Abstract
Objectives
Studies have typically investigated health and educational consequences of malaria among school-aged children in areas of high malaria transmission, but few have investigated these issues in moderate transmission settings. This study investigates the patterns of and risks for Plasmodium falciparum and anaemia and their association with cognitive and education outcomes on the Kenyan coast, an area of moderate malaria transmission.
Methods
As part of a cluster randomised trial, a baseline cross-sectional survey assessed the prevalence of and risk factors for P. falciparum infection and anaemia and the associations between health status and measures of cognition and educational achievement. Results are presented for 2400 randomly selected children who were enrolled in the 51 intervention schools.
Results
The overall prevalence of P. falciparum infection and anaemia was 13.0% and 45.5%, respectively. There was marked heterogeneity in the prevalence of P. falciparum infection by school. In multivariable analysis, being male, younger age, not sleeping under a mosquito net and household crowding were adjusted risk factors for P. falciparum infection, whilst P. falciparum infection, being male and indicators of poor nutritional intake were risk factors for anaemia. No association was observed between either P. falciparum or anaemia and performance on tests of sustained attention, cognition, literacy or numeracy.
Conclusion
The results indicate that in this moderate malaria transmission setting, P. falciparum is strongly associated with anaemia, but there is no clear association between health status and education. Intervention studies are underway to investigate whether removing the burden of chronic asymptomatic P. falciparum and related anaemia can improve education outcomes.
Keywords: malaria, plasmodium, anaemia, sustained attention, cognition, educational achievement, school children, Kenya
Introduction
The health of school children has received increasing attention over the last two decades, and there are increased efforts to implement school health programmes, delivering anthelmintics and micronutrients (Bundy et al. 2006; Bundy 2011). Less emphasis has been given to malaria as a health problem facing school children despite them experiencing some of the highest age-specific rates of Plasmodium falciparum infection (Smith et al. 2007; Hay et al. 2008; Brooker et al. 2009), which can have a number of direct and indirect consequences, including anaemia (Draper 1960). The control of malaria is associated with significant improvements in haemoglobin levels among both young children (Korenromp et al. 2004) and children of school age (Draper 1960; Geerligs et al. 2003; Clarke et al. 2008; Barger et al. 2009). Malaria may have additional consequences for children’s cognitive performance and ultimately educational achievement (Al Serouri et al. 2000; Holding & Snow 2001; Kihara et al. 2006; Vitor-Silva et al. 2009; Fernando et al. 2010). For instance, malaria has been related to increased absenteeism (Brooker et al. 2000; Fernando et al. 2003a; Kimbi et al. 2005), grade repetition (Thuilliez 2010) and poorer educational achievement (Fernando et al. 2003a, b). Studies in Ghana, Kenya and Sri Lanka suggest that malaria prevention can improve school attendance, sustained attention and educational achievement (Colbourne 1955; Fernando et al. 2006; Jukes et al. 2006; Clarke et al. 2008).
The consequences of malaria for school children and the benefits of school-based malaria control are likely to vary in different settings, particularly according to intensity of malaria transmission and the relative contribution of other causes of anaemia and poor education outcomes. A previous study in an area of perennial high malaria transmission in western Kenya (Clarke et al. 2008) investigated the impact of intermittent preventive treatment for malaria in schools and found a large impact on children’s concentration in class and a 48% reduction in the rates of anaemia. However, no effect on educational achievement was observed. To investigate this result further and find out whether the benefits of malaria control are observed in settings with different intensities of malaria transmission and different educational standards, an ongoing cluster randomised trial is investigating the impact of an alternative school-based malaria intervention, intermittent screening and treatment (IST), in an area of moderate malaria transmission on the coast of Kenya (Brooker et al. 2010). The current paper presents data from the baseline cross-sectional survey of this trial and investigates risk factors for P. falciparum infection and anaemia as well as correlates of cognition, attention and educational achievement.
Methods
The study design and methods of the intervention trial have been previously detailed (Brooker et al. 2010) and are briefly summarized below. The IST intervention under investigation in the trial comprises mobile health teams consisting of laboratory technicians and nurses visiting schools each term. Children are asked to provide a finger prick blood sample to test for the presence of malaria parasites using ParaCheck-Pf malaria rapid diagnostic tests (RDT) (Orchid Biomedical Systems, Goa, India). Children (with or without malaria symptoms) found to be RDT-positive are treated with artemether-lumefantrine (Coartem®, Novartis), an artemisinin-based combination therapy. The current investigation uses baseline cross-sectional data collected between February and March 2010 in the 51 intervention schools that were allocated to receive the intermittent screening and treatment for malaria. No baseline data were collected on P. falciparum infection for the 50 control schools not receiving the malaria intervention owing to ethical considerations about screening for malaria and not providing treatment. Results reported here on P. falciparum infection are based on expert microscopy. The reliability of the RDTs employed for the IST will be evaluated in future analyses.
Reporting of the current study has been verified in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist.
Study setting
The surveys were conducted in Kwale and Msambweni districts on the south Kenyan coast, where malaria transmission is seasonal following the two rainy seasons (April-July and September-November) (Snow et al. 1993). In economic and educational terms, the districts are ranked the seventh poorest in Kenya and consistently have some of the worst-performing schools in the national school examinations (RTI International, 2008). In 2009, mass albendazole treatment was provided to all schools as part of the national school deworming programme. No systematic treatment for schistosomiasis has been provided to date and no specific school-based malaria control efforts are ongoing.
Recruitment
A school census of all 101 schools included in the trial was conducted by trained personnel and was used as a sampling frame from which 25 children from class 1 and 30 children from class 5 were randomly selected using random number tables. Fewer children were selected from class 1 because of the extra educational assessments undertaken with these children and the feasibility of conducting the tests in a single day.
Health surveys
Finger prick blood samples were obtained from all children to assess haemoglobin concentration (Hb) using a portable haemoglobinometer (Hemocue, Ängelholm, Sweden). Children with severe anaemia (Hb < 80 g/l) were referred to the nearest health facility for iron therapy as per the national guidelines. Height and weight were measured and axillary temperature was digitally recorded. Thick and thin blood smears, prepared for malaria microscopy, were stained with 2% Giemsa for 30 minutes Parasite densities were determined from thick blood smears by counting the number of asexual parasites per 200 white blood cells, assuming a white blood cell count of 8000/μl. A smear was considered negative after reviewing 100 high-powered fields. Thin blood smears were reviewed for species identification. Two independent microscopists read the slides, with a third microscopist resolving discrepancies.
Assessments of cognition and educational achievement
These have been detailed elsewhere (Brooker et al. 2010). In brief, age-appropriate tests of sustained attention were conducted in each class: the pencil-tap test and the code transmission test adapted from the Test of everyday attention for children (TEA-Ch) group (Manly et al. 1999) for class 1 and class 5 children, respectively. Non-verbal reasoning was assessed in class 1 by the Raven’s Progressive Matrices task (Raven et al. 1998). A range of class-specific literacy and numeracy tests were conducted in individualized and small-group settings. The tests were extensively piloted and adapted to the context. Test–retest reliabilities of 0.7 were required for inclusion of tests in the battery.
Risk factors
During the informed consent process, a questionnaire was administered to parents/guardians to record household information on residence, family size, ownership of possessions, mosquito net use by them and their children, recent deworming of the child, house construction and education level of the parent. For children in class 1, additional information on household literacy, the language spoken in the household and reading practices was recorded. At each school, a questionnaire was administered to the head teacher to collect information on school demography, sanitation facilities, presence of school feeding and other health programmes. School locations were mapped using a Global Positioning System (GPS) receiver (eTrex Garmin Ltd., Olathe, KS, USA). Elevation of schools was recorded and used as a geographical marker of distance from the coast.
Statistical analysis
Data were double-entered using customized data entry screens in Microsoft Access (Microsoft Corporation, Seattle, USA). Consistency checks were performed and all discrepancies and queries were verified against the original paper forms. Health data were linked by school location and mapped using ArcGIS 9.3.1 (Environmental Systems Research Institute Inc., Redlands, CA, USA).
Plasmodium falciparum infection was defined on the basis of duplicate slide readings. Anaemia was defined using age- and sex-corrected WHO thresholds (Benoist et al. 2008), with no correction made for altitude. The anthropometric indices, z-scores of height-for-age (HAZ), weight-for-age (WAZ) and body mass index-for-age (BMIZ), were calculated using the AnthroPlus software for children aged 5–19 years (WHO, 2007), assuming a mid-year age for each child because of doubts over the correct date of birth. Weight-for-age z-score was only calculated for children aged 5–10 years. Children were classified as stunted, underweight or thin if HAZ, WAZ and BMIZ, respectively, were less than two standard deviations below the reference median. Age of the children was provided by themselves and by their parents. Ages provided by the children were used to calculate anaemia and anthropometric indices as they were considered more reliable. A sensitivity analysis using parent-reported ages for all multivariable models indicated minimal sensitivity. Age was modelled as a categorical variable for the P. falciparum and anaemia risk factor analyses and as a continuous variable for the attention and education analyses owing to the smaller age ranges observed once stratified by class. Household asset data were used to derive an index of socio-economic status (SES), based on the entire trial population. The principal component analysis (PCA) approach proposed by Filmer and Pritchett (Filmer & Pritchett 2001) was used. Variables included into the PCA included ownership of a bicycle, motorcycle, mobile phone, radio, television, as well as presence of electricity, pit latrine, and brick and cement construction materials. The first principal component explained 30.6% of the overall variability and gave greatest weight to the household construction materials followed by ownership of a television. The resultant scores were divided into quintiles so that households could be classified according to relative SES. No internal validation of the index was undertaken. Finally, elevation (a proxy for distance from the coastline) was divided into tertiles.
Analyses were performed using STATA version 11.0 (STATA Corporation, College Station, TX, USA). The outcomes of interest examined were prevalence of P. falciparum and of anaemia (binary outcomes) and scores for spelling, number identification, numeracy, comprehension, code transmission and pencil-tapping tasks (continuous outcomes). Univariable associations between the health-related outcomes and risk factors were assessed using multilevel logistic regression, accounting for school-level clustering (Rabe-Hesketh & Skrondal 2008). Variables demonstrating an association at the 10% significance level were subsequently included into a multivariable, multilevel logistic regression model, accounting for school-level clustering. Stepwise elimination was used to create the final model using a 5% significance level for retention in the model. Age and sex were treated as a priori risk factors and retained in multivariable models. A priori interactions between net use with age and sex and between school feeding and elevation (distance from the coast) were investigated.
Analysis of the cognitive and education outcomes was stratified by class and focused on associations with P. falciparum infection and with anaemia, additionally accounting for age and sex as a priori risk factors. For the pencil-tap assessment of sustained attention in class 1 children, the analysis was split into two because of the significant proportion of children who were disengaged and scored zero. The proportion of children engaged in the task was examined by different variables using multilevel logistic regression accounting for school-level clustering. For each of the spelling assessments in classes 1 (score 0–20) and 5 (score 0–43); the numeracy in classes 1 (score 0–20) and 5 (score 0–38); the Ravens assessment in class 1 (score 0–20); the sentence comprehension in class 5 (score 0–40); the code transmission assessment of sustained attention in class 5 children (score 0–20); and the analysis of children who were engaged in the pencil-tap task (score 1–20), the effect of explanatory variables was quantified by mean differences in test performance using linear regression. Bootstrapping was used to account for non-normality of the scores, whereby schools were resampled to account for school-level clustering (Efron & Tibshirani 1994). Bias-corrected confidence intervals based on the bootstrap resamples were obtained. Significant (P < 0.1) variables identified in univariable analysis were considered for the multivariable model which employed stepwise elimination.
Ethical considerations
The study was approved by the Kenya Medical Research Institute and National Ethics Review Committee (SSC No. 1543), the London School of Hygiene and Tropical Medicine Ethics Committee (5503), and the Harvard University Committee on the Use of Human Subjects in Research (F17578-101). Meetings were held in participating schools to explain the nature and purpose of the trial to parents or legal guardians, and written informed consent was obtained.
Results
Of the 3850 children randomly selected to participate in the study, 2400 (1160 in class 1 and 1240 in class 5) were included in the analysis (Figure 1), with a mean of 48 children per school (range: 26–60). No systematic differences in individual and household characteristics were observed between included children and those children excluded because of missing health data (Table S1, online-only). The mean age of children in the present analysis was 10.3 years (range: 5–18 years) and the male/female ratio was 0.95 (Table 1).
Figure 1.
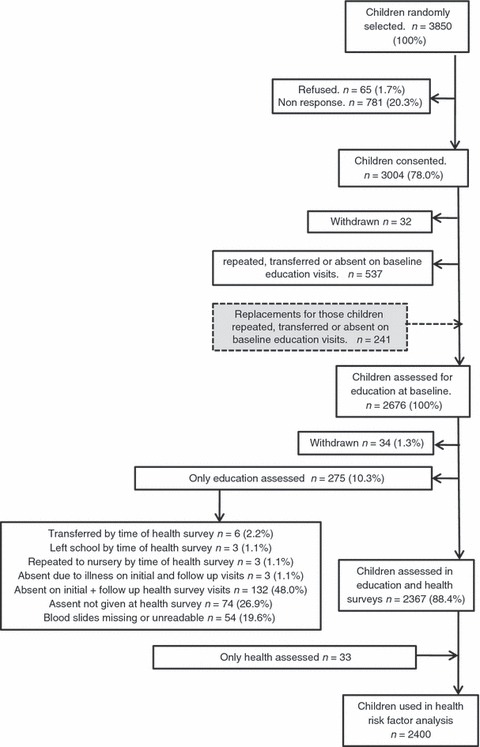
Data flow diagram for the education and health surveys conducted in school children in 51 schools on the south coast of Kenya, 2010.
Table 1.
Univariable analysis for associations between Plasmodium falciparum infection and anaemia and potential risk factors for both health outcomes among school children in 51 schools on the south coast of Kenya, 2010.
| Variable | Number of children (%)* n = 2400 | Number of children (%) with Plasmodium falciparum n = 311 | Odds ratio (95% CI) | P-value† | Number of children (%) with anaemia n = 1091 | Odds ratio (95% CI) | P-value† |
|---|---|---|---|---|---|---|---|
| Child-level | |||||||
| Sex | |||||||
| Male | 1167 (48.6) | 173 (14.8) | 1 | 566 (48.5) | 1 | 0.001 | |
| Female | 1233 (51.4) | 138 (11.2) | 0.67 (0.51–0.87) | 0.003 | 525 (42.6) | 0.76 (0.65–0.90) | |
| Age (per additional year)‡ | |||||||
| 10.34 (2.81) | 0.92 (0.87–0.96) | <0.001 | 1.00 (0.97–1.03) | 0.863 | |||
| Age groups (years) | |||||||
| 5–9 | 940 (39.2) | 139 (14.8) | 1 | 458 (48.7) | 1 | 0.002 | |
| 10–12 | 830 (34.6) | 120 (14.5) | 0.90 (0.67–1.21) | 337 (40.6) | 0.71 (0.59–0.87) | ||
| 13–18 | 630 (26.2) | 52 (8.3) | 0.42 (0.29–0.61) | <0.0001 | 296 (47.0) | 0.96 (0.78–1.18) | |
| Plasmodium falciparum infection status | |||||||
| Not infected | 2089 (87.0) | 914 (43.8) | 1 | <0.001 | |||
| Infected | 311 (13.0) | – | – | 177 (56.9) | 1.66 (1.28–2.15) | ||
| Plasmodium falciparum density (p/μl) | |||||||
| No infection (0) | 2089 (87.0) | 914 (43.8) | 1 | <0.0001 | |||
| Low (1–999) | 237 (9.9) | – | – | 124 (52.3) | 1.37 (1.03–1.82) | ||
| Medium/high (≥1000) | 74 (3.1) | 53 (71.6) | 3.28 (1.93–5.57) | ||||
| WAZ (z-scores)§,¶,** | |||||||
| Not wasted | 709 (75.8) | 102 (14.4) | 1 | 343 (48.4) | 1 | 0.726 | |
| Wasted | 227 (24.2) | 37 (16.3) | 1.37 (0.86–2.17) | 0.188 | 113 (49.8) | 1.06 (0.77–1.46) | |
| HAZ (z-scores)§ | |||||||
| Not stunted | 1790 (74.8) | 223 (12.5) | 1 | 794 (44.4) | 1 | 0.016 | |
| Stunted | 603 (25.2) | 88 (14.6) | 1.31 (0.97–1.76) | 0.083 | 294 (48.8) | 1.27 (1.04–1.54) | |
| BMIZ (z-scores)§ | |||||||
| Not thin | 1950 (81.5) | 262 (13.4) | 1 | 895 (45.9) | 1 | 0.363 | |
| Thin | 442 (18.5) | 49 (11.1) | 0.85 (0.60–1.22) | 0.377 | 193 (43.7) | 0.91 (0.73–1.12) | |
| Child been dewormed in last year¶ | |||||||
| No | 442 (19.5) | 72 (16.3) | 1 | 181 (41.0) | 1 | 0.13 | |
| Yes | 1824 (80.5) | 220 (12.1) | 0.70 (0.50–0.97) | 0.033 | 843 (46.2) | 1.19 (0.95–1.48) | |
| Household-level | |||||||
| Education level of household head | |||||||
| No schooling | 814 (34.3) | 131 (16.1) | 1 | 392 (48.2) | 1 | 0.047 | |
| Primary | 1228 (51.8) | 153 (12.5) | 0.83 (0.63–1.11) | 523 (42.6) | 0.79 (0.66–0.95) | ||
| Secondary | 255 (10.8) | 17 (6.7) | 0.50 (0.28–0.89) | 0.056 | 126 (49.4) | 1.05 (0.78–1.41) | |
| College/degree | 74 (3.1) | 6 (8.1) | 0.54 (0.22–1.34) | 34 (46.0) | 0.91 (0.56–1.50) | ||
| Water source | |||||||
| Uncovered (stream/river/dam) | 318 (13.4) | 63 (19.8) | 1 | 135 (42.5) | 1 | 0.742 | |
| Covered (well/borehole/piped) | 2066 (86.7) | 246 (11.9) | 0.76 (0.49–1.20) | 0.24 | 948 (45.9) | 1.05 (0.80–1.38) | |
| SES | |||||||
| Poorest | 577 (24.2) | 89 (15.4) | 1 | 270 (46.8) | 1 | 0.207 | |
| Poor | 504 (21.1) | 67 (13.3) | 0.94 (0.64–1.38) | 240 (47.6) | 1.01 (0.79–1.29) | ||
| Median | 423 (17.7) | 61 (14.4) | 1.00 (0.67–1.48) | 0.206 | 171 (40.4) | 0.75 (0.58–0.98) | |
| Less poor | 459 (19.3) | 48 (10.5) | 0.83 (0.55–1.27) | 206 (44.9) | 0.89 (0.69–1.15) | ||
| Least poor | 422 (17.7) | 44 (10.4) | 0.66 (0.42–1.03) | 197 (46.7) | 0.93 (0.72–1.22) | ||
| Number of people in the house‡ | |||||||
| 7.06 (2.52) | – | 1.06 (1.00–1.12) | 0.036 | – | 1.01 (0.98–1.05) | 0.39 | |
| Number of children in the house‡ | |||||||
| 4.82 (2.18) | – | 1.04 (0.98–1.11) | 0.183 | – | 1.03 (0.99–1.07) | 0.196 | |
| Child sleeps under a net | |||||||
| No | 880 (37.2) | 140 (15.9) | 1 | 406 (46.1) | 1 | 0.554 | |
| Yes | 1489 (62.8) | 166 (11.2) | 0.62 (0.47–0.82) | <0.001 | 666 (44.7) | 0.95 (0.80–1.13) | |
| If yes, is the net treated? | |||||||
| No | 278 (8.7) | 34 (12.2) | 1 | 129 (46.4) | 1 | 0.643 | |
| Yes | 1161 (78.2) | 127 (11.0) | 1.06 (0.67–1.68) | 0.879 | 518 (44.6) | 0.93 (0.71–1.22) | |
| Don’t know | 46 (3.1) | 5 (10.9) | 0.83 (0.27–2.58) | 18 (39.1) | 0.74 (0.39–1.41) | ||
| Number of nets in the house¶ | |||||||
| No nets | 360 (17.0) | 55 (15.3) | 1 | 170 (47.2) | 1 | 0.423 | |
| 1–2 nets | 655 (30.9) | 98 (15.0) | 1.01 (0.67–1.50) | 286 (43.7) | 0.84 (0.64–1.10) | ||
| 3–4 nets | 810 (38.2) | 85 (10.5) | 0.65 (0.43–0.99) | 0.003 | 362 (44.7) | 0.90 (0.69–1.17) | |
| ≥5 nets | 295 (13.9) | 26 (8.8) | 0.46 (0.27–0.80) | 136 (46.1) | 0.96 (0.70–1.33) | ||
| School-level | |||||||
| School malaria control activities | |||||||
| No | 1814 (75.6) | 238 (13.1) | 1 | 835 (46.0) | 1 | 0.531 | |
| Yes | 586 (24.4) | 73 (12.5) | 1.20 (0.48–3.00) | 0.697 | 256 (43.7) | 0.90 (0.66–1.24) | |
| School feeding programme | |||||||
| No | 1115 (46.5) | 139 (12.5) | 1 | 555 (49.8) | 1 | 0.017 | |
| Yes | 1285 (53.5) | 172 (13.4) | 0.89 (0.40–1.97) | 0.775 | 536 (41.7) | 0.73 (0.57–0.94) | |
| Elevation (metres) | |||||||
| 0–50 | 708 (29.5) | 120 (17.0) | 1 | 370 (52.3) | 1 | 0.013 | |
| 51–100 | 919 (38.3) | 119 (13.0) | 0.59 (0.24–1.50) | 410 (44.6) | 0.73 (0.54–0.99) | ||
| 101–200 | 773 (32.2) | 72 (9.3) | 0.39 (0.15–1.05) | 0.176 | 311 (40.2) | 0.61 (0.45–0.84) | |
Displayed as number and percentage except for continuous variables, displayed as mean and standard deviation (SD).
P-value is from likelihood ratio test comparing multilevel logistic regression models (adjusting for school-level clustering), with and without character of interest.
Modelled as continuous variable.
WAZ – weight-for-age HAZ – height-for-age BMI – body mass index-for-age. Wasted, stunted and thin defined as WAZ, HAZ and BMIZ z-scores < 2 SD.
Characteristics with missing data vary (all below 2% missing except deworming 5.6% missing, number of nets owned 11.7% missing, and WAZ 61% missing).
WAZ only calculated for children aged 5 years to 10 years.
Plasmodium falciparum and anaemia
The overall prevalence of P. falciparum was 13.0% (95% confidence interval [CI]: 8.9–17.0%); only 11 infected children had documented fever. Infection prevalence varied markedly by school, ranging from 0 to 75.0% (Figure 2b), with no infected children found in seven schools and a prevalence exceeding 40% in three schools. Overall, 45.5% (95% CI: 42.0–48.9%) of children were anaemic, and 1.1% (95% CI: 0.7–1.5) were severely anaemic. The mean haemoglobin concentration was 117.5g/l (95% CI: 116.4–118.6). Marked heterogeneity was also observed in the school-level prevalence of anaemia (range: 26.3–80.0%) (Figure 2c).
Figure 2.
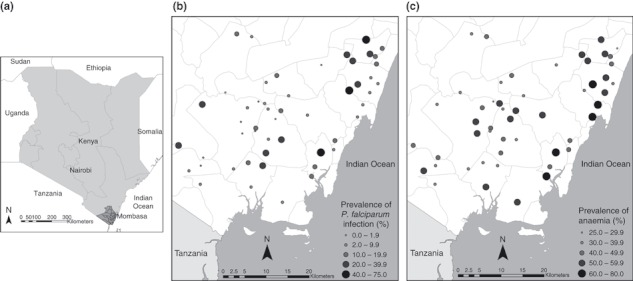
(a) The location of the study site in Kenya. (b) The geographical distribution of Plasmodium falciparum infection in 51 schools on the south coast of Kenya, 2010. (c) The geographical distribution of anaemia (adjusted for age and sex) in 51 schools on the south coast of Kenya, 2010.
Risk factors for Plasmodium falciparum infection and anaemia
The relative frequencies of individual, household and school-level risk factors for P. falciparum infection and anaemia are shown in Table 1. Overall, 62.8% (95% CI: 58.7–67.0) of children reported sleeping under a mosquito net, but usage varied markedly by school (range: 26.4–93.3%). In univariable analysis, P. falciparum infection was significantly associated with being male, younger age, stunting, absence of deworming, education level of household head, increased number of people in the household, fewer mosquito nets in the household and not sleeping under a mosquito net. In the multivariable model, higher odds of P. falciparum infection were significantly associated with being male, younger age groups, increasing number of people living in the child’s household and the child not sleeping under a net (Table 2).
Table 2.
Multivariable risk factor analysis for Plasmodium falciparum infection and anaemia among school children in 51 schools on the south coast of Kenya, 2010
| Variable | P. falciparum infection* | Anaemia† | ||||
|---|---|---|---|---|---|---|
| Adjusted odds ratio‡ | 95% confidence interval | P-value§ | Adjusted odds ratio‡ | 95% confidence interval | P-value§ | |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.68 | 0.51–0.89 | 0.005 | 0.8 | 0.67–0.95 | 0.009 |
| Age (years) | ||||||
| 5–9 | 1 | 1 | ||||
| 10–12 | 0.87 | 0.64–1.18 | 0.71 | 0.58–0.87 | ||
| 13–18 | 0.37 | 0.25–0.54 | <0.001 | 0.97 | 0.78–1.20 | 0.002 |
| Child sleeps under a net | ||||||
| No | 1 | |||||
| Yes | 0.6 | 0.45–0.79 | <0.001 | – | – | – |
| Number of people in the house¶ | ||||||
| 1.07 | 1.01–1.14 | 0.014 | – | – | – | |
| P. falciparum density (p/μl) | ||||||
| No infection | 1 | |||||
| Low (1–999) | 1.41 | 1.05–1.89 | ||||
| Medium/high (≥1000) | – | – | – | 3.68 | 2.12–6.38 | <0.001 |
| HAZ (z-scores) | ||||||
| Not stunted | 1 | |||||
| Stunted | – | – | – | 1.26 | 1.03–1.54 | 0.022 |
| Education level of household head | ||||||
| No schooling | 1 | |||||
| Primary | – | – | – | 0.78 | 0.64–0.94 | |
| Secondary | 1.12 | 0.83–1.50 | 0.014 | |||
| College/degree | 0.89 | 0.53–1.48 | ||||
| Effect of elevation (metres) by absence/presence of school feeding programme**,§§ | ||||||
| No school feeding | ||||||
| 0–50 | 1 | |||||
| 51–100 | 0.58 | 0.40–0.83 | ||||
| 101–200 | 0.58 | 0.34–1.00 | ||||
| School feeding | ||||||
| 0–50 | 1 | 0.003‡‡ | ||||
| 51–100 | 1.3 | 0.79–2.15 | ||||
| 101–200 | 1.32 | 0.63–2.76 | ||||
| Effect of school feeding programme by elevation (metres)**,†† | ||||||
| 0–50 | ||||||
| No school feeding | 1 | |||||
| School feeding | 0.46 | 0.28–0.76 | ||||
| 51–100 | ||||||
| No school feeding | 1 | |||||
| School feeding | 1.05 | 0.72–1.51 | 0.003‡‡ | |||
| 101–200 | ||||||
| No school feeding | 1 | |||||
| School feeding | 0.82 | 0.48–1.39 | ||||
n = 2369 observations included for children with complete data for all variables.
n = 2364 observations included for children with complete data for all variables.
Adjusted for variables included in final multivariable regression model as shown.
P-value derived from likelihood ratio test in multivariable multilevel, logistic regression model, adjusted for school-level clustering.
Modelled as continuous variable.
There was statistical evidence of an interaction between elevation of schools and presence of a school feeding programme on anaemia; therefore, the stratum-specific results are reported for both school feeding and elevation (P-value derived from likelihood ratio test comparing the model with school feeding and elevation variables separately with the model also including the interaction between the two variables is P = 0.042).
P-value is derived from likelihood ratio test comparing the model with both the school feeding and elevation variables and their interaction term with the model without either of the variables.
Of those children without access to a school feeding programme, 515 attend schools at elevations of 0–50m, 442 attend schools at elevations of 51–100m, 137 attend schools at elevations of 101–200m. Of those children with access to a school feeding programme, 176 attend schools at elevations of 0–50m, 466 attend schools at elevations of 51–100m, 628 attend schools at elevations of 101–200m.
At elevation group 50–100 m, 176 children have school feeding and 515 do not. At group 51–100 m, 466 children have school feeding and 442 do not. At elevation group 101–200 m, 628 children have school feeding and 137 do not.
In univariable analysis, anaemia was significantly associated with male sex, younger age, P. falciparum infection, being stunted, education level of household head, not attending a school with an active school feeding programme and attending school at lower elevation, closer to the coast. In multivariable analysis, increased odds of anaemia were significantly associated with P. falciparum infection, with the odds increasing with increasing parasite density (AOR [adjusted odds ratio], 3.68; 95% CI: 2.12–6.38 P < 0.001) for children with high intensity infection vs. those with no infection, and for children who were stunted. Significantly lower odds of anaemia were associated with children who were female, and with being aged 10–12 years old vs. 5–9 years old. The effect of a school feeding programme on anaemia was modified by elevation of school (distance from coast) and thus is presented by stratum-specific odds ratios. School feeding was associated with lower odds of anaemia in schools closest to the coast (AOR, 0.46; 95% CI: 0.28–0.76; P = 0.003), with no evidence of an association for schools positioned further from the coast.
Associations with cognition and educational achievement
Results from the univariable analysis of the associations between cognition and educational achievement and health and other factors are presented in online-only Tables S2, S3 and S4. Results from multivariable analyses are presented in Tables 3 (attention and literacy assessments) and 4 (cognitive, comprehension and numeracy assessments), which report significant associations between scores and several child-level variables. In all tasks, increasing age was associated with higher scores among children in class 1, but with lower scores among children in class 5. For several tasks, girls were found to have lower scores than boys. Neither P. falciparum infection, irrespective of parasite density, nor anaemia was found to be associated with any cognitive or educational outcome. Interestingly, poor engagement in the attention task for class 1 was associated with eating breakfast and attending a school with school feeding, and better spelling performance in class 5 was found among children who were classified as thin on the basis of BMI.
Table 3.
Multivariable risk factor analysis – associations of Plasmodium falciparum infection and anaemia with a test of sustained attention and a test of literacy in children in classes 1 and 5 on the south coast of Kenya, 2010.
| Variable | Attention assessments | Literacy assessments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pencil tap – Class 1 | Code transmission – Class 5 | Spelling – Class 1 | Spelling – Class 5 | |||||||
| Adjusted OR for engagement (95% CI) n = 1122 | P-value* | Mean adjusted difference in performance in children who were engaged†,‡(95% CI) n = 998 | P-value§ | Mean adjusted difference in performance (95% CI)† n = 1227 | P-value § | Mean adjusted difference in performance (95% CI)† n = 1127 | P-value§ | Mean adjusted difference in performance (95% CI)† n = 1216 | P-value§ | |
| Child-level | ||||||||||
| P. falciparum density (p/μl) | ||||||||||
| No infection (0) | 1 | |||||||||
| Low (1–999) | 1.00 (0.53–1.80) | 0.053 | −0.05 (−1.01, 1.12) | 0.06 (−1.42, 1.52) | 0.63 (−0.56, 2.12) | −1.00 (−2.90, 0.89) | ||||
| High (≥1000) | 6.38 (0.85–47.87) | −0.00 (−1.28, 1.23) | 1 | −0.97 (−3.40, 1.03) | 0.638 | 1.14 (0.08, 2.28) | 0.102 | −2.49 (−6.80, 1.31) | 0.205 | |
| Anaemia status | ||||||||||
| Not anaemic | 1 | |||||||||
| Anaemic | 1.20 (0.82–1.77) | 0.353 | 0.17 (−0.41, 0.77) | 0.169 | 0.34 (−0.25, 0.95) | 0.25 | 0.35 (−0.18, 0.98) | 0.343 | 0.69 (−0.28, 1.68) | 0.17 |
| Sex | ||||||||||
| Male | 1 | |||||||||
| Female | 0.80 (0.55–1.17) | 0.249 | −0.62 (−1.16, 0.00) | 0.037 | −0.61 (−1.37, 0.07) | 0.102 | 0.43 (−0.13, 1.02) | 0.268 | −1.32 (−2.14, −0.46) | 0.003 |
| Age (years)¶ | ||||||||||
| 1.16 (1.03–1.31) | 0.014 | 0.44 (0.22, 0.62) | <0.001 | −0.30 (−0.47, −0.10) | 0.003 | 0.27 (0.06, 0.47) | 0.025 | −1.32 (−1.65, −1.03) | <0.001 | |
| BMIZ (z-score) | ||||||||||
| Not thin | ||||||||||
| Thin | – | – | – | – | – | – | – | – | 1.12 (0.13, 2.11) | 0.026 |
| Eat breakfast before school | ||||||||||
| No | 1 | – | – | – | – | – | – | – | – | |
| Yes | 0.46 (0.28–0.74) | 0.001 | ||||||||
| Household-level | ||||||||||
| SES quintile | ||||||||||
| Poorest | ||||||||||
| Poor | −0.17 (−1.17, 0.72) | 0.09 (−0.59, 0.91) | −0.14 (−1.32, 1.34) | |||||||
| Median | – | – | −0.85 (−1.80, 0.04) | – | – | 0.49 (−0.33, 1.38) | 2.30 (1.24, 3.52) | <0.001 | ||
| Less poor | −0.92 (−2.00, 0.17) | 0.026 | 1.20 (0.43, 2.17) | 0.006 | 1.42 (0.30, 2.60) | |||||
| Least poor | −1.27 (−2.33, −0.16) | 1.48 (0.59, 2.46) | 3.32 (1.95, 4.80) | |||||||
| School-level | ||||||||||
| School feeding programme | ||||||||||
| No | 1 | |||||||||
| Yes | 0.62 (0.39–0.98) | 0.039 | – | – | – | – | – | – | – | – |
| Seating in classroom | ||||||||||
| Desks or tables and chairs | ||||||||||
| Floor | – | – | – | – | – | – | −1.76 (−3.55, −0.48) | 0.026 | – | – |
| Division | ||||||||||
| Diani | ||||||||||
| Lunga Lunga | −0.18 (−1.16, 0.74) | −3.53 (−5.79, −1.49) | ||||||||
| Msambweni | – | – | −1.20 (−2.40, −0.08) | 0.016 | – | – | – | – | −3.95 (−6.17, −2.13) | <0.001 |
| Kubo | −1.58 (−2.78, −0.39) | −3.79 (−6.19, −1.33) | ||||||||
P-value derived from likelihood ratio test of model with and without variable of interest in multivariable multilevel logistic regression analysis (adjusting for school-level clustering).
Positive values indicate an increased score over reference group, and negative values indicate a decreased score over reference group (95% CI is the bias-corrected confidence interval).
Only children found to be engaged in task are included.
P-value is from multivariable Wald test derived from multivariable linear regression, bootstrapped and adjusted for school-level clustering.
Modelled as a continuous variable.
The bold numbers refer to those with a P-value of 0.05 or less in the multivariable model.
Table 4.
Multivariable risk factor analysis – associations of Plasmodium falciparum infection and anaemia with a test of cognition and numeracy in children in classes 1 and 5 on the south coast of Kenya, 2010.
| Variable | Cognitive non-verbal reasoning assessment | Comprehension assessment | Numeracy assessments | |||||
|---|---|---|---|---|---|---|---|---|
| Ravens test – Class 1 | Silly sentences – Class 5 | Number identification – Class 1 | Written numeracy – Class 5 | |||||
| Mean difference in performance in children who were engaged (95% CI)* n = 1118 | P-value† | Mean difference in performance (95% CI)* n = 1211 | P-value† | Mean difference in performance (95% CI)* n = 1119 | P-value† | Mean difference in performance(95% CI)* n = 1219 | P-value† | |
| Child level | ||||||||
| P. falciparum density (p/μl) | ||||||||
| No infection (0) | ||||||||
| Low (1–999) | −0.48 (−0.97, 0.02) | −0.03 (−2.07, 0.88) | −0.10 (−0.60, 0.37) | 0.07 (−1.28, 1.16) | ||||
| Medium/high (≥1000) | 0.23 (−0.51, 1.23) | 0.151 | −1.09 (−3.78, 2.95) | 0.799 | −0.15 (−0.84, 0.57) | 0.876 | 0.51 (−1.18, 2.57) | 0.866 |
| Anaemia status | ||||||||
| Not anaemic | ||||||||
| Anaemic | −0.01 (−0.27, 0.25) | 0.936 | 0.14 (−0.65, 0.86) | 0.96 | 0.34 (0.00, 0.70) | 0.053 | −0.21 (−0.89, 0.47) | 0.54 |
| Sex | ||||||||
| Male | ||||||||
| Female | −0.08 (−0.33, 0.14) | 0.524 | −1.08 (−1.84,−0.18) | 0.005 | 0.03 (−0.28, 0.32) | 0.841 | 0.09 (−0.64, 0.74) | 0.8 |
| Age (years)‡ | ||||||||
| 0.13 (0.02, 0.25) | 0.029 | −0.56 (−0.88,−0.27) | <0.001 | 0.26 (0.13, 0.38) | <0.001 | −0.02 (−0.26, 0.19) | 0.87 | |
| Household-level | ||||||||
| SES quintile | ||||||||
| Poorest | ||||||||
| Poor | −0.19 (−1.24, 0.92) | |||||||
| Median | – | – | 1.54 (0.37, 2.74) | – | – | – | – | |
| Less poor | 1.07 (0.01, 2.15) | <0.001 | ||||||
| Least poor | 2.43 (1.18, 3.68) | |||||||
| No. of children in household | ||||||||
| −0.24 (−0.40,−0.08) | 0.026 | – | – | – | – | |||
| Education of household head | ||||||||
| No schooling | ||||||||
| Primary | – | – | −0.06 (−0.81, 0.64) | – | – | – | – | |
| Secondary | 1.11 (−0.35, 1.93) | 0.012 | ||||||
| College/degree | 3.33 (1.10, 4.88) | |||||||
| Parent is literate | ||||||||
| No | ||||||||
| Yes | – | – | – | – | 0.49 (0.18, 0.82) | 0.003 | – | – |
| School-level | ||||||||
| Seating arrangement in classroom | ||||||||
| Desks or tables and chairs | ||||||||
| Floor | – | – | – | – | −0.78 (−1.34,−0.30) | 0.005 | – | – |
| Division | ||||||||
| Diani | ||||||||
| Lunga Lunga | −0.87 (−1.34,−0.45) | −2.48 (−3.85,−0.88) | ||||||
| Msambweni | 0.51 (−0.31, 1.43) | <0.001 | – | – | – | – | −1.69 (−3.53,−0.00) | <0.001 |
| Kubo | −0.47 (−1.08, 0.22) | −3.76 (−6.38,−1.94) | ||||||
Positive values indicate an increased score over reference group, and negative values indicate a decreased score over reference group (95% CI is the bias-corrected confidence interval).
P-value is from multivariable Wald test derived from multivariable linear regression, bootstrapped and adjusted for school-level clustering.
Modelled as a continuous variable.
The bold numbers refer to those with a p-value of 0.05 or less in the multivariable model.
A number of household factors were also associated with the scores. Higher household socio-economic status was associated with higher scores in the comprehension task in class 5 and the spelling in both classes. Lower scores were associated with living in a house with a high number of children for the class 5 comprehension task. Higher parental education levels were associated with higher scores in the class 5 comprehension and class 1 numeracy tasks. School environment and educational administrative zones were found to be associated with several of the tasks, with lower literacy and numeracy scores associated with children learning in classrooms without desks, and significantly higher spelling and numeracy scores in class 5 as well as attention and cognitive scores in class 1 found in children schooling in coastal Diani zone.
Discussion
The evidence presented here shows that in this moderate malaria transmission setting, there is marked variation in the prevalence of P. falciparum, reaching 75% in some schools. There was also evidence that infection is strongly associated with anaemia, with the odds higher with increasing density of infection. The results also show potentially important variation in the malaria burden between the sexes and age groups and by school. The scale of observed health problems strongly supports the need for school health programmes aimed at reducing the health burden of malaria in school children. Despite this health burden, the analysis of educational data suggested no association between current health status and measurements of sustained attention and educational achievement.
The geographical heterogeneity observed in the prevalence of P. falciparum infection is likely to reflect a complexity of factors that influence vector distribution and density as well as vector–human contact and human infection (Greenwood 1989). The principal malaria vectors in the study are Anopheles gambiae s.l. and An. funestus, which in our study area have been shown to exhibit strong spatial and temporal heterogeneity related, in part, to variation in rainfall (Mbogo et al. 2003) and, more recently, variation in mosquito net use and type of household construction (Mutuku et al. 2011). Human–vector contact and human infection may also be influenced by proximity to vector breeding sites (Clarke et al. 2002; Pullan et al. 2010) and variation in personal protection measures (Snow et al. 1998) and net use (Pullan et al. 2010). Future geostatistical analysis will investigate the environmental correlates of the observed variation in infection patterns. Such geographical heterogeneity in infection risk has particular implications for the targeting of malaria interventions as well as for the possible impact of intervention (Kleinschmidt et al. 2007). School-level variation in the prevalence of anaemia may reflect the observed geographical variation in the prevalence of P. falciparum infection, but is also likely to be due to differences in food availability and the prevalence of helminth infection, important aetiological factors for anaemia.
The protective effect of sleeping under a mosquito net is consistent with previous cross-sectional findings (Noor et al. 2008; Pullan et al. 2010), whilst the strong association between P. falciparum infection and anaemia has been observed in other school-aged populations in East Africa (Stoltzfus et al. 1997; Olsen et al. 1998; Leenstra et al. 2004; Koukounari et al. 2008). The impact of chronic P. falciparum infection on haemoglobin levels is attributed to increased red blood cell destruction and decreased red blood cell production (Abdalla et al. 1980; Phillips & Pasvol 1992; Menendez et al. 2000), with high-density infections intensifying these processes. However, anaemia is multifactorial, and the findings of this study highlight additional contributory factors: stunting, indicative of poor nutritional intake for a sustained period during the childhood growth phase, was associated with increased odds of anaemia. This nutritional relationship is supported by the finding that at sea level, in the schools nearest the coast where the soil is infertile and the crop-growing potential is poor, the presence of a school feeding programme at the child’s school appears to be associated with a 50% decrease in odds of anaemia. Few studies to date have measured the effect of school feeding on anaemia (Jomaa et al. 2011), although provision of iron-fortified porridge and biscuits and cakes as part of school feeding programmes has been shown to be associated with a reduction in anaemia in Kenya, South Africa and Peru (Jacoby et al. 1998; van Stuijvenberg et al. 1999; Andang’o et al. 2007). Micronutrient deficiency is commonly found among school-aged children in malaria-endemic areas (Best et al. 2010), and infection with P. falciparum is bound to further increase the stress on the haemoglobin status in individuals who are already anaemic (Topley 1968; Nussenblatt & Semba 2002).
The lack of observed association between health status and sustained attention and education may not necessarily reflect an absence of effect of malaria on education. First, asymptomatic P. falciparum can persist for over three months and as children may be constantly re-infected, it is probable that infection has a cumulative effect on cognitive function over an extended period of time. Thus, the single time point of our cross-sectional design may not sufficiently capture the effects of recurrent, chronic infection over an extended period (Jukes et al. 2002; Thuilliez 2010). Second, the cross-sectional design meant that we were unable to capture information on past clinical attacks, which have previously been shown to be related to poor educational achievement (Fernando et al. 2003b). Third, malaria is just one of many contributing factors to poorer cognitive and educational performance, with socio-economic status and the educational environment of children’s homes playing an important role, as highlighted in the present study.
The association found in both classes between higher literacy and attention scores and indicators of SES is supported by previous findings where SES has been found to be strongly related to psychometric and education test scores in school children (Jukes et al. 2002). Increased SES is likely to be associated with increased stimulation, increased access to reading material and ownership of school-related materials, factors previously shown to be associated with increased academic achievement (Walker et al. 1998). This is supported by the fact that increased education of household heads and increased literacy were associated with improved performance in comprehension in class 5 and numeracy in class 1. As expected, there was a positive relationship between age and assessment scores for children in class 1. By contrast, increasing age was associated with lower scores in assessments in class 5. This seemingly contradictory observation could be attributed to the older children in class 5 having repeated earlier years because of poor educational performance, as is frequently seen in low-income countries (Heyneman & Loxley 1983; Fehrler et al. 2009). Also, poor children enrol in school later (Fentiman et al. 1999). The poorer scores in attention (class 1) and literacy (class 5) assessments observed in females are consistent with the recognized disparity between sexes in access to education and support in many low-income settings (Jukes et al. 2008). The strong variation in educational performance by administrative division is an indicator that there are aspects of the school divisional organization and management, such as the availability of books, the teacher–child contact time and the quality of teaching, which may influence educational outcomes (Heyneman & Loxley 1983; Boissiere 2004; Bhargava et al. 2005). The importance of the school environment is further demonstrated by the lower literacy and numeracy scores observed in class 1 children who learn in classrooms with no desks.
In conclusion, we have found a strong geographical variation in the prevalence of P. falciparum infection, underscoring the need for geographical targeting of malaria interventions. The observed strong association between infection and anaemia provides evidence of the, presumably cumulative, negative effects of asymptomatic P. falciparum infection on the haemoglobin status of school children. The ongoing trial of IST will provide an indication of how much of this effect can be reversed and whether malaria control can also improve the cognitive and educational performance of children in the current setting. The trial will also help understand how much of the observed poor performance is attributed to health factors and how much is attributed to teaching quality and household factors.
Acknowledgments
We would like to thank the school children, school teachers, district education officials and the district health management team for their support, and Tansy Edwards for statistical advice. The work is funded by the International Initiative for Impact Evaluation and the World Bank, through the Norwegian Education Trust Fund, the multi-donor Education Programme Development Fund (EPDF) and the Spanish Impact Evaluation Fund. The results reported here contributed to the World Bank Africa Programme for Education Impact Evaluation and the Malaria Impact Evaluation Programme. Additional funding is provided by the Wellcome Trust through a Masters Training Fellowship to George Okello (092765) and a Research Career Development Fellowship to Simon Brooker (081673).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1 Characteristics of study children with health data only or health and education data (included in analysis) and study children with education data only (excluded from analysis).
Table S2 Univariable analyses for associations of Plasmodium falciparum infection and anaemia and additional potential risk factors with a test of cognition (Ravens test), numeracy (Number Identification test) and a test of literacy (Spelling test) in class 1 children on the south coast of Kenya, 2010.
Table S3. Univariable analyses for associations of Plasmodium falciparum infection and anaemia and additional potential risk factors with a test of sustained attention (pencil tapping) in class 1 children on the south coast of Kenya, 2010.
Table S4. Univariable analyses for associations of Plasmodium falciparum infection and anaemia and additional potential risk factors with a test of cognition (Silly Sentences), numeracy (Written Numeracy test), literacy (Spelling test) and sustained attention (Code Transmission test) in class 5 children on the south coast of Kenya, 2010.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abdalla S, Weatherall DJ, Wickramasinghe SN, Hughes M. The anaemia of P. falciparum malaria. British Journal of Haematology. 1980;46:171–183. doi: 10.1111/j.1365-2141.1980.tb05956.x. [DOI] [PubMed] [Google Scholar]
- Al Serouri AW, Grantham-McGregor SM, Greenwood B, Costello A. Impact of asymptomatic malaria parasitaemia on cognitive function and school achievement of schoolchildren in the Yemen Republic. Parasitology. 2000;121:337–345. doi: 10.1017/s0031182099006502. [DOI] [PubMed] [Google Scholar]
- Andang’o PE, Osendarp SJ, Ayah R, et al. Efficacy of iron-fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet. 2007;369:1799–1806. doi: 10.1016/S0140-6736(07)60817-4. [DOI] [PubMed] [Google Scholar]
- Barger B, Maiga H, Traore OB, et al. Intermittent preventive treatment using artemisinin-based combination therapy reduces malaria morbidity among school-aged children in Mali. Tropical Medicine and International Health. 2009;14:784–791. doi: 10.1111/j.1365-3156.2009.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2003. In: WHO, editor. WHO Global Database on Anaemia. Geneva: World Health Organisation; 2008. pp. 1–40. [Google Scholar]
- Best C, Neufingerl N, van Geel L, van den Briel T, Osendarp S. The nutritional status of school-aged children: why should we care? Food Nutrition Bulletin. 2010;31:400–417. doi: 10.1177/156482651003100303. [DOI] [PubMed] [Google Scholar]
- Bhargava A, Jukes M, Ngorosho D, Khilma C, Bundy DA. Modeling the effects of health status and the educational infrastructure on the cognitive development of Tanzanian schoolchildren. American Journal of Human Biology. 2005;17:280–292. doi: 10.1002/ajhb.20142. [DOI] [PubMed] [Google Scholar]
- Boissiere M. Determinants of primary education outcomes in developing countries. In: World Bank, editor. Background paper for Evaluation of the World Bank's Support to Primary Education. World Bank Operations Evaluation Department. Washington, DC: World Bank; 2004. pp. 1–44. [Google Scholar]
- Brooker S, Guyatt H, Omumbo J, Shretta R, Drake L, Ouma J. Situation analysis of malaria in school-aged children in Kenya – what can be done? Parasitology Today. 2000;16:183–186. doi: 10.1016/s0169-4758(00)01663-x. [DOI] [PubMed] [Google Scholar]
- Brooker S, Kolaczinski JH, Gitonga CW, Noor AM, Snow RW. The use of schools for malaria surveillance and programme evaluation in Africa. Malaria Journal. 2009;8:231. doi: 10.1186/1475-2875-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Okello G, Njagi K, et al. Improving educational achievement and anaemia of school children: design of a cluster randomised trial of school-based malaria prevention and enhanced literacy instruction in Kenya. Trials. 2010;11:93. doi: 10.1186/1745-6215-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy D. Rethinking school health: a key component of education for all. Washington: The World Bank; 2011. [Google Scholar]
- Bundy D, Shaeffer S, Jukes M. School-based Health and Nutrition Programmes. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, et al., editors. Disease Control Priorities in Developing Countries. 2nd edn. Washington: Oxford University Press; 2006. pp. 1091–1108. [PubMed] [Google Scholar]
- Clarke SE, Bogh C, Brown RC, Walraven GE, Thomas CJ, Lindsay SW. Risk of malaria attacks in Gambian children is greater away from malaria vector breeding sites. Transactions of the Royal Society of Tropical Medicine & Hygiene. 2002;96:499–506. doi: 10.1016/s0035-9203(02)90419-0. [DOI] [PubMed] [Google Scholar]
- Clarke SE, Jukes MC, Njagi JK, et al. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne MJ. The effect of malaria suppression in a group of Accra school children. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1955;49:556–569. doi: 10.1016/0035-9203(55)90058-4. [DOI] [PubMed] [Google Scholar]
- Draper CC. Effect of malaria control on haemoglobin levels. British Medical Journal. 1960;1:1480–1483. doi: 10.1136/bmj.1.5184.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. Introduction to the bootstrap (monographs on statistics and applied probability) Boca Raton, FL: Chapman & Hall CRC; 1994. [Google Scholar]
- Fehrler S, Michaelowa K, Wechtler A. The effectiveness of inputs in primary education: insights from recent student surveys for Sub-Saharan Africa. Journal of Development Studies. 2009;45:1545–1578. [Google Scholar]
- Fentiman A, Hall A, Bundy A. School enrolment patterns in rural Ghana: a comparative study of the impact of location, gender, age and health on children’s access to basic schooling. Comparative Education. 1999;35:331–349. [Google Scholar]
- Fernando D, De Silva D, Wickremasinghe R. Short-term impact of an acute attack of malaria on the cognitive performance of schoolchildren living in a malaria-endemic area of Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003a;97:633–639. doi: 10.1016/s0035-9203(03)80093-7. [DOI] [PubMed] [Google Scholar]
- Fernando D, Wickremasinghe R, Mendis KN, Wickremasinghe AR. Cognitive performance at school entry of children living in malaria-endemic areas of Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003b;97:161–165. doi: 10.1016/s0035-9203(03)90107-6. [DOI] [PubMed] [Google Scholar]
- Fernando D, de Silva D, Carter R, Mendis KN, Wickremasinghe R. A randomized, double-blind, placebo-controlled, clinical trial of the impact of malaria prevention on the educational attainment of school children. American Journal of Tropical Medicine and Hygiene. 2006;74:386–393. [PubMed] [Google Scholar]
- Fernando SD, Rodrigo C, Rajapakse S. The ‘hidden’ burden of malaria: cognitive impairment following infection. Malaria Journal. 2010;9:366. doi: 10.1186/1475-2875-9-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer D, Pritchett LH. Estimating wealth effects without expenditure data – or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Geerligs PD, Brabin BJ, Eggelte TA. Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bulletin of The World Health Organisation. 2003;81:205–216. [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM. The microepidemiology of malaria and its importance to malaria control. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1989;83(Suppl):25–29. doi: 10.1016/0035-9203(89)90599-3. [DOI] [PubMed] [Google Scholar]
- Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infectious Diseases. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyneman SP, Loxley WA. The effect of primary-school quality on academic achievement across twenty-nine high- and low-income countries. American Journal of Sociology. 1983;88:1162–1194. doi: 10.1086/227799. [DOI] [PubMed] [Google Scholar]
- Holding PA, Snow RW. Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. American Journal of Tropical Medicine and Hygiene. 2001;64:68–75. doi: 10.4269/ajtmh.2001.64.68. [DOI] [PubMed] [Google Scholar]
- Jacoby ER, Cueto S, Pollitt E. When science and politics listen to each other: good prospects from a new school breakfast program in Peru. American Journal of Clinical Nutrition. 1998;67:795s–797s. doi: 10.1093/ajcn/67.4.795S. [DOI] [PubMed] [Google Scholar]
- Jomaa LH, McDonnell E, Probart C. School feeding programs in developing countries: impacts on children’s health and educational outcomes. Nutrition Review. 2011;69:83–98. doi: 10.1111/j.1753-4887.2010.00369.x. [DOI] [PubMed] [Google Scholar]
- Jukes MCH, Nokes CA, Alcock KJ, et al. Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Tropical Medicine & International Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- Jukes MC, Pinder M, Grigorenko EL, et al. Long-term impact of malaria chemoprophylaxis on cognitive abilities and educational attainment: follow-up of a controlled trial. PLoS Clinical Trials. 2006;1:e19. doi: 10.1371/journal.pctr.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes MCH, Drake L, Bundy DAP. School Health, Nutrition and Education for All. Wallingford, UK: CABI Publishing; 2008. [Google Scholar]
- Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Tropical Medicine & International Health. 2006;11:386–397. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- Kimbi HK, Awah NW, Ndamukong KJ, Mbuh JV. Malaria infection and its consequences in school children. East African Medical Journal. 2005;82:92–97. doi: 10.4314/eamj.v82i2.9261. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt I, Torrez M, Schwabe C, et al. Factors influencing the effectiveness of malaria control in Bioko Island, equatorial Guinea. American Journal of Tropical Medicine and Hygiene. 2007;76:1027–1032. [PMC free article] [PubMed] [Google Scholar]
- Korenromp EL, Armstrong-Schellenberg JR, Williams BG, Nahlen BL, Snow RW. Impact of malaria control on childhood anaemia in Africa – a quantitative review. Tropical Medicine and International Health. 2004;9:1050–1065. doi: 10.1111/j.1365-3156.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- Koukounari A, Estambale BB, Njagi JK, et al. Relationships between anaemia and parasitic infections in Kenyan schoolchildren: a Bayesian hierarchical modelling approach. International Journal for Parasitology. 2008;38:1663–1671. doi: 10.1016/j.ijpara.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenstra T, Kariuki SK, Kurtis JD, Oloo AJ, Kager PA, ter Kuile FO. Prevalence and severity of anemia and iron deficiency: cross-sectional studies in adolescent schoolgirls in western Kenya. European Journal of Clinical Nutrition. 2004;58:681–691. doi: 10.1038/sj.ejcn.1601865. [DOI] [PubMed] [Google Scholar]
- Manly T, Robertson IH, Anderson V, Nimmo-Smith I. Test of Everyday Attention for Children: TEA-Ch. Bury St Edmunds, UK: Thames Valley Test Company; 1999. [DOI] [PubMed] [Google Scholar]
- Mbogo CM, Mwangangi JM, Nzovu J, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. American Journal of Tropical Medicine and Hygiene. 2003;68:734–742. [PubMed] [Google Scholar]
- Menendez C, Fleming AF, Alonso PL. Malaria-related anaemia. Parasitology Today. 2000;16:469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- Mutuku FM, King CH, Mungai P, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malaria Journal. 2011;10:356. doi: 10.1186/1475-2875-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor A, Moloney G, Borle M, Fegan GW, Shewchuk T, Snow RW. The use of mosquito nets and the prevalence of Plasmodium falciparum infection in rural south central Somalia. PLoS ONE. 2008;3:e2081. doi: 10.1371/journal.pone.0002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt V, Semba RD. Micronutrient malnutrition and the pathogenesis of malarial anemia. Acta Tropica. 2002;82:321–337. doi: 10.1016/s0001-706x(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Olsen A, Magnussen P, Ouma JH, Andreassen J, Friis H. The contribution of hookworm and other parasitic infections to haemoglobin and iron status among children and adults in western Kenya. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1998;92:643–649. doi: 10.1016/s0035-9203(98)90795-7. [DOI] [PubMed] [Google Scholar]
- Phillips RE, Pasvol G. Anaemia of Plasmodium falciparum malaria. Baillieres Clinical Haematology. 1992;5:315–330. doi: 10.1016/s0950-3536(11)80022-3. [DOI] [PubMed] [Google Scholar]
- Pullan RL, Bukirwa H, Staedke SG, Snow RW, Brooker S. Plasmodium infection and its risk factors in eastern Uganda. Malaria Journal. 2010;9:2. doi: 10.1186/1475-2875-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Press; 2008. [Google Scholar]
- Raven JC, Styles I, Raven MA. Raven’s Progressive Matrices: CPM parallel test booklet. Oxford, UK: Oxford Psychologists Press; 1998. [Google Scholar]
- RTI International. Early Grade Reading Kenya: Baseline Assessment. Analyses and Implications for Teaching Interventions Design. Final Report. RTI International; 2008. Research Triangle Park, NC. [Google Scholar]
- Smith DL, Guerra CA, Snow RW, Hay SI. Standardizing estimates of the Plasmodium falciparum parasite rate. Malaria Journal. 2007;6:131. doi: 10.1186/1475-2875-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Schellenberg JR, Peshu N, et al. Periodicity and space-time clustering of severe childhood malaria on the coast of Kenya. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1993;87:386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]
- Snow RW, Peshu N, Forster D, et al. Environmental and entomological risk factors for the development of clinical malaria among children on the Kenyan coast. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1998;92:381–385. doi: 10.1016/s0035-9203(98)91056-2. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chwaya HM, Tielsch JM, Schulze KJ, Albonico M, Savioli L. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. American Journal of Clinical Nutrition. 1997;65:153–159. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- Thuilliez J. Fever, malaria and primary repetition rates amongst school children in Mali: combining demographic and health surveys (DHS) with spatial malariological measures. Social Science and Medicine. 2010;71:314–323. doi: 10.1016/j.socscimed.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Topley E. Common anaemia in rural Gambia. 3. A spontaneously remitting anaemia possibly precipitated by malarial parasitaemia. Transactions of the Royal Society of Tropical Medicine & Hygiene. 1968;62:602–606. doi: 10.1016/0035-9203(68)90109-0. [DOI] [PubMed] [Google Scholar]
- van Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benade AJ. Effect of iron-, iodine-, and beta-carotene-fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial. American Journal of Clinical Nutrition. 1999;69:497–503. doi: 10.1093/ajcn/69.3.497. [DOI] [PubMed] [Google Scholar]
- Vitor-Silva S, Reyes-Lecca RC, Pinheiro TRA, Lacerda MVG. Malaria is associated with poor school performance in an endemic area of the Brazilian Amazon. Malaria Journal. 2009;8:230. doi: 10.1186/1475-2875-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SP, Grantham-McGregor SM, Himes JH, Williams S, Duff EM. School performance in adolescent Jamaican girls: associations with health, social and behavioural characteristics, and risk factors for dropout. Journal of Adolescence. 1998;21:109–122. doi: 10.1006/jado.1997.0133. [DOI] [PubMed] [Google Scholar]
- WHO. Anthroplus: Growth Reference 5–19 years [Online] Geneva: WHO; 2007. http://www.who.int/growthref/tools/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


