Abstract
The family with sequence similarity 83, member D (Fam83D) encodes a mitotic spindle-associated protein. Its knockdown results in shorter spindles that fail to organize a correct metaphase plate. In this study, we demonstrated that Fam83D is coexpressed with well-known mitotic genes. Pathway analysis results also showed that cell cycle- and mitosis-related pathways are enriched with Fam83D-coexpressed genes. Furthermore, Fam83D is differentially expressed in various types of cancers. The results presented in this study suggest that Fam83D may be an important molecule for mitotic progression and equal segregation of chromosomes. Since the molecules that are involved in these mechanisms are crucial for mitosis as well as carcinogenesis, Fam83D should be considered as a novel regulator of mitosis and a putative carcinogenesis-related gene.
Keywords: Fam83D, Oncomine, coexpression, gene ontology, in silico
Introduction
The family with sequence similarity 83, member D (Fam83D, also known as CHICA) is located on chromosome 20 of the human genome (1). Fam83D contains an uncharacterized DUF1669 domain in the N terminus. The members of this domain family are found in all eukaryotes and are composed of sequences derived from hypothetical eukaryotic proteins of unknown function. Some members of this domain family are noted as being potential phospholipases, but no evidence from literature or sequence analysis was found to support this (2). Fam83D was identified as a putative mitotic spindle component in a mass spectrometry study (3). Furthermore, another study revealed that although Fam83D is primarily found in the cytoplasm during interphase, during prophase it associates with spindle microtubules, on which it remains throughout metaphase and anaphase (4). The same article also revealed that Fam83D is an interaction partner of chromokinesin KID, which is required for the generation of polar ejection forces and chromosome congression, and has roles in organizing the metaphase plate (4).
As all the mitotic spindle-associated proteins are involved in the control and regulation of cell proliferation, as well as in carcinogenesis, we further investigated Fam83D using in silico tools. Our results revealed that Fam83D is coexpressed with important mitosis-related genes, including Aurora-A, Aurora-B, Plk-1, Plk-4, Cdc20, Cdk1, Nek2, Geminin and CENP family members. All these molecules are well-known genes that have crucial roles in different stages of mitosis, from equal segregation of chromosomes to production of daughter cells. Therefore, we speculate that Fam83D is involved in mitotic processes to regulate cell division. Moreover, our results also demonstrated that this gene is differentially expressed in various cancers in concordance with the previously mentioned coexpression partners.
This is the first study concerning the correlation between Fam83D and cancer. It is well-known that differentially expressed genes in cancers are candidates for diagnostic and prognostic approaches. Therefore, this article suggests that Fam83D is a strong candidate for prognostic and diagnostic approaches and should be investigated further.
Materials and methods
Meta-analysis of Fam83D
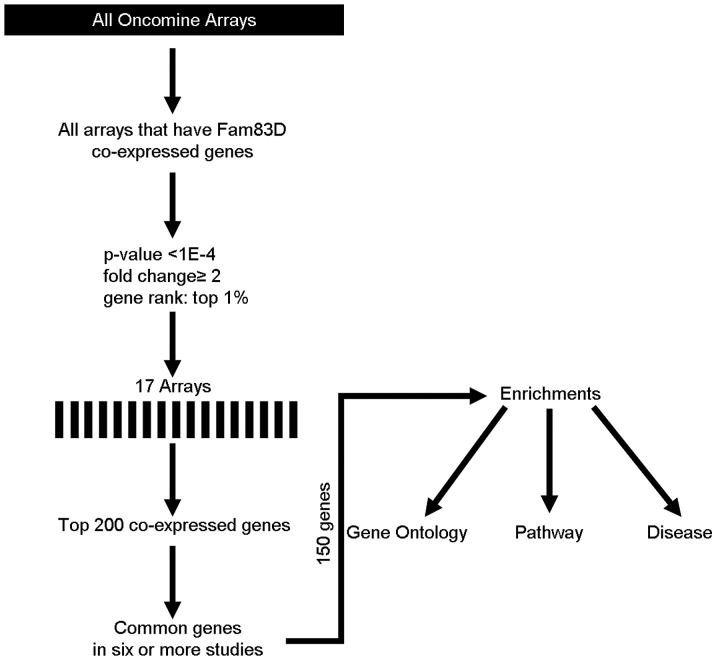
To understand the function of Fam83D, coexpression analysis was performed using the Oncomine database (http://oncomine.org) as previously described (5,6), but with minor modifications. The threshold was adjusted to P-value <1E-4; fold-change, 2 and gene rank, top 1%. Seventeen different arrays fulfilled these criteria (Table I) and the top 200 coexpressed genes were extracted and filtered to give one representative gene per study (removing duplicates and partial expressed sequence tags). These filtered gene lists were then compared to search for repeatedly coexpressed genes over multiple studies. The frequency cut-off was 6 studies (>30% of 17 studies). This generated a meta-analysis list for Fam83D. The web-based Database for Annotation, Visualization and Integrated Discovery (DAVID; http://david.abcc.ncifcrf.gov) was used to assess enriched gene ontology terms within the gene lists produced by the coexpression data analysis (7,8). The results were corrected for multiple testing using the Benjamini and Hochberg false discovery rate (FDR) correction.
Table I.
Arrays used in coexpression analysis.
| No. | Array name |
|---|---|
| 1 | Lingren Bladder |
| 2 | Lee Brain |
| 3 | Bittner Breast |
| 4 | Richardson Breast 2 |
| 5 | Meyniel Ovarian |
| 6 | Lu Breast |
| 7 | HAO Esophagus |
| 8 | Anglesio Ovarian |
| 9 | Bittner Multicancer |
| 10 | Janoueix-Lerosey Brain |
| 11 | Lee Brain 2 |
| 12 | Skrzypczak Colorectal 2 |
| 13 | Ma Breast 2 |
| 14 | Giordano Adrenal 2 |
| 15 | Yang Renal |
| 16 | Loi Breast 3 |
| 17 | Bittner Thyroid |
Correlation between Fam83D and cancer
The oncomine cancer microarray database was used to study gene expression of Fam83D in various tumor types and in their normal control tissues. Only the gene transcriptome data from the same study, generated with the same methodology, were used. All gene expression data were log-transformed, median-centered per array, and standard deviation was normalized to one per array (9). Student’s t-test was used for differential expression analysis, and only studies with P-value less than 1E-4 and fold-change greater than two were considered.
Results
Fam83D is coexpressed with genes involved in mitosis
Using the Oncomine cancer microarray database Fam83D was searched for coexpressed genes. Fig. 1 indicates the methodological workflow of the meta-analysis and the selected multi-array studies for Fam83D. Following meta-analysis, 150 genes were found to be coexpressed in six or more studies (Table II). DAVID was used to perform gene ontology (GO) term enrichment analysis to obtain characteristics of the set of significant genes from our meta-analyses. This analysis provides a list of gene functions, which are overrepresented in a gene set. Analysis of the 150 Fam83D-coexpressed genes with the DAVID functional annotation tool (GOTERM BP FAT) resulted in 181 GO categories (cut-off, P<0.05; count ≥2 and fold enrichment >1.5) (data not shown). To produce a more comprehensive and structured view of the annotation terms, a DAVID clustering analysis under high-stringency conditions was performed, resulting in 42 annotation clusters matching the statistical criteria (P<0.0001, count ≥10 and fold enrichment >1.5) (Table III). Subsequently, the aforementioned DAVID annotation tool was used for identification of putative KEGG pathways associated with Fam83D-coexpressed genes. Consequently, five pathways associated with the cell cycle, mitosis and related signaling pathways were significantly enriched with Fam83D-coexpressed genes (P<0.05 and fold enrichment >1.5) (Table IV). In addition, DAVID was used for predicting putative diseases that linked with Fam83D-coexpressed genes using the Genetic Association Database. The results revealed that breast and colorectal cancers were significantly enriched with these genes (P<0.05 and fold enrichment >1.5) (Table V).
Figure 1.
Methodological workflow of Fam83D meta-analysis.
Table II.
Fam83D-coexpressed genes.
| 1 ANLN | 51 DLGAP5 | 101 MYBL2 |
| 2 APOBEC3B | 52 DSCC1 | 102 NCAPG |
| 3 ATAD2 | 53 DTL | 103 NCAPG2 |
| 4 AURKA | 54 E2F7 | 104 NCAPH |
| 5 AURKB | 55 E2F8 | 105 NDC80 |
| 6 BIRC5 | 56 ECT2 | 106 NEK2 |
| 7 BUB1 | 57 ERCC6L | 107 NUF2 |
| 8 BUB1B | 58 ESPL1 | 108 NUSAP1 |
| 9 C11orf82 | 59 EXO1 | 109 IP5 |
| 10 C15orf42 | 60 EZH2 | 110 PBK |
| 11 C16ORF75 | 61 FAM54A | 111 PHF19 |
| 12 CASC5 | 62 FAM64A | 112 PLK1 |
| 13 CCNA2 | 63 FANCI | 113 PLK4 |
| 14 CCNB1 | 64 FBXO5 | 114 POLE2 |
| 15 CCNB2 | 65 FEN1 | 115 PRC1 |
| 16 CDC20 | 66 FOXM1 | 116 PTTG1 |
| 17 CDC25A | 67 GGH | 117 RACGAP1 |
| 18 CDC25B | 68 GIN | 118 RAD51 |
| 19 CDC25C | 69 GINS2 S1 | 119 RAD54L |
| 20 CDC45 | 70 GINS4 | 120 RECQL4 |
| 21 CDC6 | 71 GMNN | 121 RFC3 |
| 22 CDC7 | 72 GPSM2 | 122 RFC4 |
| 23 CDCA2 | 73 GTSE1 | 123 RNASEH2A |
| 24 CDCA3 | 74 HELLS | 124 RRM2 |
| 25 CDCA5 | 75 HJURP | 125 SGOL2 |
| 26 CDCA7 | 76 HMMR | 126 SHCBP1 |
| 27 CDCA8 | 77 KIAA0101 | 127 SLC7A5 |
| 28 CDK1 | 78 KIF11 | 128 SMC4 |
| 29 CDKN3 | 79 KIF14 | 129 SPAG5 |
| 30 CDT1 | 80 KIF15 | 130 SPC24 |
| 31 CENPA | 81 KIF18B | 131 SPC25 |
| 32 CENPE | 82 KIF20A | 132 STIL |
| 33 CENPF | 83 KIF23 | 133 TACC3 |
| 34 CENPI | 84 KIF2C | 134 TFRC |
| 35 CENPJ | 85 KIF4A | 135 TIMELESS |
| 36 CENPK | 86 KIFC1 | 136 TK1 |
| 37 CENPM | 87 KPNA2 | 137 TOP2A |
| 38 CENPN | 88 LMNB1 | 138 TPX2 |
| 39 CENPW | 89 MAD2L1 | 139 TRIM59 |
| 40 CEP55 | 90 MASTL | 140 TRIP13 |
| 41 CHEK1 | 91 MCM10 | 141 TROAP |
| 42 CKAP2 | 92 MCM2 | 142 TTK |
| 43 CKAP2L | 93 MCM4 | 143 TYMS |
| 44 CKS1B | 94 MCM6 | 144 UBE2C |
| 45 CKS2 | 95 MCM7 | 145 UBE2S |
| 46 DBF4 | 96 MCM8 | 146 UBE2T |
| 47 DEPDC1 | 97 MELK | 147 UHRF1 |
| 48 DEPDC1B | 98 MKI67 | 148 WHSC1 |
| 49 DHFR | 99 MLF1IP | 149 ZNF367 |
| 50 DIAPH3 | 100 MYBL1 | 150 ZWINT |
Table III.
Functional enrichment of Fam83D-coexpressed genes.
| Term | Count | % | P-value | Fold | FDR |
|---|---|---|---|---|---|
| GO:0007049 - Cell cycle | 88 | 59.1 | 1.90E-74 | 11.2 | 1.31E-71 |
| GO:0000279 - M phase | 65 | 43.6 | 9.23E-68 | 19.5 | 3.19E-65 |
| GO:0022403 - Cell cycle phase | 69 | 46.3 | 3.78E-67 | 16.5 | 8.71E-65 |
| GO:0022402 - Cell cycle process | 73 | 49 | 2.29E-63 | 12.8 | 3.96E-61 |
| GO:0000278 - Mitotic cell cycle | 62 | 41.6 | 1.39E-59 | 16.5 | 1.92E-57 |
| GO:0007067 - Mitosis | 53 | 35.6 | 7.11E-59 | 23.8 | 8.19E-57 |
| GO:0000280 - Nuclear division | 53 | 35.6 | 7.11E-59 | 23.8 | 8.19E-57 |
| GO:0000087 - M phase of mitotic cell cycle | 53 | 35.6 | 2.01E-58 | 23.4 | 1.99E-56 |
| GO:0048285 - Organelle fission | 53 | 35.6 | 7.15E-58 | 22.9 | 6.18E-56 |
| GO:0051301 - Cell division | 53 | 35.6 | 1.10E-51 | 17.7 | 8.47E-50 |
| GO:0006260 - DNA replication | 31 | 20.8 | 8.29E-28 | 16.1 | 5.73E-26 |
| GO:0007059 - Chromosome segregation | 22 | 14.8 | 1.82E-24 | 26.8 | 1.14E-22 |
| GO:0006259 - DNA metabolic process | 40 | 26.8 | 3.13E-24 | 7.81 | 1.80E-22 |
| GO:0051726 - Regulation of cell cycle | 33 | 22.1 | 7.82E-23 | 9.84 | 4.16E-21 |
| GO:0007017 - Microtubule-based process | 29 | 19.5 | 1.31E-21 | 11.3 | 6.46E-20 |
| GO:0007051 - Spindle organization | 15 | 10.1 | 6.83E-18 | 32.9 | 3.15E-16 |
| GO:0000070 - Mitotic sister chromatid segregation | 14 | 9.4 | 1.12E-17 | 38.4 | 4.82E-16 |
| GO:0000819 - Sister chromatid segregation | 14 | 9.4 | 1.71E-17 | 37.4 | 6.93E-16 |
| GO:0007346 - Regulation of mitotic cell cycle | 21 | 14.1 | 3.98E-17 | 13.6 | 1.53E-15 |
| GO:0010564 - Regulation of cell cycle process | 19 | 12.8 | 5.90E-17 | 16.5 | 4.00E-15 |
| GO:0000226 - Microtubule cytoskeleton organization | 20 | 13.4 | 3.60E-16 | 13.4 | 1.15E-14 |
| GO:0000075 - Cell cycle checkpoint | 15 | 10.1 | 3.02E-13 | 16.3 | 9.93E-12 |
| GO:0051276 - Chromosome organization | 27 | 18.1 | 1.98E-12 | 5.5 | 6.22E-11 |
| GO:0007126 - Meiosis | 13 | 8.72 | 2.54E-10 | 13.1 | 7.63E-09 |
| GO:0051327 - M phase of meiotic cell cycle | 13 | 8.72 | 2.54E-10 | 13.1 | 7.63E-09 |
| GO:0051321 - Meiotic cell cycle | 13 | 8.72 | 3.23E-10 | 12.8 | 9.29E-09 |
| GO:0007093 - Mitotic cell cycle checkpoint | 10 | 6.71 | 3.39E-10 | 23 | 9.37E-09 |
| GO:0007010 - Cytoskeleton organization | 23 | 15.4 | 3.87E-10 | 5.21 | 1.03E-08 |
| GO:0051329 - Interphase of mitotic cell cycle | 13 | 8.72 | 4.58E-10 | 12.5 | 1.17E-08 |
| GO:0051325 - Interphase | 13 | 8.72 | 6.43E-10 | 12.1 | 1.59E-08 |
| GO:0006974 - Response to DNA damage stimulus | 21 | 14.1 | 9.27E-10 | 5.56 | 2.21E-08 |
| GO:0007088 - Regulation of mitosis | 10 | 6.71 | 4.08E-09 | 17.6 | 9.40E-08 |
| GO:0051783 - Regulation of nuclear division | 10 | 6.71 | 4.08E-09 | 17.6 | 9.40E-08 |
| GO:0006261 - DNA-dependent DNA replication | 10 | 6.71 | 5.64E-09 | 17 | 1.26E-07 |
| GO:0008283 - Cell proliferation | 21 | 14.1 | 1.34E-08 | 4.76 | 2.89E-07 |
| GO:0048015 - Phosphoinositide-mediated signaling | 11 | 7.38 | 1.75E-08 | 12.3 | 3.67E-07 |
| GO:0006323 - DNA packaging | 11 | 7.38 | 2.71E-07 | 9.28 | 5.50E-06 |
| GO:0051640 - Organelle localization | 10 | 6.71 | 3.45E-07 | 10.7 | 6.81E-06 |
| GO:0033554 - Cellular response to stress | 21 | 14.1 | 9.19E-07 | 3.66 | 1.76E-05 |
| GO:0006281 - DNA repair | 15 | 10.1 | 1.01E-06 | 5.22 | 1.88E-05 |
| GO:0007018 - Microtubule-based movement | 10 | 6.71 | 1.98E-06 | 8.74 | 3.61E-05 |
| GO:0033043 - Regulation of organelle organization | 11 | 7.38 | 6.71E-05 | 5.01 | 0.001188 |
Fold, fold enhancement; FDR, false discovery rate.
Table IV.
Pathway-based enrichment of Fam83D-coexpressed genes.
| Term | Count | % | P-value | Fold | FDR |
|---|---|---|---|---|---|
| hsa04110: Cell cycle | 24 | 16.1 | 1.16E-25 | 20.3 | 3.24E-24 |
| hsa03030: DNA replication | 9 | 6.04 | 7.12E-10 | 26.5 | 9.97E-09 |
| hsa04114: Oocyte meiosis | 12 | 8.05 | 2.66E-09 | 11.6 | 2.48E-08 |
| hsa04914: Progesterone-mediated oocyte maturation | 10 | 6.71 | 5.97E-08 | 12.3 | 4.18E-07 |
| hsa04115: p53 signaling pathway | 6 | 4.03 | 3.66E-04 | 9.35 | 0.002048 |
Fold, fold enrichment; FDR, false discovery rate.
Table V.
Disease-based enrichment of Fam83D-coexpressed genes.
| Term | Count | % | P-value | Fold | FDR |
|---|---|---|---|---|---|
| Breast cancer | 13 | 8.7 | 1.91E-06 | 4.9 | 1.39E-04 |
| Colorectal cancer | 6 | 4.0 | 0.029838 | 3.2 | 0.669009 |
Fold, fold enrichment; FDR, false discovery rate.
Fam83D is differentially expressed in various cancers
We investigated the expression of Fam83D in cancer using publicly available gene expression data from Oncomine (Table VI). Fam83D has been found to be upregulated in various tumors including in breast cancer compared to normal breast (10); in colorectal cancer compared to normal colon or rectum in three independent studies (11–13); in gastric cancer compared to gastric mucosa in two independent studies (14,15); in hepatocellular carcinoma compared to normal liver in two independent studies (16,17); in lung cancer compared to normal lung in two independent studies (18,19) and in vulva intraepithelial neoplasia compared to normal vulva (20). Conversely, downregulation of Fam83D was found in glioblastoma compared to neural stem cells (21); in esophageal cancer compared to normal esophagus (22) and in leukemia compared to peripheral blood mononuclear cells (23).
Table VI.
Differential expression of Fam83D in cancer types compared to their normal counterparts, using the Oncomine cancer microarray database.
Discussion
The main function of the cell cycle is to accurately duplicate the entire genome and segregate a copy of each chromosome precisely into two daughter cells. Maintenance of a correct chromosome number is essential for the survival of an organism. Errors in the cell division may lead to loss or gain of chromosomes and consequently to aneuploidy. In mitotically dividing cells, aneuploidy is a hallmark of cancer and many cancer cells are characterized by high rates of chromosomal instability (CIN). CIN leads to the persistent generation of new chromosomal variations, to tumor progression and to the development of more aggressive phenotypes (24). Centrosomes have important roles in equal segregation of chromosomes through the establishment of bipolar spindle formation during mitosis. Many studies have reported that centrosome-located proteins are involved in the regulation of centrosome organization (25,26). Moreover, it has been demonstrated that deregulation of the centrosome organization machinery is a clear source of centrosome amplification (27). There is a growing line of evidence to suggest that most solid tumors and many hematopoietic malignancies contain cells with centrosome abnormalities (28–30). For example, the centrosomal mitotic kinases Aurora-A, Plk-1, Plk-4 and Nek2 are all Fam83D-coexpressed genes (Table II), involved in multiple mitotic events. These range from centrosome maturation to centrosome separation, spindle formation and cytokinesis, and their deregulation has been linked to centrosome abnormalities and consequently carcinogenesis (31–35). Therefore, all centrosome and bipolar spindle-associated proteins are considered as putative cancer-related molecules. Santamaria et al have demonstrated that Fam83D localizes to the mitotic spindle, and Fam83D-depleted cells form shorter spindles and fail to organize a correct metaphase plate (4). In this study, we showed that Fam83D is coexpressed with many centrosome-located and mitosis-related genes, which are involved in normal cell cycle progression as well as in carcinogenesis. Notably, the majority of the coexpressed genes were key molecules for entry into mitosis, mitotic progression and cytokinesis. All these processes are related to centrosome organization and important to the faithful segregation of chromosomes. Therefore, we suggested that Fam83D may be involved in equal segregation of chromosomes during mitosis. In concordance with this hypothesis, our results also revealed that Fam83D is differentially expressed in some cancers that are directly linked to centrosome abnormalities, such as bladder (36), breast (37), lung (38), colorectal (30) or hepatocellular (39) carcinomas and leukemia (40).
In conclusion, we performed a meta-analysis for Fam83D using in silico approaches. Our results revealed that this molecule may be important for centrosome organization, mitotic processes and also in carcinogenesis. In silico studies support wet-lab approaches to finding new diagnostic, therapeutic and prognostic factors by using various tools, software and large-scale databases. However, the results of in silico studies generally need confirmation by lab experiments. Therefore, further investigation of the results presented in this study by experimental approaches may increase our understanding of centrosome organization, mitosis and carcinogenesis.
References
- 1.Deloukas P, Matthews LH, Ashurst J, et al. The DNA sequence and comparative analysis of human chromosome 20. Nature. 2001;414:865–871. doi: 10.1038/414865a. [DOI] [PubMed] [Google Scholar]
- 2.Finn RD, Mistry J, Tate J, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer G, Korner R, Hanisch A, Ries A, Nigg EA, Sillje HH. Proteome analysis of the human mitotic spindle. Mol Cell Proteomics. 2005;4:35–43. doi: 10.1074/mcp.M400158-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Santamaria A, Nagel S, Sillje HH, Nigg EA. The spindle protein CHICA mediates localization of the chromokinesin Kid to the mitotic spindle. Curr Biol. 2008;18:723–729. doi: 10.1016/j.cub.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BJ. Meta-analysis of SUMO1. BMC Res Notes. 2008;1:60. doi: 10.1186/1756-0500-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson BJ, Giguere V. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Mol Cancer. 2008;7:49. doi: 10.1186/1476-4598-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 8.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 12.Sabates-Bellver J, Van der Flier LG, de Palo M, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 13.Skrzypczak M, Goryca K, Rubel T, et al. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 2010;5:e13091. doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Leung SY, Yuen ST, et al. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003;14:3208–3215. doi: 10.1091/mbc.E02-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Errico M, de Rinaldis E, Blasi MF, et al. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 2009;45:461–469. doi: 10.1016/j.ejca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Cheung ST, So S, et al. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurmbach E, Chen YB, Khitrov G, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 18.Garber ME, Troyanskaya OG, Schluens K, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou J, Aerts J, den Hamer B, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One. 2010;5:e10312. doi: 10.1371/journal.pone.0010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santegoets LA, Seters M, Helmerhorst TJ, et al. HPV related VIN: highly proliferative and diminished responsiveness to extracellular signals. Int J Cancer. 2007;121:759–766. doi: 10.1002/ijc.22769. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Kim SM, Park YY, Park ES, et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One. 2010;5:e15074. doi: 10.1371/journal.pone.0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haferlach T, Kohlmann A, Wieczorek L, et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 2010;28:2529–2537. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 25.Cizmecioglu O, Arnold M, Bahtz R, et al. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J Cell Biol. 2010;191:731–739. doi: 10.1083/jcb.201007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cizmecioglu O, Warnke S, Arnold M, Duensing S, Hoffmann I. Plk-2 regulated centriole duplication is dependent on its localization to the centrioles and a functional polo-box domain. Cell Cycle. 2008;7:3548–3555. doi: 10.4161/cc.7.22.7071. [DOI] [PubMed] [Google Scholar]
- 27.Zyss D, Gergely F. Centrosome function in cancer: guilty or innocent? Trends Cell Biol. 2009;19:334–346. doi: 10.1016/j.tcb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Brinkley BR. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 2001;11:18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- 29.Carroll PE, Okuda M, Horn HF, et al. Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene. 1999;18:1935–1944. doi: 10.1038/sj.onc.1202515. [DOI] [PubMed] [Google Scholar]
- 30.Pihan GA, Purohit A, Wallace J, et al. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- 31.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk-4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 32.Hayward DG, Fry AM. Nek-2 kinase in chromosome instability and cancer. Cancer Lett. 2006;237:155–166. doi: 10.1016/j.canlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Lu LY, Wood JL, Ye L, et al. Aurora-A is essential for early embryonic development and tumor suppression. J Biol Chem. 2008;283:31785–31790. doi: 10.1074/jbc.M805880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XQ, Zhu YQ, Lui KS, Cai Q, Lu P, Poon RT. Aberrant Polo-like kinase 1-Cdc25A pathway in metastatic hepatocellular carcinoma. Clin Cancer Res. 2008;14:6813–6820. doi: 10.1158/1078-0432.CCR-08-0626. [DOI] [PubMed] [Google Scholar]
- 35.Lu LY, Wood JL, Minter-Dykhouse K, et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6876. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Matsuyama H, Furuya T, et al. Centrosome hyperamplification predicts progression and tumor recurrence in bladder cancer. Clin Cancer Res. 2004;10:6449–6455. doi: 10.1158/1078-0432.CCR-04-0773. [DOI] [PubMed] [Google Scholar]
- 37.Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung CK, Jung JH, Lee KY, et al. Centrosome abnormalities in non-small cell lung cancer: correlations with DNA aneuploidy and expression of cell cycle regulatory proteins. Pathol Res Pract. 2007;203:839–847. doi: 10.1016/j.prp.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima T, Moriguchi M, Mitsumoto Y, et al. Centrosome aberration accompanied with p53 mutation can induce genetic instability in hepatocellular carcinoma. Mod Pathol. 2004;17:722–727. doi: 10.1038/modpathol.3800115. [DOI] [PubMed] [Google Scholar]
- 40.Giehl M, Fabarius A, Frank O, et al. Centrosome aberrations in chronic myeloid leukemia correlate with stage of disease and chromosomal instability. Leukemia. 2005;19:1192–1197. doi: 10.1038/sj.leu.2403779. [DOI] [PubMed] [Google Scholar]