Abstract
This study aims to present the clinical features and treatment of a case of maxillary ameloblastic carcinoma. Ameloblastic carcinoma is a rare malignant odontogenic carcinoma that has metastatic potential. Due to its rare incidence, there are few studies focusing on its radiological characteristics. When ameloblastic carcinoma demonstrates an aggressive appearance, it may be diagnosed as a malignant tumor; however, in cases showing a non-aggressive appearance, it is difficult to distinguish ameloblastic carcinoma from ameloblastoma. We report a case of ameloblastic carcinoma of the maxilla in a 59-year-old male patient, including the clinical signs, radiological images and pathological features. A partial area was surgically excised under local anesthesia and the material was sent to the Laboratory of Oral Pathology. The histological sections revealed a fragmented odontogenic tumor of epithelial origin, consisting of solid parenchyma and also revealed basal cells resembling ameloblasts, occasionally arranged in palisades. Certain parts of the architecture resembled that of an ameloblastoma; however, the cytology of other areas confirmed the diagnosis of ameloblastic carcinoma of the maxilla. The patient was scheduled for definitive surgery, including a right maxillectomy and radiotherapy. The patient was followed up every 3 months. After 2 years follow-up, there were no clinical or radiological signs of recurrence.
Keywords: ameloblastic carcinoma, maxilla, surgery, oral, case report
Introduction
Ameloblastic carcinoma is a rare odontogenic carcinoma. Although ameloblastomas are well studied and documented (over 3,600 cases of ameloblastomas have been described in the literature), little is known about their malignant features, as fewer than 60 cases of ameloblastic carcinoma have been reported (1–4).
Malignant variants of ameloblastoma include metastasizing ameloblastoma, which microscopically appears benign but has metastasized, and ameloblastic carcinoma, which exhibits malignant histopathological features. Ameloblastic carcinoma may be classified into two types: a primary odontogenic malignancy and a secondary type resulting from the malignant transformation of ameloblastoma. Most secondary ameloblastic carcinomas result from the malignant transformation of a primary lesion following repeated postsurgical recurrences. Therefore, it is rare to find an untreated secondary type presenting the histological features of malignant transformation from an earlier benign lesion (5,6).
In order to provide a better understanding of the many terms for ameloblastic malignant lesions, Hall et al (7) presented the Elzay classification (1982) of malignant odontogenic tumors, which has been accepted by many pathologists, and its nomenclature remains in use today. The term malignant ameloblastoma was used to describe a tumor that is a histologically typical or classic ameloblastoma, but which metastasizes. The ameloblastic carcinoma is characterized as a tumor that has certain features of ameloblastoma but demonstrates traditional histological features of malignancy and acts much more aggressively than ameloblastoma.
In the 2005 World Health Organization (WHO) histological classification of odontogenic tumors, ameloblastic carcinoma was included as an odontogenic carcinoma with histological features of ameloblastoma, but with cytological atypia with or without metastasis (8).
Kruse et al (9) recommended a modified classification in which a primary ameloblastoma is followed by secondary metastasis with histopathological features of malignancy and without evidence of malignancy in the primary location.
This carcinoma occurs in a wide range of age groups, but the mean age is 30.1 years, as in ameloblastomas. The most common site of occurrence is the posterior portion of the mandible. It is very rare in the maxillary region. There is no apparent gender predilection, but some authors have described predominance in males. The most common indication described has been swelling, although others include associated pain, rapid growth, trismus and dysphonia (1,2,4,7,10).
The radiographic appearance of the ameloblastic carcinomas described in the literature is generally consistent with that of ameloblastomas, except perhaps for the presence of some focal radiopacities, apparently reflecting dystrophic calcifications. Signs of osseous destruction are found in ameloblastic carcinomas as well as in ameloblastomas. These lytic phenomena may be assessed by CT and MRI imaging (5).
The most common site of metastasis is the lung, but brain or bony locations have also been reported. These tumors are prone to numerous recurrences that justify a long follow-up (5).
Being a rare disease, there are no treatment guidelines; however, the standard treatment is usually a complete surgical resection with or without radiotherapy (2,11).
The early, aggressive and complete removal of the tumor appears to offer the best chance of survival (7). Some authors (1,2) recommend surgical treatment usually involving maxillary resection with 2 to 3 cm bony margins and consideration of contiguous neck dissection, both prophylactic and therapeutic.
This study aims to present the clinical features and treatment of a maxillary ameloblastic carcinoma case. The study was approved by Ethics Committee of Univag Academic Center, Mato Grosso, Brazil. Informed consent was obtained from the patient.
Case report
A 59-year-old males was examined by a dental surgeon in the city of Tangará da Serra, Mato Grosso, Brazil. The patient reported to have been experiencing bone pain and swelling for two months. The surgeon identified the injured area and performed a curettage. After 2 months, the pain reoccurred, and the patient was again treated surgically, but gained no relief. The swelling gradually increased, reaching a size which caused the patient difficulty in eating, speaking and swallowing for 2–3 months prior to him seeking assistance. A systems review was inconclusive. The patient was not a user of tobacco or alcohol.
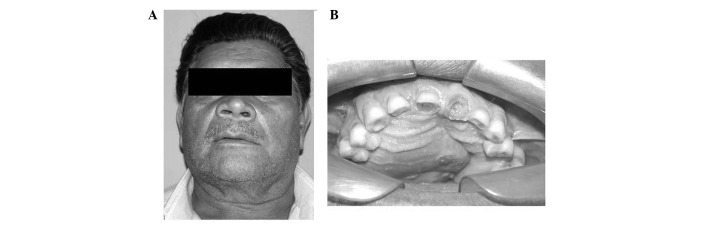
Extraoral examination revealed facial asymmetry, increased right-side volume, alar nose elevation and erasing of the nasolabial furrow (Fig. 1A). The overlying skin was smooth and normal. No ulceration was observed. The facial lymph nodes were nonpalpable. The results of the rest of the head and neck examination were normal.
Figure 1.
Clinical examination. (A) Extraoral image revealing facial asymmetry, right side volume increase, alar nose elevation and the erasing of the nasolabial furrow. (B) Intraoral examination showing an involved right side of the maxilla, extending to the left side.
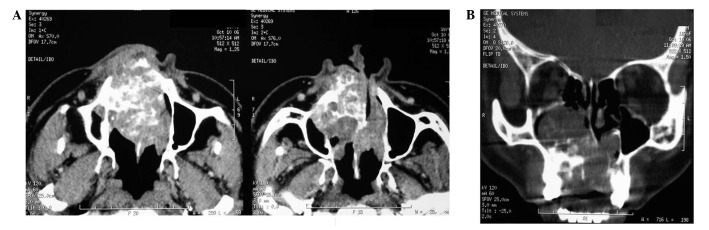
In the intraoral physical examination, there was an increase in volume in the hard palate (Fig. 1B). The central and upper right lateral incisors presented mobility. A panoramic view showed a multilocular radiolucent lesion mainly in the right side of the maxilla, extending across the midline to involve the left side. Axial slice computed tomography (CT) scans were carried out, revealing an oval corticated lucency occupying a large portion of the right maxillary sinus (Fig. 2A) and expansion and destruction of the alveolar cortical plate, involving the nasal cavity and the eyeball. The coronal slice showed a large, expansile, multilobulated cystic lesion of the maxilla (Fig. 2B). Based on these findings, a diagnosis of ameloblastoma involving the right maxilla was made. Fine-needle aspiration of the mass proved inconclusive, and incisional biopsy was also performed. A partial area was surgically excised under local anesthesia and the material was sent to the laboratory of oral pathology.
Figure 2.
Computerized tomography image. (A) The axial slice revealed the presence of an oval corticated lucency occupying a large portion of the right maxillary sinus and expansion and destruction of the alveolar cortical plate, involving the nasal cavity and the eyeball. (B) The coronal slice showed a large, expansile, multilobulated cystic lesion of the maxilla.
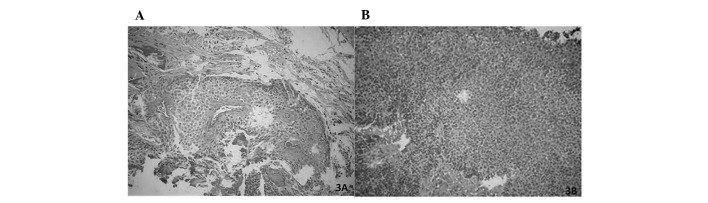
The histological sections revealed a fragmented odontogenic tumor of epithelial origin, consisting of solid parenchyma and also revealed basal cells resembling ameloblasts, occasionally arranged in palisades. The most central cells were arranged more loosely resembling the stellate reticulum of the enamel organ. Metaplasia was also noted. The stroma consisted of loose fibrous connective tissue (Fig. 3A). The odontogenic parenchyma was composed of epithelial cells showing intense cellular pleomorphism, numerous hyper-chromatic cells, loss of the nuclear/nucleolus and nucleus/ cytoplasm and areas of necrosis (Fig. 3B). Certain parts of the architecture resembled that of an ameloblastoma; however, the cytology of other areas confirmed the diagnosis of ameloblastic carcinoma of the maxilla.
Figure 3.
Microscopic examination. (A) The histological sections revealed a fragmented odontogenic tumor of epithelial origin, consisting of solid parenchyma, and showing basal cells resembling ameloblasts, occasionally arranged in palisades. (B) The odontogenic parenchyma was composed of epithelial cells showing intense cellular pleomorphism, numerous hyperchromatic cells, loss of the nucleolus and cytoplasm, and areas of necrosis.
The patient was scheduled for definitive surgery, including a right maxillectomy and radiotherapy. The patient was followed up every 3 months. After 2 years follow-up, there were no clinical or radiological signs of recurrence; however, the patient is currently suffering from discomfort when eating and talking due to bucosinusal communication. Treatment is in the prosthetic rehabilitative phase.
Discussion
Ameloblastic carcinoma is an extremely rare, aggressive malignant epithelial odontogenic tumor with a poor prognosis. Two thirds of these tumors arise from the mandible while one third originate in the maxilla. The most common symptom is a rapidly progressing, painful swelling (4,5,11).
Clinically, these carcinomas are more aggressive than most typical ameloblastomas. Perforation of the cortical plate, extension into the surrounding soft tissue and numerous recurrent lesions and metastasis, usually to cervical lymph nodes, are associated with ameloblastic carcinomas (5). The facial lymph nodes in this case were nonpalpable.
The lung is the most common area of distant metastasis, but metastasis to the skull and lymph nodes has also been described. In the present case, the patient had no metastasis at the time of diagnosis (12); however, metastases may also occur following treatment, emphasizing the significance of clinical and radiographical follow-up (5). A systems review was inconclusive.
In 2007, 37 cases of ameloblastic carcinoma in patients ranging in age from 15 to 84 years were reviewed (13). The male to female ratio was 5:3, with the majority of cases occurring in patients aged 50–60. The present case involved a 59-year-old male. In the 2007 study (13), 12 of the 37 tumors were located in the maxilla. Expansion or a hard mass was the main complaint, followed by pain or discomfort. Others signs and symptoms included trismus, dysphonia and paresthesia. The reported cases of spindle-cell ameloblastic carcinoma occurred more often in the mandible than in the maxilla, at a ratio of 3:1. In the present case, the lesion was present in the right-side maxilla, extending across to the left side. The patient in this case presented with swelling in the right maxilla, facial asymmetry, associated pain, rapid growth, dysphagia and dysphonia.
The radiographical features of ameloblastic carcinoma are similar to ameloblastomas. In the majority of cases, a radiolucent intraosseous lesion is revealed (1,5), as was demonstrated in the present case. In our case study, the radiographical appearance of the lesion was consistent with that of an ameloblastoma except for the presence of some focal radiopacities, apparently reflecting the dystrophic calcifications revealed by CT.
An additional consideration in the differential diagnosis is a squamous cell carcinoma arising in the lining of an odontogenic cyst. Histologically, this lesion tends to more closely resemble oral squamous cell carcinoma than what we have described for ameloblastic carcinoma. However, it is important to point out that ameloblastic carcinoma may arise from the cystic lining. The squamous odontogenic tumor may also be mistaken for ameloblastic carcinoma. It is composed of islands of squamous epithelium that lack stellate reticulum-like zones and peripheral palisading. In addition, microcystic changes and dystrophic calcifications are occasionally observed in this lesion. However, the epithelium of the squamous odontogenic tumor lacks any cytologic evidence of malignant disease (14). Thus, the term ameloblastic carcinoma may be applied to our case, which showed focal histological evidence of malignant disease including cytologic atypia and mitoses along with the indisputable features of classic ameloblastoma.
It is generally accepted that maxillary ameloblastomas should be treated as radically as possible due to the spongy maxillary bone architecture (9); however, controversy still exists regarding the treatment of ameloblastic carcinoma. As ameloblastic carcinomas are rare, there is no consensus on their treatment. Certain authors have suggested surgery plus radiotherapy, while others doubt the effectiveness of this combination. There are few reports on chemotherapy regimens for ameloblastic carcinoma (1). Preoperative radiotherapy has been suggested to decrease the tumor size and may be used to treat some rapidly growing tumors prior to radical surgery. The role of chemotherapy has not yet been proven (11). In the present case, treatment consisted of surgical resection and adjuvant radiation.
The prognosis is dominated by the possibility of local recurrences, even after a long relapse, and distant metastases. Metastases generally occur in the lung but may also occur in the bone and the brain. A systematic assessment of the chest by periodic imaging is recommended (3).
Reconstruction of the post-resection defect should proceed as normal following any head or neck carcinoma resection; however, sufficient time should be allotted prior to reconstruction due to potential tumor recurrence (4).
Microscopically, the benign and malignant ameloblastoma are very similar, often only differentiated correctly when a contained metastasis in the lung is revealed by histopathological examination of images. Ameloblastic carcinoma presents with atypical mitotic figures, intense cellular pleomorphism among other areas of necrosis, which are not usually observed in ameloblastoma and malignant ameloblastoma, and features which enable differentiation and a more precise diagnosis, as was observed in the present case.
We have had no report of metastasis in the case presented in this study, although we must keep in mind the possibility that this may still occur. It is essential that, in the future, these lesions are accurately identified and followed up so that their natural history and prognosis may be further defined.
In conclusion, ameloblastic carcinoma is a very rare malignant odontogenic tumor with characteristic histopathological and clinical features, which requires aggressive surgical treatment and surveillance.
Diagnosis at an early stage and close periodical screening for metastasis are necessary to improve patient prognosis.
In addition to local long-term control, specific attention should be paid to potential pulmonary involvement.
References
- 1.Nai GA, Grosso RN. Fine-needle aspiration biopsy of ameloblastic carcinoma of the mandible: a case report. Braz Dent J. 2011;22:254–257. doi: 10.1590/s0103-64402011000300013. [DOI] [PubMed] [Google Scholar]
- 2.Jensen AD, Ecker S, Ellerbrock M, Nikoghosyan A, Debus J, Münter MW. Carbon ion therapy for ameloblastic carcinoma. Radiat Oncol. 2011;6:1–5. doi: 10.1186/1748-717X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuzaki H, Katase N, Hara M, Asaumi J, Yanagi Y, Unetsubo T, Hisatomi M, Konouchi H, Nagatsuka H. Ameloblastic carcinoma: a case report with radiological features of computed tomography and magnetic resonance imaging and positron emission tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:40–47. doi: 10.1016/j.tripleo.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Pundir S, Saxena S, Rathod V, Aggrawal P. Ameloblastic carcinoma: Secondary dedifferentiated carcinoma of the mandible - Report of a rare entity with a brief review. J Oral Maxillofac Pathol. 2011;15:201–204. doi: 10.4103/0973-029X.84501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benlyazid A, Lacroix-Triki M, Aziza R, Gomez-Brouchet A, Guichard M, Sarini J. Ameloblastic carcinoma of the maxilla: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:17–24. doi: 10.1016/j.tripleo.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Karakida K, Aoki T, Sakamoto H, Takahashi M, Akamatsu T, Ogura G, Sekido Y, Ota Y. Ameloblastic carcinoma, secondary type: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:33–37. doi: 10.1016/j.tripleo.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Hall JM, Weathers DR, Unni KK. Ameloblastic carcinoma: An analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:799–807. doi: 10.1016/j.tripleo.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Jindal C, Palaskar S, Kaur H, Shankari M. Low-grade spindle-cell ameloblastic carcinoma: report of an unusual case with immunohistochemical findings and review of the literature. Curr Oncol. 2010;17:52–57. doi: 10.3747/co.v17i5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruse ALD, Zwahlen RA, Grätz KW. New classification of maxillary ameloblastic carcinoma based on an evidence-based literature review over the last 60 years. Head Neck Oncol. 2009;1:31. doi: 10.1186/1758-3284-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suomalainen A, Hietanen J, Robinson S, Peltola JS. Ameloblastic carcinoma of the mandible resembling odontogenic cyst in a panoramic radiograph. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:638–642. doi: 10.1016/j.tripleo.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Ozlugedik S, Ozcan M, Basturk O, Deren O, Kaptanoglu E, Adanali G, Unal A. Ameloblastic carcinoma arising from anterior skull base. Skull Base. 2005;15:269–272. doi: 10.1055/s-2005-918621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abiko Y, Nagayasu H, Takeshima M, Yamazaki M, Nishimura M, Kusano K, Kitajo H, Saitoh M, Kawakami T, Chiba I, Kaku T. Ameloblastic carcinoma ex ameloblastoma: report of a case-possible involvement of CpG island hypermethylation of the p16 gene in malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:72–76. doi: 10.1016/j.tripleo.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Akrish S, Buchner A, Shoshani Y, Vered M, Dayan D. Ameloblastic carcinoma: report of a new case, literature review and comparison to ameloblastoma. J Oral Maxillofac Surg. 2006;65:777–783. doi: 10.1016/j.joms.2005.11.116. [DOI] [PubMed] [Google Scholar]
- 14.Avon SL, McComb J, Crokie C. Ameloblastic carcinoma: case report and literature review. J Can Dent Assoc. 2003;69:573–576. [PubMed] [Google Scholar]