Abstract
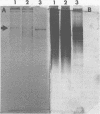
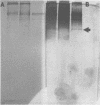
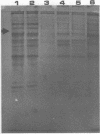
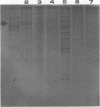
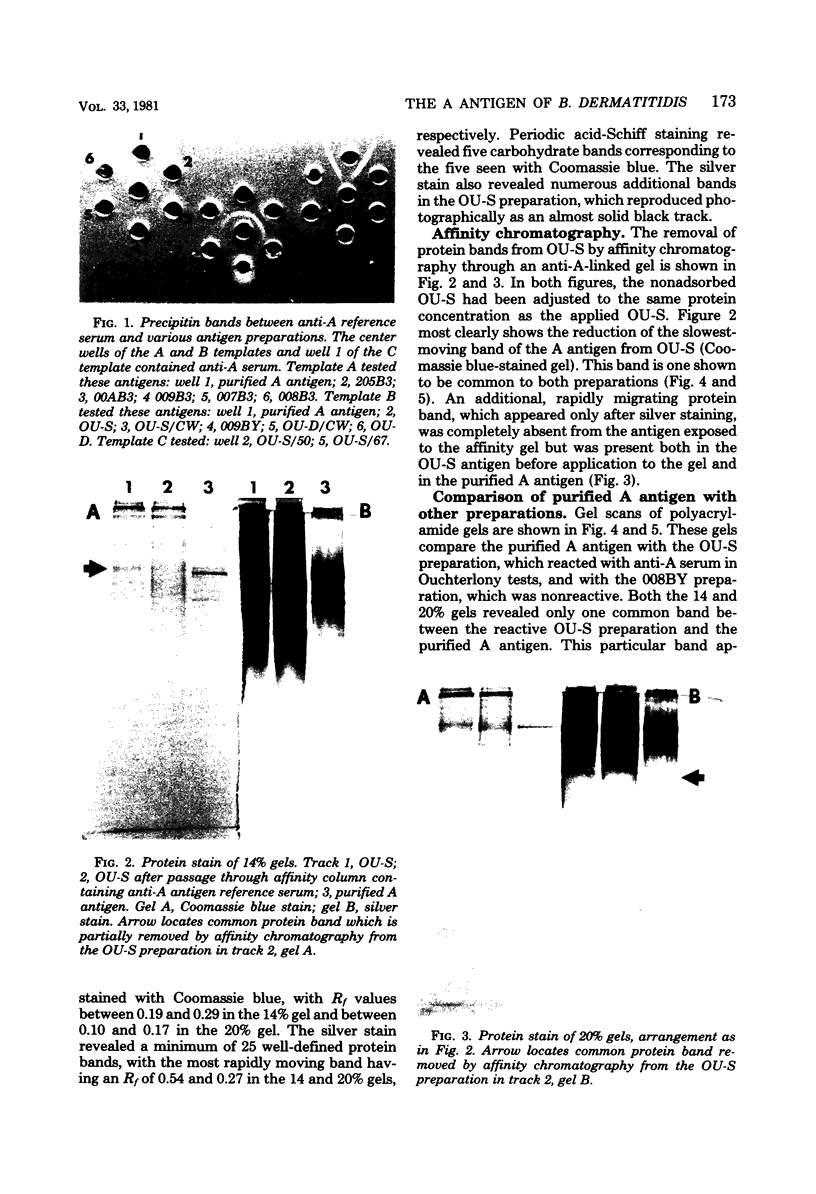
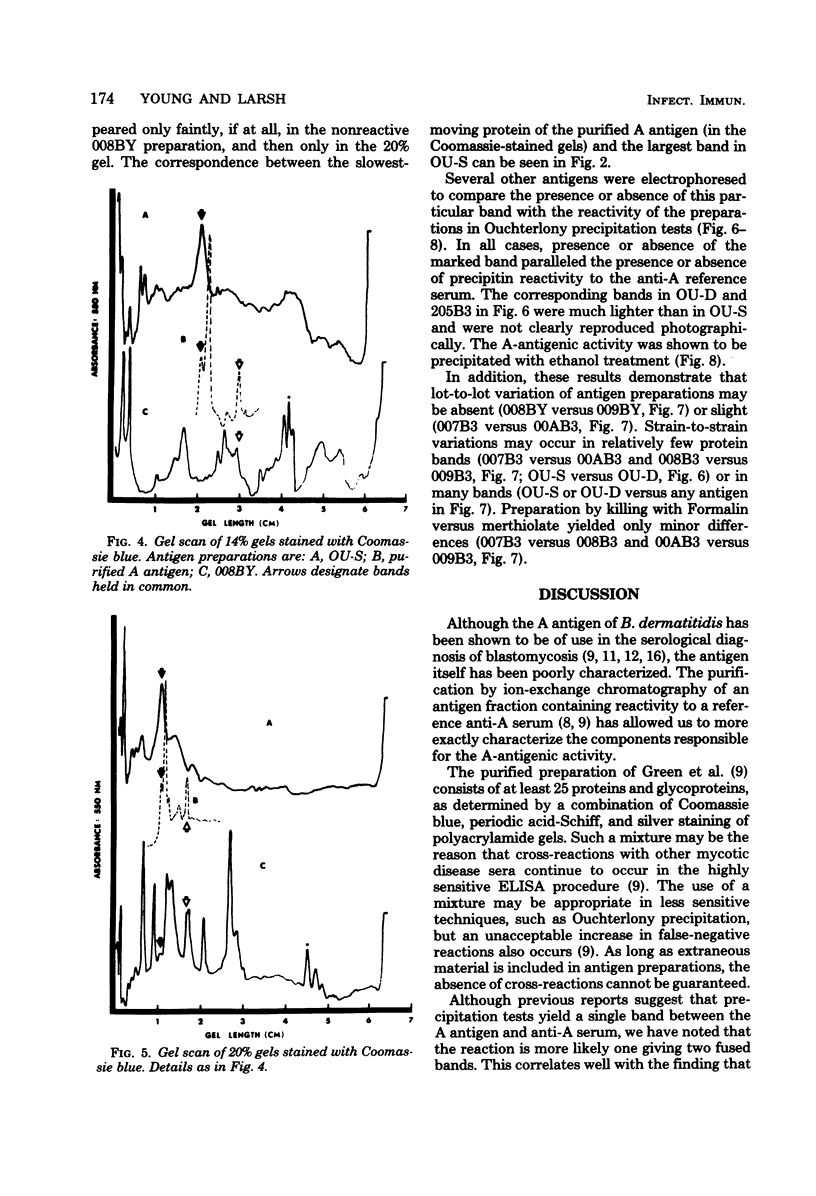
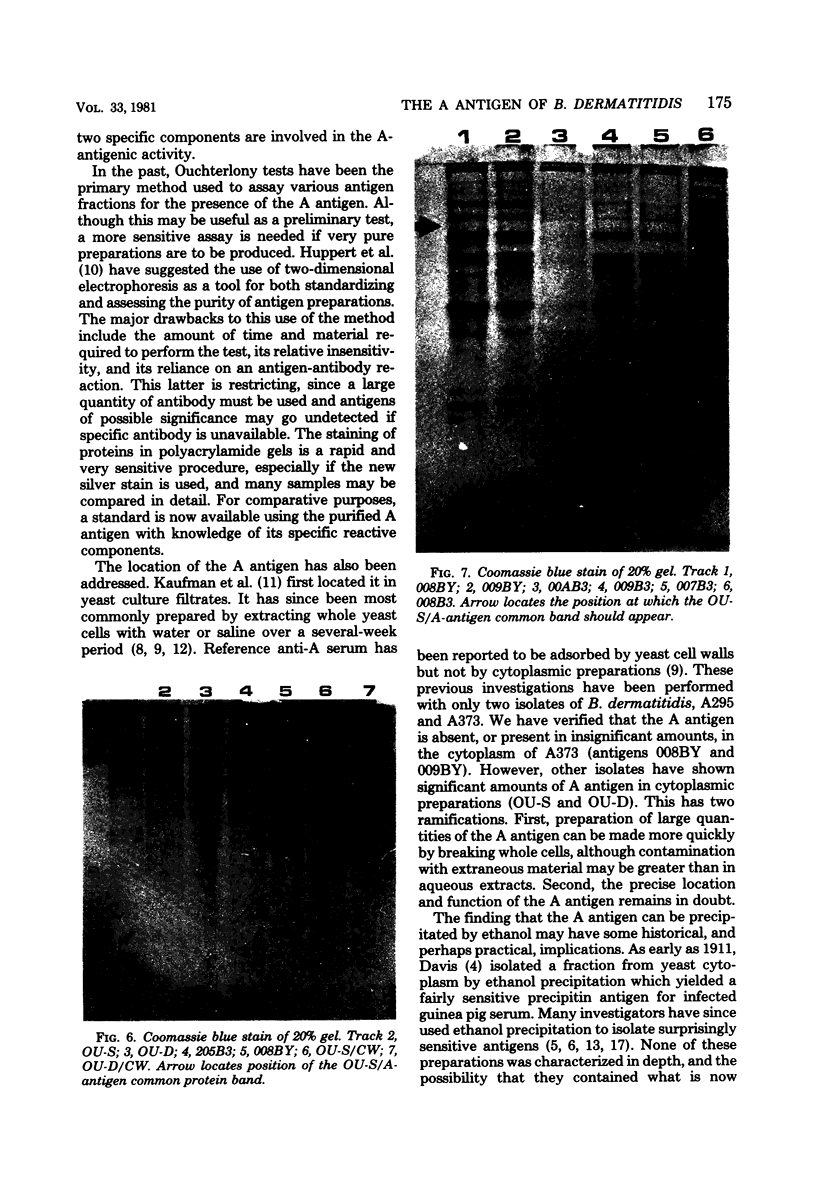
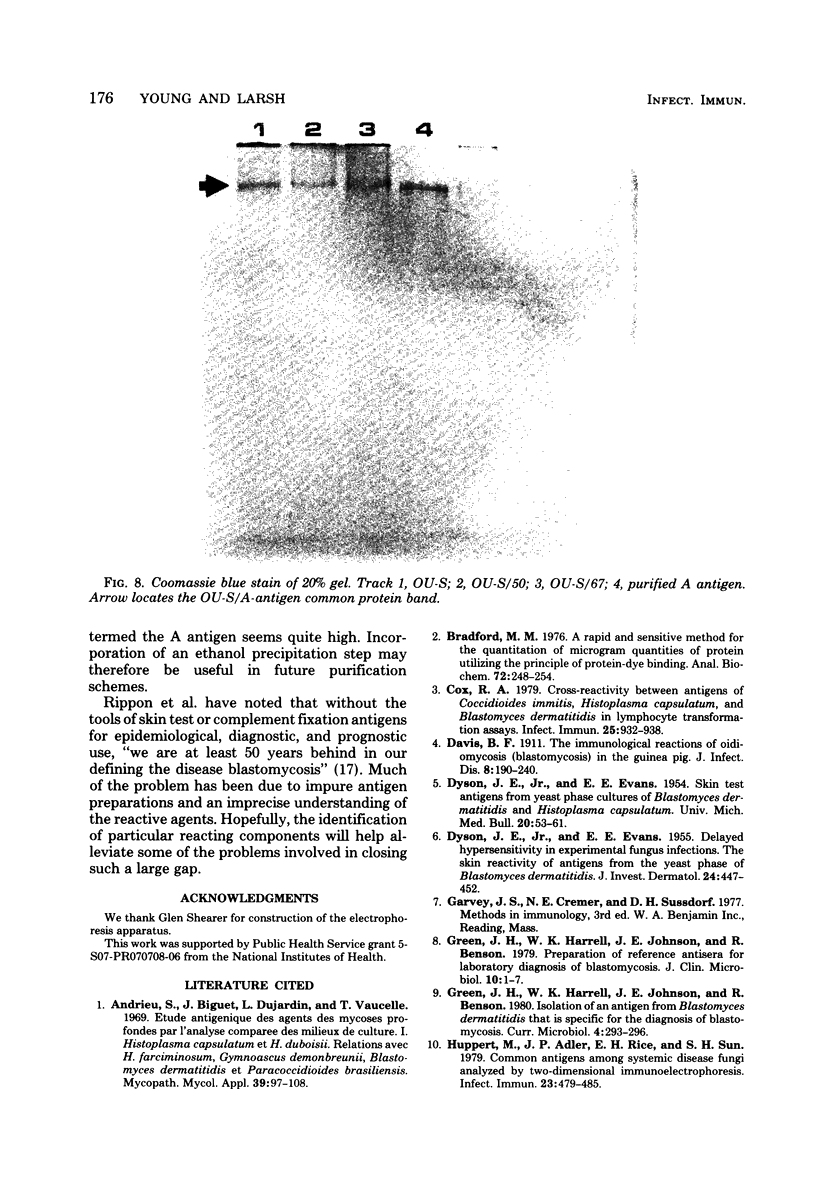
A purified A-antigen preparation of Blastomyces dermatitidis was determined to be composed of five major glycoprotein bands, visible with Coomassie blue and periodic acid-Schiff staining of polyacrylamide gels. At least 20 additional protein bands were detected by using a silver stain, which was 100 times more sensitive than the Coomassie method. Two components of this mixture were determined to be associated with the A-antigenic activity of B. dermatitidis. Of several antigen preparations examined in Ouchterlony precipitation tests, those reactive with a reference anti-A antiserum contained the slowest moving of the Coomassie blue bands. The antigen preparations without precipitin reactivity lacked this protein band. Two protein bands were shown to disappear from an antigen preparation after incubation with an affinity gel linked to the reference anti-A serum. One of the bands was the slowest Coomassie blue band, and the other was a fast-migrating protein detectable only with the silver stain. Characterization of the components responsible for the A-antigenic activity has important applications in the production and standardization of serological reagents for the diagnosis of blastomycosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrieu S., Biguet J., Dujardin L., Vaucelle T. Etude antigenique des agents des mycoses profondes par l'analyse comparee des milieux de culture. I. Histoplasma capsulatum et H. duboisil. Relations avec H. farciminosum, Gymnoascus demonbreunii, Blastomyces dermatitidis et Paracoccidioides brasiliensis. Mycopathol Mycol Appl. 1969 Nov 28;39(2):97–108. doi: 10.1007/BF02053482. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cox R. A. Cross-reactivity between antigens of Coccidioides immitis, Histoplasma capsulatum and Blastomyces dermatitidis in lymphocyte transformation assays. Infect Immun. 1979 Sep;25(3):932–938. doi: 10.1128/iai.25.3.932-938.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYSON J. E., Jr, EVANS E. E. Delayed hypersensitivity in experimental fungus infections: the skin reactivity of antigens from the yeast phase of Blastomyces dermatitidis. J Invest Dermatol. 1955 Apr;24(4):447–454. doi: 10.1038/jid.1955.60. [DOI] [PubMed] [Google Scholar]
- DYSON J. E., Jr, EVANS E. E. Skin test antigens from yeast phase cultures of Blastomyces dermatitidis and Histoplasma capsulatum. Med Bull (Ann Arbor) 1954 Mar;20(3):53–61. [PubMed] [Google Scholar]
- Green J. H., Harrell W. K., Johnson J. E., Benson R. Preparation of reference antisera for laboratory diagnosis of blastomycosis. J Clin Microbiol. 1979 Jul;10(1):1–7. doi: 10.1128/jcm.10.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT R. A., MARCUS S. Polysaccharide skin test antigens derived from Histoplasma capsulatum and Blastomyces dermatitidis. Am Rev Tuberc. 1958 Jun;77(6):983–989. doi: 10.1164/artpd.1958.77.6.983. [DOI] [PubMed] [Google Scholar]
- Kaufman L., McLaughlin D. W., Clark M. J., Blumer S. Specific immunodiffusion test for blastomycosis. Appl Microbiol. 1973 Sep;26(3):244–247. doi: 10.1128/am.26.3.244-247.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Ogita Z. I., Markert C. L. A miniaturized system for electrophoresis on polyacrylamide gels. Anal Biochem. 1979 Nov 1;99(2):233–241. doi: 10.1016/s0003-2697(79)80001-9. [DOI] [PubMed] [Google Scholar]
- Rippon J. W., Anderson D. N., Jacobsohn S., Soo Hoo M., Garber E. D. Blastomycosis: specificity of antigens reflecting the mating types of Ajellomyces dermatitidis. Mycopathologia. 1977 Feb 18;60(2):65–72. doi: 10.1007/BF00490374. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]