tumor promoters (8). Increasing evidence suggests that COX-2 participates in carcinogenesis and cancer progression. COX-2 functions through activating carcinogens, inhibiting apoptosis, promoting angiogenesis, modulating immunological responses and influencing tumor invasion by activation of matrix metalloproteinases (MMPs) (9). The correlation between COX-2 and tumor lymphangiogenesis has been discovered in breast (10), gastric (11), prostate (12) and lung cancer (13), suggesting that COX-2 plays a role in tumor lymphangiogenesis. However, whether COX-2 expression is correlated with lymphangiogenesis in lymphoma has not been reported.
The aims of the current study were to i) investigate the expression of COX-2 and VEGF-C and lymphangiogenesis in non-Hodgkin’s lymphoma (NHL) and their correlations with the clinical features of patients and ii) investigate the correlations among COX-2, VEGF-C and lymphangiogenesis in NHL.
Materials and methods
Patients and samples
Formalin-fixed and paraffin-embedded tissue blocks of 75 patients diagnosed with NHL in Qingdao Municipal Hospital (Qingdao, China) between 2002 and 2010 were used in the current study. The NHL group included 46 males and 29 females. The median age was 60.0 years (range, 17–86). The patients’ conditions were staged according to the Ann Arbor staging system. Samples were classified according to World Health Organization classification of lymphomas. Samples were further grouped according to the aggressiveness of the NHL subtypes. There were 14 cases of indolent lymphoma (8 cases of small lymphocytic lymphoma, 1 case of mucosa-associated lymphoid tissue lymphoma, 2 cases of follicular lymphoma and 3 cases of lymphoplasmacytic lymphoma), 57 cases of aggressive lymphoma (56 cases of diffuse large B-cell lymphoma and 1 case of extranodal NK/T-cell lymphoma, nasal type) and 4 cases of very aggressive lymphoma (3 cases of Burkitt lymphoma and 1 case of T-lymphoblastic lymphoma). Patients with aggressive or very aggressive lymphoma were grouped as aggressive lymphoma in the following analyses. Informed consent was obtained from each patient or the patient’s family for use of the samples. This study was approved by the local Ethics Committee.
Immunohistochemical staining and assessment
A total of 75 NHL samples were examined for the expression of lymph vessel endothelial hyaluronan receptor-1 (LYVE-1), VEGF-C and COX-2. Briefly, sections of 4-μm thickness were cut from paraffin blocks and mounted on slides coated with 3-aminopropyl-triethoxysilane (Maxin-bio, Fuzhou, China). The slides were dewaxed in xylene and rehydrated through a graded ethanol series. Antigen retrieval was performed by microwave treatment at 98°C for 10 min in citric buffer (pH 6.0) for LYVE-1 and VEGF-C detection or EDTA (pH 9.0) for COX-2 detection. The slides were then cooled at room temperature for 30 min. Endogenous peroxidase activity was blocked by immersion in 3% hydrogen peroxide for 10 min at room temperature. This was followed by addition of rabbit anti-COX-2 monoclonal antibody (Maxin-bio, Fuzhou, China) at a dilution of 1:200, rabbit anti-LYVE-1 polyclonal antibody (Abcam Ltd., Cambridge, UK) at a dilution of 1:200 or rabbit anti-VEGF-C polyclonal antibody (Zhongshan Goldbridge Biotech, Beijing, China) at a dilution of 1:200. The slides were then incubated overnight at 4°C. The secondary antibody of the Maxvision system, peroxidase-labeled polymer conjugated to anti-rabbit/mouse immunoglobulin antibody (Maxin-bio), was then applied and incubated at room temperature for 15 min. Subsequently, the chromogenic substrate 3,3′-diaminobezidine tetrahydrochloride (DAB, Maxin-bio) was added. Finally, the slides were briefly counterstained with hematoxylin. PBS was used as a negative control in place of the primary antibody.
COX-2 staining was considered to be positive when >20% of the lymphoma cells showed positive cytoplasmic staining. The definition of positive staining of VEGF-C was immunore-activity in ≥10% of the lymphoma cells.
VEGF-C expression was evaluated using a scoring system based on staining intensity and percentage of positive lymphoma cells. The staining intensity was classified into four categories: 0, negative; 1, weak; 2, moderate; 3, strong. The score of a slide was calculated by multiplying the staining intensity by the percentage of positive lymphoma cells. The median score was used as the cut-off to divide samples into high and low VEGF-C expression groups.
Lymphatic vessel density (LVD) was determined using Weidner’s method (14) with minor modifications. Any brown-stained endothelial cell or cell cluster clearly separated from the adjacent cells, tissue elements and microvessels was considered to be a lymph vessel. The sections were first scanned at ×100 magnification to identify two regions with the greatest number of distinct brown staining regions (hot spots). The two hot spots were then counted at ×200 magnification with a microscope ocular grid equal to 0.24 mm2 of the examination area. The median LVD was used as a cut-off to separate the NHL cases into negative and positive groups. The slides were reviewed independently by two investigators blinded to clinical details.
Statistical analysis
The Chi-square test was applied to analyze the correlations among LVD, COX-2 and VEGF-C and their associations with the clinical features of the patients. P<0.05 was considered to indicate a statistically significant result.
Results
Expression of LYVE-1 and its correlation with clinical variables
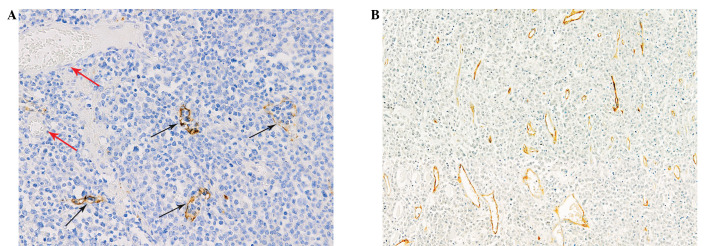
The lymphatic vessels were lined with a single layer of LYVE-1-positive endothelial cells, while blood vessels containing red blood cells were negative for LYVE-1 staining (Fig. 1A). Lymphatic vessels with open lumina and lymphatic endothelial cells or cell clusters without a lumen structure were observed (Fig. 1B). The lymphatic vessels were distributed unevenly throughout the sections investigated. The median LVD in this study was 5.5 (range, 0–36). There were 40 (53.3%) cases of NHL with a LVD counting greater than or equal to the median. LVD was positive in 59.0% of the cases with aggressive morphology, while the LVD positive rate of indolent lymphoma was only 28.6% (P=0.039). The 5 cases with LVD >25 were all of aggressive histology. The LVD showed no correlation with the clinical parameters, including gender, age, lactate dehydrogenase (LDH) levels, β2 microglobulin (β2M) levels, extranodal involvement, disease stage, B symptoms and international prognostic index (IPI; Table I).
Figure 1.
LYVE-1-stained lymphatic vessels in DLBCL. (A) Lymphatic vessels showed positive staining (black arrows) while blood vessels (red arrows) containing erythrocytes were negative. Original magnification, ×200. (B) Lymphatic vessels with open lumina and linear lymphatic vessels without lumen structure were both positive for LYVE-1. Original magnification, ×100. LYVE-1, lymph vessel endothelial hyaluronan receptor-1; DLBCL, diffuse large B-cell lymphoma.
Table I.
Correlation of LVD, COX-2 and VEGF-C with clinical parameters in NHL.
| Clinical parameters | n | LVD
|
P-value | COX-2
|
P-value | VEGF-C
|
P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | High | Low | |||||
| Age (years) | ||||||||||
| <60 | 36 | 17 | 19 | 0.308 | 17 | 19 | 0.308 | 17 | 19 | 0.725 |
| ≥60 | 39 | 23 | 16 | 23 | 16 | 20 | 19 | |||
| Gender | ||||||||||
| Male | 46 | 23 | 23 | 0.466 | 24 | 22 | 0.800 | 23 | 23 | 0.884 |
| Female | 29 | 17 | 12 | 16 | 13 | 14 | 15 | |||
| LDH | ||||||||||
| Normal | 18 | 9 | 9 | 0.637 | 10 | 8 | 0.930 | 9 | 9 | 0.754 |
| High | 46 | 26 | 20 | 25 | 21 | 25 | 21 | |||
| β2M | ||||||||||
| Normal | 20 | 12 | 8 | 0.353 | 13 | 7 | 0.203 | 9 | 11 | 0.406 |
| High | 28 | 13 | 15 | 13 | 15 | 16 | 12 | |||
| En inv | ||||||||||
| No | 31 | 16 | 15 | 0.777 | 16 | 15 | 0.777 | 19 | 12 | 0.116 |
| Yes | 40 | 22 | 18 | 22 | 18 | 17 | 23 | |||
| Stagea | ||||||||||
| I | 15 | 7 | 8 | 0.737 | 7 | 8 | 0.517 | 5 | 10 | 0.383 |
| II | 18 | 9 | 9 | 7 | 11 | 9 | 9 | |||
| III | 22 | 11 | 11 | 11 | 11 | 10 | 12 | |||
| IV | 12 | 8 | 4 | 8 | 4 | 8 | 4 | |||
| B symptoms | ||||||||||
| No | 35 | 20 | 15 | 0.893 | 20 | 15 | 0.712 | 16 | 19 | 0.407 |
| Yes | 36 | 20 | 16 | 19 | 17 | 20 | 16 | |||
| IPI | ||||||||||
| 0–2 | 46 | 27 | 19 | 0.301 | 25 | 21 | 0.571 | 26 | 20 | 0.378 |
| 3–5 | 16 | 7 | 9 | 10 | 6 | 7 | 9 | |||
| Histology | ||||||||||
| Indolent | 14 | 4 | 10 | 0.039b | 5 | 9 | 0.143 | 4 | 10 | 0.085c |
| Aggressive | 61 | 36 | 25 | 35 | 26 | 33 | 28 | |||
COX-2, cyclooxygenase-2; VEGF-C, vascular endothelial growth factor-C; NHL, non-Hodgkin’s lymphoma.
Ann Arbor staging system. LVD, lymphatic vessel density; LDH, lactate dehydrogenase; β2M, β2 microglobulin; En inv, extranodal involvement; IPI, international prognostic index.
P<0.05;
0.05<P<0.1.
Expression of COX-2 and its correlation with clinical variables
Positive expression of COX-2 was observed in 40 (53.3%) of the NHL samples. COX-2 was expressed in the cytoplasm of lymphoma cells (Fig. 2). In addition, inflammatory cells, especially plasma cells, were also positive for COX-2. COX-2 expression was not correlated with the gender, age, LDH levels, β2M levels, extranodal involvement, disease stage, B symptoms or IPI of the patients (Table I). COX-2 expression showed no difference between the indolent and aggressive subtypes of NHL.
Figure 2.
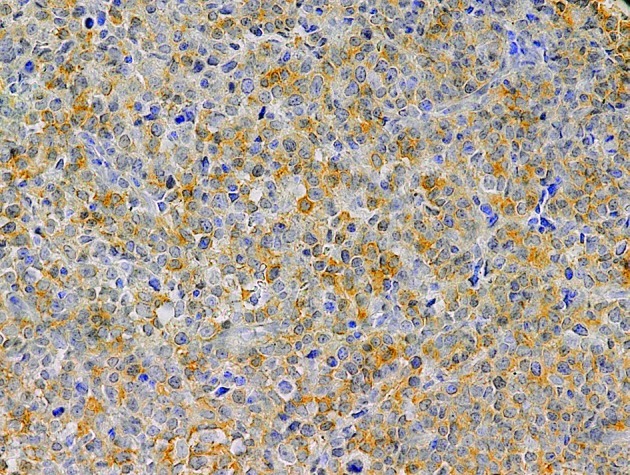
COX-2 expression in DLBCL. Original magnification, ×200. COX-2, cyclooxygenase-2; DLBCL, diffuse large B-cell lymphoma.
Expression of VEGF-C and its correlation with clinical variables
Positive VEGF-C staining was observed in 72 (96%) of the NHL samples. VEGF-C was expressed in the cytoplasm of lymphoma cells (Fig. 3). A total of 37 (49.3%) cases were graded as showing high expression and 38 (50.7%) cases were graded as low expression of VEGF-C. In addition to lymphoma cells, macrophages and stromal cells positively stained for VEGF-C were also observed. VEGF-C expression showed a trend of association with the morphology of NHL (P=0.085), with VEGF-C high expression rates of 54.1% in aggressive lymphoma and 40% in indolent lymphoma. VEGF-C expression was not correlated with the gender, age, LDH levels, β2M levels, extranodal involvement, disease stage, B symptoms, or IPI of the patients (Table I).
Figure 3.
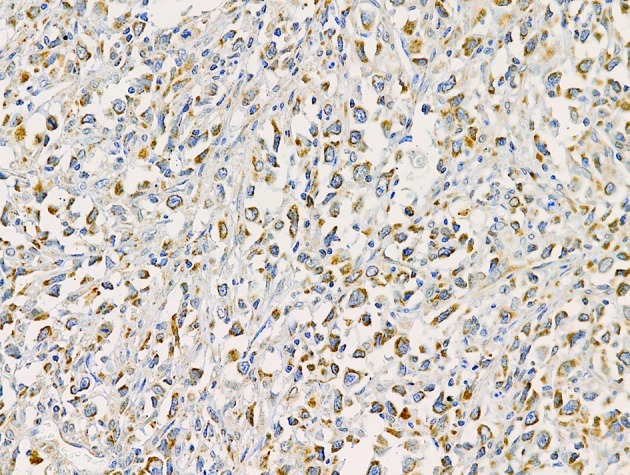
VEGF-C expression in DLBCL. Original magnification, ×200. VEGF-C, vascular endothelial growth factor-C; DLBCL, diffuse large B-cell lymphoma.
Correlation of COX-2, VEGF-C and LVD
LVD was positive in 65% of cases in the COX-2-positive group, whereas the LVD positive rate in the COX-2-negative group was only 40% (P=0.030). The LVD positive rates in the VEGF-C high and low expression groups were 75.7 and 31.6%, respectively (P=0.000; Table II). The expression levels of VEGF-C and COX-2 were significantly correlated in NHL (P=0.015; Table III).
Table II.
Correlation of LVD and COX-2 or VEGF-C in NHL.
P<0.05. LVD, lymphatic vessel density; COX-2, cyclooxygenase-2; VEGF-C, vascular endothelial growth factor-C; NHL, non-Hodgkin’s lymphoma.
Table III.
Correlation between COX-2 and VEGF-C in NHL.
| n | COX-2
|
P-value | ||
|---|---|---|---|---|
| + | − | |||
| VEGF-C | ||||
| + | 37 | 25 | 12 | 0.015a |
| − | 38 | 15 | 23 | |
P<0.05. COX-2, cyclooxygenase-2; VEGF-C, vascular endothelial growth factor-C; NHL, non-Hodgkin’s lymphoma.
Discussion
To date, the lymphangiogenesis in lymphoma has only been investigated by two studies. Kadowaki et al showed that LVD in lymphoma was significantly higher than that in non-reactive normal lymph node (15). Wróel et al showed that lymphatic sinus density (LSD) was not different between aggressive and indolent lymphomas (16). The mean LSD in reactive lymph nodes was found to be significantly higher than that in NHL (16). The correlation between VEGF-C and LVD (LSD) was demonstrated in both studies. In the two studies, lymphatic vessels with lumen structure were identified as lymphatic vessels. However, when LVD and microvessel density (MVD) were evaluated in solid tumors (13,14,17,18) and MVD assessed in lymphomas (19,20), lymphatic vessels were usually quantitated by counting endothelial cells or cell clusters clearly separated from the adjacent cells, tissue elements and microvessels regardless of whether a lumen structure was observed. In adults, new lymphatic vessels form primarily by sprouting from pre-existing vessels (21). Detry et al (22) disclosed the process of lymphangiogenesis in adults using in vivo (lymphangiogenic corneal assay and lymphangioma) and in vitro (lymphatic ring assay) models. The authors showed that the lymphatic endothelial cells migrate and organize into a cord-like structure. Subsequently, the matrix degrades to generate space and a lumen forms in the intercellular space (22). This suggests that a lumen structure may not form in the initial stage of adult lymphangiogenesis. In addition, it is possible that only lymphatic endothelial cells could be observed even when a lumen structure existed, since only a single slide, not a series of slides, was used to investigate LVD. Therefore, we calculated LVD by counting LYVE-1 brown-stained endothelial cells or cell clusters regardless of whether a lumen structure was observed. We showed that the LVD positive rate in aggressive lymphoma was higher than that in indolent lymphoma. However, no difference in LSD was identified between these two histological subtypes in the study by Wróel et al (16). The difference in definition of lymphatic vessels between the current study and that by Wróel et al may be the reason for the discrepant results obtained by the two studies. This contradiction suggests that there are more immature lymphatic vessels in aggressive than indolent lymphomas. Our results also indicate that lymphangiogenesis plays a role in the progression of NHL.
The correlation between COX-2 and lymphangiogenesis has been demonstrated in a variety of types of cancer (10–13). However, other studies failed to demonstrate this correlation (23,24). Lymphangiogenesis is a complicated multi-step process regulated by a group of growth factors, cytokines and chemokines (1). In addition to VEGF-C, other factors, including VEGF-A, angioprotein-1, hepatocyte growth factor (HGF), insulin-like growth factor 1 and 2 (IGF-1 and -2) and platelet-derived growth factors (PDGFs), may also promote lymphangiogenesis (25). Although the correlation between COX-2 and VEGF-C has been studied in diffuse large B-cell lymphoma (DLBCL) (26), the correlation between COX-2 and lymphangiogenesis in lymphoma is yet to be elucidated. To the best of our knowledge, this is the first study to demonstrate that the expression of COX-2 is significantly correlated with LVD in NHL. This suggests that COX-2 is an inducer of lymphangiogenesis and implies that lymphoma cells induce local lymphangiogenesis. In this study, the expression of VEGF-C was also associated with LVD. This is in agreement with the results of previous studies (15,16) which estimated LVD by counting lymphatic vessels with lumen structure. In this study, we counted lymphatic vessels regardless of whether a lumen structure was observed. Therefore, our results demonstrate that VEGF-C induces lymphangiogenesis in lymphoma from a different angle from the previous studies.
In the current study, VEGF-C expression showed a trend of association with aggressive histology but was not correlated with any of the clinical features investigated. VEGF-C has been shown to be correlated with prognosis but not with histology or any clinical features in NHL (27) and DLBCL (28). However, Ganjoo et al revealed that VEGF-C is correlated with LDH and IPI score, but not with progression-free or overall survival in DLBCL (29). The correlation between COX-2 expression and clinical features is also inconsistent in NHL. COX-2 expression was shown to be correlated with aggressive histology by Paydas et al (30) whereas the study by Hazar et al revealed that COX-2 was correlated with disease stage (31). In the present study, COX-2 expression was not found to be correlated with any of the clinical features investigated. Variations in patients’ characteristics, immunostaining methods or biological heterogeneity among individuals from different countries may be responsible for the discrepancies. More studies are needed to decide the true roles of VEGF-C and COX-2 in lymphomas.
In the present study, the expression levels of COX-2 and VEGF-C were correlated in NHL. However, no correlation between COX-2 and VEGF-C was identified in DLBCL in the study by Paydas et al (26). Staining for VEGF-C was positive in 96% of the samples investigated in the current study. This is in agreement with the positive rates in the studies by Kadowaki et al, Pazgal et al and Ganjoo et al, which were 92.3, 100 and 98%, respectively (15,28,29). However, the VEGF-C positive rate was 36.4% in the study by Paydas et al (26). The apparent discrepant positive rates may be the reason for the contradicting results of the correlation between COX-2 and VEGF-C obtained by the two studies. The results of the current study are in agreement with those of studies on esophageal (32,33), head and neck (34), gastric (11), prostate (12) and lung carcinoma (13). The correlation between COX-2 and VEGF-C may be due to the expression of the two genes being co-regulated or the expression of one gene being regulated by the other. Using a human lung adenocarcinoma cell line (CL1.0) transfected with a COX-2 expression system, Su et al demonstrated that COX-2 regulated the expression of VEGF-C in lung adeno-carcinoma (35). A similar regulatory function of COX-2 has also been observed in breast cancer cells (10,36). Therefore, the results in this study suggest that a lymphangiogenic pathway, i.e. COX-2 upregulating VEGF-C expression, may exist in NHL.
In conclusion, lymphangiogenesis is correlated with aggressive histology in NHL. COX-2 and VEGF-C are inducers of lymphangiogenesis and their expression levels are correlated in NHL.
Acknowledgments
We are grateful for Dan Xie and Ping Ji for their assistance in preparing slides.
References
- 1.Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insight into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25:677–694. doi: 10.1007/s10555-006-9026-y. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. Biomed Pharmacother. 2007;61:534–539. doi: 10.1016/j.biopha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Raica M, Ribatti D. Targeting tumor lymphangiogenesis: an update. Curr Med Chem. 2010;17:698–708. doi: 10.2174/092986710790514471. [DOI] [PubMed] [Google Scholar]
- 4.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 6.Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 7.Hla T, Bishop-Bailey D, Liu CH, Schaefers HJ, Trifan OC. Cyclooxygenase-1 and cyclooxygenase-2 isoenzymes. Int J Biochem Cell Biol. 1999;31:551–557. doi: 10.1016/s1357-2725(98)00152-6. [DOI] [PubMed] [Google Scholar]
- 8.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh N, Chaki R, Mandal V, Mandal S. Cox-2 as a target for cancer chemotherapy. Pharmacol Rep. 2010;62:233–244. doi: 10.1016/s1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharjee RN, Timoshenko AV, Cai J, Lala PK. Relationship between cyclooxygenase-2 and human epidermal growth factor receptor 2 in vascular endothelial growth factor C up-regulation and lymphangiogenesis in human breast cancer. Cancer Sci. 2010;101:2026–2032. doi: 10.1111/j.1349-7006.2010.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Ji J, Yuan F, Zhu L, Yan C, Yu YY, Liu BY, Zhu ZG, Lin YZ. Cyclooxygenase-2 expression is associated with VEGF-C and lymph node metastases in gastric cancer patients. Biomed Pharmacother. 2005;59(Suppl 2):S285–S288. doi: 10.1016/s0753-3322(05)80047-2. [DOI] [PubMed] [Google Scholar]
- 12.Di JM, Zhou J, Zhou XL, Gao X, Shao CQ, Pang J, Sun QP, Zhang Y, Ruan XX. Cyclooxygenase-2 expression is associated with vascular endothelial growth factor-C and lymph node metastasis in human prostate cancer. Arch Med Res. 2009;40:268–275. doi: 10.1016/j.arcmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Chen Y, Xu Z, Xu Z, Qian Y, Yu X. Prognostic significance of VEGF-C expression in correlation with COX-2, lymphatic microvessel density, and clinicopathologic characteristics in human non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2009;41:217–222. doi: 10.1093/abbs/gmp004. [DOI] [PubMed] [Google Scholar]
- 14.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki I, Ichinohasama R, Harigae H, Ishizawa K, Okitsu Y, Kameoka J, Sasaki T. Accelerated lymphangiogenesis in malignant lymphoma: possible role of VEGF-A and VEGF-C. Br J Haematol. 2005;130:869–877. doi: 10.1111/j.1365-2141.2005.05695.x. [DOI] [PubMed] [Google Scholar]
- 16.Wróel T, Mazur G, Dziegiel P, Jeleń M, Szuba A, Kuliczkowski K, Zabel M. Density of intranodal lymphatics and VEGF-C expression in B-cell lymphoma and reactive lymph nodes. Folia Histochem Cytobiol. 2006;44:43–47. [PubMed] [Google Scholar]
- 17.Mineo TC, Ambrogi V, Baldi A, Rabitti C, Bollero P, Vincenzi B, Tonini G. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumor vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M, Ma SP, Lin HZ, Ji P, Xie D, Yu JX. Intratumoral as well as peritumoral lymphatic vessel invasion correlates with lymph node metastasis and unfavorable outcome in colorectal cancer. Clin Exp Metastasis. 2010;27:123–132. doi: 10.1007/s10585-010-9309-0. [DOI] [PubMed] [Google Scholar]
- 19.Citak EC, Oguz A, Karadeniz C, Akyurek N. Immunohistochemical expression of angiogenic cytokines in childhood Hodgkin lymphoma. Pathol Res Pract. 2008;204:89–96. doi: 10.1016/j.prp.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Gratzinger D, Zhao S, Marinelli RJ, Kapp AV, Tibshirani RJ, Hammer AS, Hamilton-Dutoit S, Natkunam Y. Microvessel density and expression of vascular endothelial growth factor and its receptors in diffuse large B-cell lymphoma subtypes. Am J Pathol. 2007;170:1362–1369. doi: 10.2353/ajpath.2007.060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holopainen T, Bry M, Alitalo K, Saaristo A. Perspective on lymphangiogenesis and angiogenesis in cancer. J Surg Oncol. 2011;103:484–488. doi: 10.1002/jso.21808. [DOI] [PubMed] [Google Scholar]
- 22.Detry B, Bruyère F, Erpicum C, Paupert J, Lamaye F, Maillard C, Lenoir B, Foidart J, Thiry M, Noël A. Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol. 2011;12:29–39. doi: 10.1186/1471-2121-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longatto-Filho A, Pinheiro C, Pereira SM, Etlinger D, Moreira MA, Jubé LF, Queiroz GS, Baltazar F, Schmitt FC. Lymphatic vessel density and epithelial D2-40 immunoreactivity in pre-invasive and invasive lesions of the uterine cervix. Gynecol Oncol. 2007;107:45–51. doi: 10.1016/j.ygyno.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Gou HF, Chen XC, Zhu J, Jiang M, Yang Y, Cao D, Hou M. Expression of COX-2 and VFGF-C in gastric cancer: correlations with lymphangiogenesis and prognostic implications. J Exp Clin Cancer Res. 2011;30:14–21. doi: 10.1186/1756-9966-30-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13:1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paydas S, Ergin M, Seydaoglu G, Erdogan S, Yavuz S. Prognostic significance of angiogenic/lymphangiogenic, anti-apoptotic, inflammatory and viral factors in 88 cases with diffuse large B cell lymphoma and review of the literature. Leuk Res. 2009;33:1627–1635. doi: 10.1016/j.leukres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Paydas S, Seydaoglu G, Ergin M, Erdogan S, Yavuz S. The prognostic significance of VEGF-C and VEGF-A in non-Hodgkin lymphomas. Leuk Lymphoma. 2009;50:366–373. doi: 10.1080/10428190802706665. [DOI] [PubMed] [Google Scholar]
- 28.Pazgal I, Boycov O, Shpilberg O, Okon E, Bairey O. Expression of VEGF-C, VEGF-D and their receptor VEGFR-3 in diffuse large B-cell lymphomas. Leuk Lymphoma. 2007;48:2213–2220. doi: 10.1080/10428190701632822. [DOI] [PubMed] [Google Scholar]
- 29.Ganjoo KN, Moore AM, Orazi A, Sen JA, Johnson CS, An CS. The importance of angiogenesis markers in the outcome of patients with diffuse large B cell lymphoma: a retrospective study of 97 patients. J Cancer Res Clin Oncol. 2008;134:381–387. doi: 10.1007/s00432-007-0294-x. [DOI] [PubMed] [Google Scholar]
- 30.Paydas S, Ergin M, Erdogan S, Seydaoglu G. Cyclooxygenase-2 expression in non-Hodgkin’s lymphomas. Leuk Lymphoma. 2007;48:389–395. doi: 10.1080/10428190601059787. [DOI] [PubMed] [Google Scholar]
- 31.Hazar B, Ergin M, Seyrek E, Erdoğan S, Tuncer I, Hakverdi S. Cyclooxygenase-2 (Cox-2) expression in lymphomas. Leuk Lymphoma. 2004;45:1395–1399. doi: 10.1080/10428190310001654032. [DOI] [PubMed] [Google Scholar]
- 32.Von Rahden BHA, Stein HJ, Puhringer F, Koch I, Langer R, Piontek G, Siewert JR, Hofler H, Sarbia M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038–5044. doi: 10.1158/0008-5472.CAN-04-1107. [DOI] [PubMed] [Google Scholar]
- 33.Byeon JS, Jung HY, Lee YJ, Lee D, Lee GH, Myung SJ, Yang SK, Hong WS, Kim JH, Min YI, Kim JS. Clinicopathological significance of vascular endothelial growth factor-C and cyclooxygenase-2 in esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2004;19:648–654. doi: 10.1111/j.1440-1746.2004.03348.x. [DOI] [PubMed] [Google Scholar]
- 34.Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005;18:153–160. doi: 10.1038/modpathol.3800244. [DOI] [PubMed] [Google Scholar]
- 35.Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, Wei LH, Yang PC, Kuo ML. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- 36.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]