Abstract
Hepatocellular carcinoma (HCC), a worldwide malignancy, is prevalent in Asian countries. For individuals with unresectable HCC, the effect of chemotherapy or the present target therapy is limited. There is an urgent need to find innovative new therapies. It is believed that sonic hedgehog (Shh) pathway activation may be essential for hepatocarcinogenesis. In the present study, we conducted an in vivo animal study using an Shh pathway inhibitor to elucidate the effect of treatment upon mice with HCC. Eighty C57BL/6 mice were divided into 4 groups (groups A, B, C and D, with group A serving as a control; n=20 for each). We injected mouse hepatoma Mistheton Lectin-1 cells (5×106 cells/20 μl) into the left liver of each mouse in groups B, C and D. In the second week, we analyzed each mouse to assess the tumor growth status. Following the tumor injection, group B did not receive any additional intraperitoneal (i.p.) injection, group C received cyclopamine 10 mg/kg/day i.p. and group D received cyclopamine 30 mg/kg/day i.p. every day for 10 days. After an interval of 4 weeks, harvesting and analysis of the liver was performed for each mouse. Tumor size measurement and real-time PCR of Shh pathway factors (Shh, Ptch-1, Gli-1 and Smoh) for livers of group A and tumors of group B, C and D were undertaken. The decrease in the tumor size of group D was found to be statistically significant (P= 0.047) when compared with groups B or C. The decrease of Shh mRNA of both groups C and D had borderline significance when compared with group B. However, Gli-1 mRNA of group D has statistically significant difference (P=0.044) when compared with group A, B or C. Inhibition of the Shh pathway significantly decreases the size and Gli-1 mRNA expression of the tumor. The Shh pathway may be an effective treatment target for HCC in the future.
Keywords: hepatocellular carcinoma, sonic hedgehog pathway
Introduction
Hepatocellular carcinoma (HCC), one of the most prevalent malignancies worldwide, is recently rapidly increasing in the United States, and is particularly prevalent in Taiwan and other Asian counties (1–5). For individuals with unresectable HCC, the effect of either chemotherapy or the present target therapy is limited. The need to find new therapies is urgent.
Previous studies have suggested that abnormal activation of the sonic hedgehog (Shh) signaling pathway may be essential for carcinogenesis in certain cancer types, including HCC (6–18). Cyclopamine, a well-known antagonist of Smoothened (Smoh), may inhibit the Shh pathway. The effect of cyclopamine on hepatocarcinogenesis has been described in a previous study (19). However, the in vivo effect remains unknown. We conducted this study to investigate the treatment effect of cyclopamine upon HCC in an in vivo model of mice.
Materials and methods
Treatment groups
Eighty C57BL/6 mice (6–8 weeks old, 19–24 g) were purchased and divided into 4 groups (A, B, C and D) with 20 mice in each. Group A formed the control group. Under isoflurane general anesthesia, we injected mouse hepatoma cells, i.e., Mistheton Lectin-1 (ML-1) cells (5×106 cells/20 μl), into the left liver of mice in groups B, C and D. In the second week, we analyzed each mouse to assess the tumor growth status. Following the initial tumor injection, group B did not receive any additional drug injections. Group C received cyclopamine 10 mg/kg/day i.p and group D received cyclopamine 30 mg/kg/day i.p. The injections were administered every day for 10 days. After an interval of 4 weeks, exploration and harvesting of the liver was performed for each group. The tumor size was measured for groups B, C and D. Real-time PCR analysis of Shh pathway factors [Shh, patched homolog-1 (Ptch-1), glioma-associated oncogene homolog-1 (Gli-1) and Smoh) of the livers in group A and of the tumors in groups B, C and D were undertaken. The experiment was conducted under the Guidelines for the Care and Use of Laboratory Animals of the Far Eastern Memorial Hospital, Taiwan. The institutional licensing committee had approved the experiments undertaken.
Definition of effective reduction of tumor size following treatment
The maximal diameter of the tumor size of each mouse (groups B, C and D) was measured at the final evaluation. Effective reduction of the tumor size following treatment was defined if there was a statistically significant reduction in the tumor size as measured at the end of the study compared to the tumor size prior to treatment (measured in the second week after ML-1 cell injection).
Detection of mouse mRNA of Shh, Ptch-1, Gli-1 and Smoh
The examination included extraction of RNA and reverse transcription, and amplification of cDNA of Shh, Ptch-1, Gli-1, Smoh and housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by real-time PCR.
Extraction of RNA and reverse transcription PCR
We homogenized each resected cancer and liver tissue completely in 1 ml TRIzol® reagent (Invitrogen, Carlsbad, CA, USA), and added 0.2 ml chloroform and agitated it vigorously by hand for 15–30 sec, then incubated them on ice for 20 min. The samples were then centrifuged at 13,000 rpm for 20 min at 4°C. Following centrifugation, the mixture was separated into a lower red, phenol-chloroform phase, an interphase and a colorless upper aqueous phase. RNA remained exclusively in the aqueous phase. We transferred the aqueous phase to a fresh tube, and precipitated the RNA from the aqueous phase by mixing in 0.5 ml of isopropanol. The samples were incubated on ice for 20 min and were centrifuged at 13,000 rpm for 20 min at 4°C. The RNA precipitation, often invisible before centrifugation, formed a gel-like pellet on the side and bottom of the tube. We removed the supernatant and washed the RNA pellet once with 75% ethanol, adding at least 1 ml 75% ethanol. We centrifuged it at 13,000 rpm for 5 min at 4°C. The RNA pellet was dried and RNA was dissolved in RNase-free water. Then it was incubated for 10 min at 60°C and was stored at −80°C.
cDNA was synthesized from 2 mg mRNA. The reverse transcription reaction solution consisted of 2.0 ml 10X RT buffer, 0.8 ml 100 mM dNTP mixed with 2.0 ml 10X RT random primers and 1.0 ml MultiScribe™ Reverse Transcriptase (Applied Biosystems, Foster City, CA, USA). The RNA solution was mixed with the reverse transcription reaction solution (total volume 20 ml) and incubated at 25°C for 10 min, 37°C for 120 min and 85°C for 5 sec. It was stored at −20°C.
Amplification of cDNA of Shh, Ptch-1, Gli-1, Smoh and GAPDH by real-time PCR
Total RNA was extracted from each liver tissue and HCC tissue using TRIzol reagent. RT-PCR was performed using high-capacity cDNA reverse transcription kits (Applied Biosystems). In brief, 2–5 μg total RNA was used in a 20-μl reverse transcription assay. Subsequently, the cDNA was diluted at 1:4 for real-time PCR assays which were carried out in a 96-well plate in the LightCycler 480 (Roche Diagnostics, Mannheim, Germany) using SYBR-Green I Master dye (Roche Diagnostics). Each real-time PCR assay (10 μl) contained 3 μl water, 0.5 μl forward and reverse primers, respectively, 5 μl 2X SYBR-Green I Master and 1 μl diluted cDNA. All primer sequences used for real-time analysis are listed in Table I. Real-time PCR parameters were cycled as follows: hot start at 95°C for 1 min, followed by 45 cycles of denaturing at 95°C for 10 sec, annealing at 58°C for 5 sec and extension at 72°C for 20 sec. PCR products were detected using 2% agarose gel to confirm the expected sizes. To normalize the total amount of cDNA in each reaction, GAPDH was coamplified as the internal control. Each sample was analyzed 3 times and quantified with the analysis software for LightCycler (Roche Diagnostics).
Table I.
Sequences of primer pairs.
| Gene | Direction | Primers (5′-3′) |
|---|---|---|
| GAPDH | sense | 5′-CACCACCAACTGCTTAG-3′ |
| antisense | 5′-CTTCACCACCTTCTTGATG-3′ | |
| Shh | sense | 5′-AAAGCTGACCCCTTTAGCCTA-3′ |
| antisense | 5′-TTCGGAGTTTCTTGTGATCTTCC-3′ | |
| Ptch-1 | sense | 5′-CCGTTCAGCTCCGCACAGA-3′ |
| antisense | 5′-CTCACTCGGGTGGTCCCATAAA-3′ | |
| Gli-1 | sense | 5′-TGTGGCGAATAGACAGAGGT-3′ |
| antisense | 5′-TGCCAGATATGCTTCAGCA-3′ | |
| Smoh | sense | 5′-GAGCGTAGCTTCCGGGACTA-3′ |
| antisense | 5′-CTGGGCCGATTCTTGATCTCA-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Shh, sonic hedgehog; Ptch-1, patched homolog-1; Gli-1, glioma-associated oncogene homolog-1; Smoh, smoothened homolog.
Statistical analysis
Comparisons between groups were performed with a Chi-square test (or Fisher’s exact test) for continuous variables. The least significant difference (LSD) pair-wise multiple comparison was used for multivariate analysis of associated factors. All statistical analyses were performed using the SPSS version 17.0 (Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant result.
Results
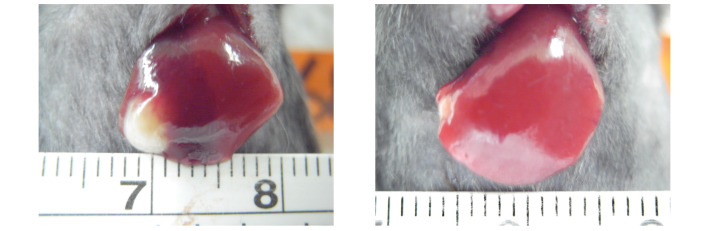
No tumors were observed in the livers in group A mice at any point in this study. At the end of the second week following injection of ML-1 cells, tumors developed successfully in left lobe of the liver of all the mice in groups B, C and D. At the end of the study, the reduction of the tumor size in group D was found to be significant, from 0.152±0.219 cm2 (mean ± SD) before treatment to 0.003±0.009 cm2 (mean ± SD) after treatment (P=0.047). The tumor size in group C reduced from 0.152±0.219 cm2 to 0.071±0.187 cm2 without statistical significance (P=0.267). Fig. 1 shows the decrease of tumor size in one mouse from group D.
Figure 1.
The size of liver tumor of one mouse in group D decreased from 3×2 mm2 (left) to 1.2×1 mm2 (right). Left: at second week after ML-1 cell injection, before cyclopamine treatment. Right: at fourth week after treatment with cyclopamine (i.p. 30 mg/kg/day × 10 days)
Table II shows the mRNA expression of Shh, Ptch-1, Gli-1 and Smoh in the 4 groups. Compared with group B, the value of Shh mRNA of HCC in groups C and D decreased. However, the difference of each had only borderline significance (P=0.062 and 0.071; Table II). Compared with group B, the decrease of Ptch-1 mRNA expression in groups C and D also had no statistical significance. However, compared with group B, the decrease of Gli-1 mRNA in group D had statistical significance (P=0.044). Compared with group B, the decrease of Smoh mRNA in groups C and D was not statistically significant (Table II).
Table II.
Comparison of the mRNA expression of Shh, Ptch-1, Gli-1 and Smoh of liver (group A) and HCC (groups B, C and D).
| Group | Shh | Ptch-1 | Gli-1 | Smoh |
|---|---|---|---|---|
| Group A | ||||
| Median | 0.58 | 1.715 | 0.74 | 2.87 |
| Mean ± SD | 0.5575±0.0810 | 1.7325±0.4918 | 0.7125±0.3165 | 3.1333±1.2952 |
| 95% CI | 0.4286–0.6864 | 0.9500–2.5150 | 0.2089–1.2161 | −0.0842–6.3509 |
| Range | 0.45–0.62 | 1.22–2.28 | 0.31–1.06 | 1.99–4.54 |
| Group B | ||||
| Median | 1.23 | 1.29 | 1.22 | 2.905 |
| Mean ± SD | 1.2720±0.4535 | 1.9920±1.8249 | 1.2825±0.1839 | 3.0875±1.5321 |
| 95% CI | 0.7090–1.8350 | −0.2739–4.2579 | 0.9898–1.5752 | 0.6496–5.5254 |
| Range | 0.67–1.89 | 0.85–5.22 | 1.15–1.54 | 1.59–4.95 |
| Group C | ||||
| Median | 0.595 | 0.685 | 1.07 | 2.145 |
| Mean ± SD | 0.7175±0.4310 | 0.7950±0.3397 | 1.1600±0.2762 | 3.2125±3.2488 |
| 95% CI | 0.0317–1.4033 | 0.2545–1.3355 | 0.4738–1.8462 | −1.9571–8.3821 |
| Range | 0.36–1.32 | 0.52–1.29 | 0.94–1.47 | 0.75–7.81 |
| Group D | ||||
| Median | 0.77 | 0.61 | 0.94 | 1.4475 |
| Mean ± SD | 0.7700±0.2993 | 0.6840±0.3818 | 0.9380±0.2128 | 0.8640±0.6661 |
| 95% CI | 0.3984–1.1416 | 0.2099–1.1581 | 0.6738–1.2022 | 0.0369–1.6911 |
| Range | 0.43–1.12 | 0.23–1.10 | 0.71–1.15 | 0.30–1.82 |
| P-value | B vs C: 0.062 | B vs C: 0.145 | B vs C: 0.484 | B vs C: 0.932 |
| B vs D: 0.071 | B vs D: 0.097 | B vs D: 0.044 | B vs D: 0.130 | |
| C vs D: 0.848 | C vs D: 0.887 | C vs D: 0.200 | C vs D: 0.112 |
Group A, control group without ML-1 cell implantation or drug treatment. Group B, ML-1 cell implantation, without any treatment. Group C, ML-1 cell implantation and treatment with cyclopamine 10 mg/kg/per day for 7 days. Group D, ML-1 cell implantation and treatment with cyclopamine 30 mg/kg/per day for 7 days. HCC, hepatocellular carcinoma; Shh, sonic hedgehog; Ptch-1, patched homolog-1; Gli-1, glioma-associated oncogene homolog-1; Smoh, smoothened homolog; SD, standard deviation; 95% CI, 95% confidence interval.
Discussion
In this study, we found that cyclopamine treatment, either at a low or high dose, may decrease the size of liver tumors in mice in vivo. The effect of high-dose therapy was significant.
We successfully established the growth of ML-1 hepatoma in the livers of groups B, C and D mice. A higher expression level of Shh, Gli-1 and Smoh mRNA was observed in group B when compared with group A, which suggests that the activation of the Shh pathway occurred during HCC development in mice. This corresponds with certain authors’ findings that compared with paired adjacent noncancerous liver tissue, Shh, Ptch-1, Gli-1 and Smoh were overexpressed in human HCC tissues (17,20). Similarly, Patil et al used quantitative real-time RT-PCR and revealed an increased level of expression of Gli-1 and Smoh in HCC samples compared with non-tumor liver tissues (16). Che et al found that in over 50% of human HCC, the mRNA of Shh pathway target genes Ptch-1, Gli-1 and Smoh were expressed (17). Tada et al demonstrated that hedgehog signaling components were expressed in hepatoma cell lines in various degrees (21). These findings suggested that the hedgehog pathway was frequently activated or deregulated in human HCCs (14–17,21). In vitro, certain authors considered that some hedgehog signal-responsive progenitor cells function as cancer stem cells, leading to carcinogenesis (22–24).
The detailed molecular mechanisms and the effect of the timing of Shh pathway activation upon HCC are not well understood. Some authors have hypothesized that activation of the Shh pathway is important both in the development and the progression of HCC (14–18). Cheng et al found that the Shh signaling pathway correlated with the proliferation and invasiveness of HCC cells (20). In addition, some authors reported an association between the factors of Shh signaling pathways and invasiveness of human HCC (17,20).
Tada et al regarded the overexpression of Smoh or Shh as being positive regulators and the major trigger for the activation of this signaling pathway (21). The authors demonstrated that overexpression and/or tumorigenic activation of the Smoh protooncogene mediates c-myc overexpression, which plays a critical role in hepatocarcinogenesis (21). Smoh has been suggested as being a prognostic factor in hepatocarcinogenesis (21).
Cyclopamine is the inhibitor of Smoh. Cyclopamine has been reported to inhibit the growth of HCC cells or hepatoblastoma cells (19,25,26). Chen et al revealed that cyclopamine markably decreased cell viability, induced apoptosis and downregulated Bcl-2 expression in HCC cells (19). Kim et al treated three hepatoma cell lines with KAAD-cyclopamine, resulting in a decrease of the expression of hedgehog target genes and cell growth, leading to apoptosis (25). Cheng et al showed that the blockade of the Shh signaling pathway by KAAD-cyclopamine induced a reduction of DNA synthesis leading to a marked inhibition of cell growth and a significant attenuation in invasiveness and motility of HCC cells (20). Collectively, the studies support the hypothesis that inhibition of the Shh pathway by cyclopamine may inhibit both the development and invasiveness of HCC.
However, the majority of these studies were carried out in vitro. By contrast, our present study is in vivo.
From our study, high-dose cyclopamine therapy not only effectively decreases the tumor size but also significantly decreases the expression of Gli-1 mRNA in the tumors. The reason for the significant decrease of Gli-1 mRNA and not the mRNA of Smoh, Ptch-1 or Smoh is unknown. We attribute this result to three possible mechanisms.
The first is that the interactions among these factors of the Shh pathways are complex. Ptch-1 activation predisposes a cell to proliferative and expansive behavior (22,27). Some elements of the interaction between Smoh and Ptch-1 are not fully understood. Smoh is an intracellular substrate that migrates to the cellular membrane where it is activated following engagement of Ptch-1 by Shh. At the cellular membrane, the activated Smoh triggers the downstream transcription of Gli-1 proteins (22,27). Aberrant activation of the Shh pathway leads Gli-1 into the nucleus to promote gene transcription and to maintain the biological behaviors of cancer cells.
However, the change of the mRNA expression may be dynamic. The timing of tumor harvesting affects the values of the factor.
The second mechanism may be that the significance of Shh pathway activation may be different among different stages of the same cancer and among different malignancies at the same stage. For example, a previous study reported that the proliferation of extrahepatic biliary tract cancer cell lines could also be suppressed by inhibition of the Shh pathway (28). However, the degrees of Shh and Gli-1 expression were independent of tumor stage and cancer cell differentiation (28). Activation of the Shh pathway also occurs in different stages of the same cancer. Huang et al suggested that the activation occurs in the early stage of HCC (14), whereas Thayer et al considered the hedgehog is both an early and late mediator in pancreatic carcinogenesis (13). The activation of the Shh pathway occurring in advanced stages of other cancers is also noted (8,10,13). The detailed cause of these discrepancies needs further elucidation.
The third possibility affecting the level of expression of Shh pathway factors is the hypothesized concept of cancer stem cells which have the capacity of self-renewal and unlimited replication (29–31). Bailey et al also identified the so-called cancer stem cell of the pancreas (32) and Tian et al studied lung cancer and observed that the Shh pathway is activated mainly in the cancer stem cells and not in every cancer cell (23). The effect of cyclopamine upon the Shh pathway may have occurred only in the cancer stem cells of our HCC mice and not in all cancer cells. Cyclopamine may affect the mRNA expression. The key target factor of the Shh pathway in the inhibition of cancer remains controversial (24,33,34). Interference with Shh-Gli-1 signaling may inhibit the proliferation of prostate cancer cells (35). Chen et al considered that the downregulation of Bcl-2 was important in HCC following cyclopamine treatment (19). Kim et al reported that the suppression of Gli-2 expression is significant (25).
There are some limitations of the present study. One is that the most effective and tolerable dose of cyclopamine for the treatment of HCC in mice requires further study. The second is that the side-effects of this drug at higher doses in humans need to be understood. The third is that it remains unknown whether the treatment outcome would be improved if a longer treatment period was used.
We conclude that cyclopamine may effectively inhibit HCC in mice in vivo. The results also indicate that blockade of the Shh signaling pathway may potentially be an effective treatment target for HCC.
Acknowledgments
The authors are grateful for the support of the Far Eastern Memorial Hospital and the financial support from a research grant from the National Science Council, Executive Yuan, Taiwan (NSC-97-2314-B-195-008-MY3).
References
- 1.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 4.Chen MF, Hwang TL, Jeng LB, et al. Postoperative recurrence of hepatocellular carcinoma: 205 consecutive patients who underwent hepatic resection in 15 years. Arch Surg. 1994;129:738–742. doi: 10.1001/archsurg.1994.01420310070012. [DOI] [PubMed] [Google Scholar]
- 5.Jeng KS, Sheen IS, Tsai YC. Does the presence of circulating hepatocellular carcinoma cells indicate a risk of recurrence after resection? Am J Gastroenterol. 2004;99:1503–1509. doi: 10.1111/j.1572-0241.2004.30227.x. [DOI] [PubMed] [Google Scholar]
- 6.Bale AE, Yu KP. The hedghog pathway and basal cell carcinomas. Hum Mol Genet. 2001;10:757–762. doi: 10.1093/hmg/10.7.757. [DOI] [PubMed] [Google Scholar]
- 7.Lupi O. Correlations between the sonic hedgehog pathway and basal cell carcinoma. Int J Dermatol. 2007;46:1113–1117. doi: 10.1111/j.1365-4632.2007.03391.x. [DOI] [PubMed] [Google Scholar]
- 8.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signaling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 9.Warkins DN, Peacock CD. Hedgehog signalling in foregut malignancy. Biochem Pharmacol. 2004;68:1055–1060. doi: 10.1016/j.bcp.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Chen K, Huang S, et al. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26:1698–1705. doi: 10.1093/carcin/bgi130. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Sheng T, Zhang Y, et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006;118:139–148. doi: 10.1002/ijc.21295. [DOI] [PubMed] [Google Scholar]
- 12.Chari NS, McDonnell TJ. The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol. 2007;14:344–352. doi: 10.1097/PAP.0b013e3180ca8a1d. [DOI] [PubMed] [Google Scholar]
- 13.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, He J, Zhang X, et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27:1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 15.Sicklick JK, Li YX, Jayaraman A, et al. Dysregulation of the hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–757. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 16.Patil MA, Zhang J, Ho C, Cheung ST, et al. Hedgehog signaling in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:111–117. doi: 10.4161/cbt.5.1.2379. [DOI] [PubMed] [Google Scholar]
- 17.Che L, Ren J, Yuan YH, et al. Expression of genes related to sonic hedgehog signaling in human hepatocellular carcinomas. Beijing Da Xue Xue Bao. 2008;40:616–623. (In Chinese). [PubMed] [Google Scholar]
- 18.Fu X, Wang Q, Chen X, et al. Expression patterns and polymorphisms of PTCH in Chinese hepatocellular carcinoma patients. Exp Mol Pathol. 2008;84:195–199. doi: 10.1016/j.yexmp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Chen XL, Cheng QY, She MR, et al. Expression of sonic hedgehog signaling components in hepatocellular carcinoma and cyclopamine-induced apoptosis through Bcl-2 downregulation in vitro. Arch Med Res. 2010;41:315–323. doi: 10.1016/j.arcmed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Cheng WT, Xu K, Tian DY, Zhang ZG, Liu LJ, Chen Y. Role of Hedgehog signaling pathway in proliferation and invasiveness of hepatocellular carcinoma cells. Int J Oncol. 2009;34:829–836. doi: 10.3892/ijo_00000209. [DOI] [PubMed] [Google Scholar]
- 21.Tada M, Kanai F, Tanaka Y, et al. Down-regulation of hedgehog-interacting protein through genetic and epigenetic alterations in human hepatocellular carcinoma. Clin Cancer Res. 2008;14:3768–3776. doi: 10.1158/1078-0432.CCR-07-1181. [DOI] [PubMed] [Google Scholar]
- 22.Taipale J, Beachy PA. The Hedgehog and Wnt signaling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 23.Tian F, Mysliwietz J, Ellwart J, et al. Effects of the hedgehog pathway inhibitor GDC-0449 on lung cancer cell lines are mediated by side populations. Clin Exp Med. 2012;12:25–30. doi: 10.1007/s10238-011-0135-8. [DOI] [PubMed] [Google Scholar]
- 24.Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445–451. doi: 10.1093/carcin/bgq280. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, Marcusson EG. Selective down-regulation of glioma-associated oncogene 2 inhibits the proliferation of hepatocellular carcinoma cells. Cancer Res. 2007;67:3583–3593. doi: 10.1158/0008-5472.CAN-06-3040. [DOI] [PubMed] [Google Scholar]
- 26.Eichenmüller M, Gruner I, Hagl B, et al. Blocking the hedgehog pathway inhibits hepatoblastoma growth. Hepatology. 2009;49:482–490. doi: 10.1002/hep.22649. [DOI] [PubMed] [Google Scholar]
- 27.Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006;66:2081–2088. doi: 10.1158/0008-5472.CAN-05-2146. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Park JY, Park SW, et al. The sonic hedgehog pathway as a treatment target for extrahepatic biliary tract cancer. Mol Med Report. 2012;5:12–16. doi: 10.3892/mmr.2011.598. [DOI] [PubMed] [Google Scholar]
- 29.Tai MH, Chang CC, Kiupel M, et al. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 30.Jordan CT. Searching for leukemia stem cells - not yet the end of the road? Cancer Cell. 2006;10:253–254. doi: 10.1016/j.ccr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura M, Kubo M, Nagai S, et al. New therapeutic strategy for cancer targeting the hedgehog signaling pathway. Gan To Kagaku Ryoho. 2007;34:1914–1916. (In Japanese). [PubMed] [Google Scholar]
- 34.Bigelow RLH, Chari NS, Unden AB, et al. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez P, Hernández AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with sonic hedgehog-Gli-1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]