Abstract
Merkel cell carcinoma (MCC) is a rare malignant skin neoplasm with the potential for local recurrence, spreading to regional lymph nodes (LNs) and distant metastases. Although it has been identified in various anatomical sites, LN metastatic MCC in the absence of a primary site is extremely rare. The present case reports a 54-year-old male who initially underwent histological examination of a biopsy specimen from the right inguinal LNs. A diagnosis of metastatic small cell carcinoma was made. Nine months later, this diagnosis was changed to MCC with multiple metastases following observation of a tumour mass in the right dorsal thigh. Additionally, in the present study a summary is provided of 23 published cases of MCC with initial LN metastasis in the absence of a primary site, with details of clinical characteristics, natural history and pertinent therapy of this uncommon tumour. The present patient with LN metastatic MCC in the absence of a primary site and the other reported cases demonstrate that although multimodal treatment with surgery, radiotherapy (RT) and chemotherapy provides excellent local control, local recurrence and distant metastases commonly develop in this uncommon tumour. LN metastatic MCC in the absence of a primary site is a highly malignant disease and the role of adjuvant postoperative RT and/or chemotherapy remains to be fully determined.
Keywords: merkel cell carcinoma, metastasis, chemotherapy, radiotherapy
Introduction
Merkel cell carcinoma (MCC) in the skin was first described by Toker in 1972 (1). It is an uncommon skin neoplasm of the elderly population (2). The majority of patients with MCC present with localised disease at diagnosis and few patients have regional lymph node (LN) involvement and distant metastases (3). MCC was historically considered to be an indolent skin tumour, but it has now been demonstrated to be highly aggressive, with recurrence and metastasis (4). Although it has been previously reported in various anatomical sites (5), LN metastatic MCC in the absence of a primary site is extremely rare (6) and for this reason there is no standard approach to its management.
Curative surgery, particularly Mohs micrographic surgery, is commonly recommended to manage localised MCC (7,8). Nevertheless, specific postoperative palliative treatments for MCC have emerged, including radiotherapy (RT) or chemotherapy, due to the high local failure rate and the aggressive nature of the disease. Clinical investigators have performed a large number of monotherapies or various combinations of surgery, RT and chemotherapy, but the stage, size and location of the tumours investigated has varied, making retrospective analysis difficult. Therefore, conclusions with regard to these treatment regimens in patients with MCC are controversial (9–11). It is important to identify the contribution of the treatment regimens.
In the present study we report the case of an Asian patient with LN metastatic MCC in the absence of a primary site and summarise 23 published cases of MCC with initial LN metastasis in the absence of a primary site, in which the clinical characteristics, natural history and pertinent therapy of this uncommon tumour are described.
Case reports
Clinical presentation and diagnosis of cancer
A 54-year-old male had complained of a palpable subcutaneous mass in the right groin increasing in size for ∼4 months. Written consent for publication was obtained from the patient. The mass was nontender, firm, painless, but not hard. The patient had no other symptoms but had had psoriasis for 20 years. Examination of the patient with enhanced computed tomography and positron emission tomography-computed tomography (PET-CT) demonstrated that the skull, chest and abdomen were normal, but three masses were observed in the right groin (Fig. 1A). A biopsy was performed, leading to a diagnosis of right inguinal LNs consistent with small cell carcinoma metastasis from another organ, most probably the lung. Serum tumour markers were negative for carcinoembryonic antigen, α-fetoprotein, prostate-specific antigen, CA125, CA15-3 and CA19-9, but positive for nonspecific esterase (NSE; 83.73 ng/ml; normal, <16.30 ng/ml) and the tumour marker CYFRA (5.80 ng/ml; normal, <3.30 ng/ml). The patient had a performance status of 0 and was referred to our hospital.
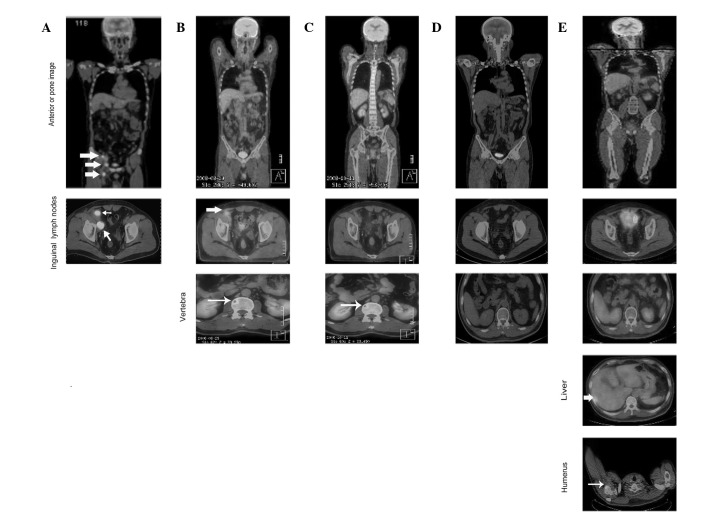
Figure 1.
Cutaneous Merkel cell carcinoma involving inguinal lymph nodes, bone, liver and humerus metastases identified by follow-up PET-CT. (A) Large lymph node metastases observed at initial presentation (large arrow). (B) Following initiation of chemotherapy (EP regimen), lymph node metastases shrunk (large arrow) but metastasis to the vertebra occurred (small arrow). (C) Following chemotherapy (IP regimen) and radiotherapy, lymph node metastases recurred and vertebral metastases shrunk (small arrow). (D) Disappearance of lymph nodes and vertebral metastases following concomitant treatment with chemotherapy (IP regimen) and radiotherapy. (E) Following 4 months of combination therapy, metastases in the liver (large arrow) and humerus (small arrow) were discovered by PET-CT follow-up. EP regimen, etoposide/cisplatin; IP regimen, irinotecan/cisplatin. PET-CT, positron emission tomography-computed tomography.
A further histological examination of the biopsy specimen was made, demonstrating metastatic small cell carcinoma composed of small cells with oval to fusiform hyperchromatic nuclei and indistinct nucleoli with frequent mitoses (Fig. 2A). Immunohistochemistry demonstrated a marked positive reaction for NSE, CD56 and protein kinase C (PKC), but was negative for CD45 and chromogranin A (CgA). The Ki-67 staining index was ∼85% (Fig. 2A). The morphological and immunohistochemical features were in keeping with metastatic small cell carcinoma. However, no primary tumour was identified in any sites following extensive investigations. A diagnosis of metastatic small cell carcinoma in right inguinal LNs without a primary site was made.
Figure 2.
Histological examination of a biopsy specimen and immunohistochemical study in (A) inguinal lymph node and (B) cutaneous mass. H&E, hematoxylin and eosin; NSE, nonspecific esterase; CD, cluster of differentiation; PCK, pan cytokeratin; CgA, chromogranin A; CK, cytokeratin; TTF-1, thyroid transcription factor-1; LCA, leucocyte common antigen; NFP, neurofilament protein.
The patient received therapy (as described in the treatment and clinical course section) and a PET-CT scan was performed at the end of therapy, which revealed a complete response. Following 4 months with no evidence of disease, a subcutaneous mass developed. Clinical examination disclosed a rapidly growing, painless, firm, grey skin nodule in the right dorsal thigh measuring 3.5x4.5x1.5 cm. PET-CT demonstrated metastatic disease in the liver and humerus. Mohs micrographic surgery was performed. Histological examination of a biopsy specimen demonstrated nodules and diffuse sheets of basophilic cells with imperceptible cytoplasm and round or oval vesicular nuclei and dispersed chromatin with a pathognomonic watery appearance (Fig. 2B). Immunohistochemistry demonstrated a marked positive reaction for NSE, CD56, PKC, epithelial membrane antigen, CK7, CK20 and CD99. Tumour cells were negative for CgA, thyroid transcription factor-1 (TTF-1), leucocyte common antigen (LCA), neurofilament protein and S100. The Ki-67 staining index was ∼70% (Fig. 2B). This combined evidence confirmed a final diagnosis of MCC with multiple metasases in inguinal LNs, bone and liver.
Treatment and clinical course
The patient was initially treated with a combination of etoposide (100 mg/m2, days 1–5) and cisplatin (50 mg/m2, days 1–3) every 3 weeks. A partial response was achieved in the LNs but progressive metastatic disease was present in T6 and T12-L2 following two cycles (Fig. 1B). The patient then received chemotherapy with irinotecan (100 mg/m2, day 1, 8, 15) and cisplatin (40 mg/m2, days 1–3) every 3 weeks as well as RT with 54 Gy/30 fractions/6 weeks to inguinal LNs (Fig. 3A) and 30 Gy/10 fractions/2 weeks to T6 and T12-L2, respectively (Fig. 3B and C). A partial response was achieved following two cycles (Fig. 1C).
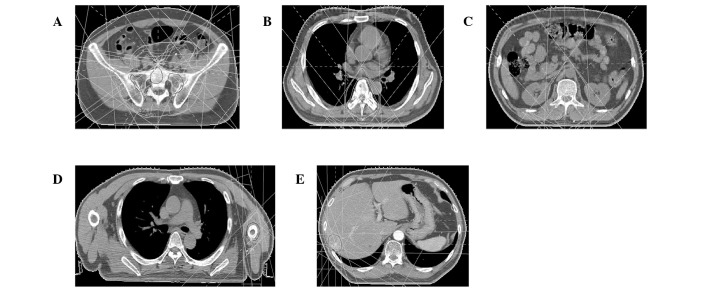
Figure 3.
Computed tomography-assisted radiation treatment of the patient. The dose was distributed at the level of the central axis. The protocol required the planning target volume to be encompassed by a 95% dose envelope. (A) Inguinal lymph nodes; (B) T6 vertebra; (C) T12–L2 vertebrae; (D) humerus; (E) liver.
The patient tolerated only two cycles of chemotherapy concomitant with RT, following which his condition deteriorated due to grade IV bone marrow suppression and poor performance status. Thereafter, the patient was treated with a third cycle of chemotherapy with reduced concentrations of irinotecan (70 mg/m2, days 1, 8, 15) and cisplatin (30 mg/m2, days 1–3). Following five cycles of chemotherapy concomitant with RT, the mass in his right groin resolved, the metastatic diseases disappeared and complete response was observed (Fig. 1D).
The patient demonstrated no evidence of disease for 4 months when a subcutaneous mass was identified. Subsequently, PET-CT follow-up revealed that the patient’s liver and humerus had metastatic disease (Fig. 1E). Mohs micrographic surgery and salvage chemotherapy plus RT (Fig. 3D and E) were then instituted, but the patient succumbed to liver failure 21 months following the onset of his illness.
Literature review
Search strategy
Articles were identified by searching PUBMED/MEDLINE between January 1966 and April 2012 using the key words: ‘Merkel cell carcinoma’ or ‘Merkel cell cancer’ or ‘MCC’ without language restriction. Computer searches were supplemented with hand searching journals up to April 2012. We also hand searched the general reviews and references from published clinical trials.
Common characteristics
Six studies were identified which reported 23 patients with LN metastatic MCC in the absence of a primary site (12–17). Of these patients, 8/23 (34.8%) came from Italy and the rest from the USA. The average ages of the patients were between 50 and 80 years; the majority were elderly, but two were aged <40 years. There were similar numbers of males and females: 13 males, 10 females. The most common sites of origin were the inguinal LNs (17/23, 73.9%), 3 cases demonstrated axillary LNs (3/23, 13.0%). The other sites of origin were neck area LNs (2/23, 8.7%), the submandibular lymph (1/23, 4.3%), the cervical LN (1/23, 4.3%) and the upper jugular area LN (1/23, 4.3%). Three histological patterns of MCC were reported: a solid (8/23, 34.8%), a diffuse (4/23, 17.4%) and a trabecular type (1/23, 4.3%). Local recurrences were identified in 5 patients (21.7%), 6 (26.1%) sustained extraregional metastases and 5 (21.7%) had distant spreading (Table I).
Table I.
Clinical findings of Merkel cell carcinoma with lymph node metastasis in absent of a primary site.
| First author (Ref.) | Case no. | Age (years) | Gender | Site | Histol. type | Skin site of primary | Local recur. (months) | Extraregio. or distant spread (months) | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaplan et al (12) | 1 | Elderly | M | Axilla LN, L and R Cervical LN, R |
S | UK | UK | Brain, liver and adrenal gland, syn. | RT | DOD (4) |
| Pilotti et al (13) | 2 | 50 | M | Inguinal LN, R | D | UK | UK | Retroperit. and pancreas, syn. | RT | ED 6) |
| 3 | 58 | M | Inguinal LN, R | S | UK | UK | UK | SE | NED (5) | |
| 4 | 65 | M | Inguinal LN, R | S | UK | UK | UK | SE | NED (18) | |
| 5 | 73 | M | Inguinal LN, R | S | UK | UK | UK | SE | ED (36) | |
| 6 | 66 | M | Inguinal LN, R | D | UK | UK | UK | SE | DOC (168) | |
| 7 | 65 | F | Inguinal LN, R | T | UK | UK | UK | UK | Cons | |
| 8 | 39 | F | Inguinal LN, R | S | UK | UK | UK | SE | Cons | |
| 9 | 53 | F | Inguinal LN, L | S | UK | 6 | UK | SE | NED (8) | |
| 10 | 78 | M | Inguinal LN, L | D | UK | UK | Supraclav. LN, L (48) | CT | DOD (60) | |
| 11 | 52 | F | Axilla LN, R | D | UK | UK | UK | SE | NED (30) | |
| Eusebi et al (14) | 12 | 65 | F | Axilla LN, L | UK | UK | UK | UK | RT+CT | NED (6) |
| 13 | 46 | M | Inguinal LN, L | UK | UK | UK | UK | UK | NED (10) | |
| 14 | 37 | M | Inguinal LN, R | UK | UK | 6 | Pelvic LN, R (12) Cervical LN, R (15) |
RT+CT | DOD (25) | |
| 15 | 46 | F | Inguinal LN, L | UK | UK | UK | UK | UK | NED (19) | |
| 16 | 64 | M | Inguinal LN, L | UK | UK | 11 | UK | UK | NED (25) | |
| 17 | 68 | F | Inguinal LN, L | UK | UK | UK | Paraortic LN, R (3) Parailliac LN, L (3) |
UK | DOD (8) | |
| 18 | 80 | F | Submandibular LN, L | UK | UK | UK | UK | UK | NED (26) | |
| 19 | 54 | F | Inguinal LN, L | UK | UK | 5 | Iliac LN, L (12) | RT+CT | ED (12) | |
| Rice et al (15) | 20 | 76 | M | Upper jugular area LN, R | S | UK | UK | UK | SE+RT | NED (23) |
| Yang et al (16) | 21 | 74 | M | Neck LN, R | UK | UK | UK | Submandibular gland, syn. | SE+RT | DOD (12) |
| Straka and Straka (17) | 22 | 71 | F | Neck LN in zone II, R | UK | UK | 10 | Submandibular gland, syn. brain (10) | SE+RT+CT | DOD (12) |
| 23 | 52 | M | Inguinal LN, L | S | UK | UK | UK | SE+CT | UK | |
| Zhao and Meng (TS) | 24 | 54 | M | Inguinal LN, R | S | Dorsi-thigh, R | UK | Vertebrae (1) Humerus and liver (12) |
SE+RT+CT | DOD (21) |
TS, this study; F, female; M, male; axilla, axillary; LN, lymph node; R, right; L, left; histol, histological; S, solid; D, diffuse; T, trabecular; UK, unknown; recur, recurrence; extraregio, extraregional; syn, synchronous; supraclav, supraclavicular; RT, radiotherapy; SE, surgical excision; CT, chemotherapy; DOD, died of disease; ED, evidence of disease; NED, no evidence of disease; DOC, died of other disease; cons, consultation case.
Treatment schedule
Of the 23 patients included in this study, 7 (30.4%) underwent surgical excision alone and the remaining patients were treated with chemotherapy alone (1/23, 4.3%), surgical excision plus chemotherapy (1/23, 4.3%), surgical excision plus RT (2/23, 8.7%), RT plus chemotherapy (3/23, 13.0%), surgical excision plus chemotherapy plus RT (2/23, 8.7%) and unknown (6/23, 26.1%).
Chemotherapy was administered to a total of 7 patients (30.4%) with LN metastatic MCC in the absence of primary site. A two-drug regimen was recieved by 5 patients, 1 received single-agent chemotherapy and in 1 patient, the chemotherapy regimen was unknown. Etopoplatin with either cisplatin or carboplatin were used for combination chemotherapy and the majority of patients had chemotherapy following surgical excision (Table I).
Overall survival rate and recurrence
With a median follow-up of 18 months, a total of 12 patients (52.17%) survived to the final follow-up: 10 patients were alive with no evidence of disease, 2 were alive with disease and the remaining 11 had succumbed to the disease. Of the 23 total patients, 5 experienced recurrences during follow-up. Due to the small sample size, no prognostic factor was identified to be correlated with poor survival rates by univariate and multivariate analyses. The survival rate of patients ranged from 4 to 168 months and the median was estimated to be 60 months (Table I).
Discussion
To the best of our knowledge, this is the first case report of an Asian patient with MCC and multiple metastases in the absence of a primary site. LN metastases as the initial clinical manifestation of MCC were unexpected, suggesting that diagnosis may difficult. Outcomes of our patient and those of the other 23 reported cases demonstrated that although multi-modal treatment with surgery, RT and chemotherapy provides excellent local control, local recurrence and distant metastases commonly develop in this uncommon tumour. Therefore, LN metastatic MCC in the absence of a primary site is a highly malignant disease and the role of adjuvant postoperative RT and/or chemotherapy remains to be fully determined.
At the time of diagnosis, patients with MCC typically present with a flesh-coloured, red or blue, firm, nontender, intracutaneous mass, which may be ulcerated (18). Owing to its nonspecific appearance, diagnosis of MCC is often made by biopsy. In the present case, an initial histological examination of a biopsy specimen from the right inguinal LNs demonstrated a metastatic small cell carcinoma. However, histological examination of a biopsy specimen from the right dorsal thigh revealed nodules and diffuse sheets of basophilic cells with imperceptible cytoplasm and round or oval vesicular nuclei, the dispersed chromatin yielding a pathognomonic watery appearance. To confirm the diagnosis, immunohistochemistry studies, including staining for CK20, CK7, TTF-1, LCA, S100 and CD99, were performed. In general, MCC cases express CK20 rather than CK7, the latter identifying bronchial small cell carcinoma (19). Notably, in the present case CK20 and CK7 were identified, an unusual presentation of this rare tumour. Immunohistochemistry produced a negative result for TTF-1, which is expressed at high levels in small cell carcinoma of the lung. Immunohistochemistry also demonstrated a marked positive reaction for CD99 rather than TTF-1, LCA, S100 in cutaneous tissues, which indicated that the differential diagnosis includes small cell carcinoma of the lung, lymphoma, peripheral primitive neuroectodermal tumour and small cell melanoma. However, an extensive analysis revealed no primary tumour at any site in the patient. Therefore, the morphology of the small cell tumour in this case, ultrastructural evidence of its neuroendocrine granules and the immunohistochemistry results were indicative of MCC.
Currently, there is no standard approach to the management of MCC in the absence of a primary site. Mohs micrographic surgery is currently considered as the primary and complementary measure for controlling this serious disease. Since the disease is highly aggressive and the high failure rate following surgery alone, RT is usually administered as temporary support for numerous patients with MCC. MCC cell lines have been demonstrated to be radiosensitive in vitro (20). Results have indicated that adjuvant RT, following initial surgery and resection for recurrent MCC and palliation is beneficial (21). A previous study reported that a radiation dose of 45 Gy had significant impact on local control and prolonged survival in nine patients, whereas a subset of 7 patients who received <45 Gy had a poorer outcome (22). Our patient received 54, 30, 50, 30 and 30 Gy for inguinal LNs, vertebrae, right dorsal thigh, humerus and liver, respectively.
MCC was initially considered to be resistant to chemotherapy, however, various agents have been used to treat MCC with variable results; the most commonly used chemotherapy regimen is etoposide/cisplatin (EP regimen) (23). However, our patient responded successfully to an irinotecan/cisplatin (IP) regimen with RT, previously following an EP regimen alone. The majority of patients with MCC are elderly and may be intolerant of high doses of chemotherapy (24). For example, our patient tolerated only two cycles of the IP regimen concomitant with RT, following which his condition deteriorated owing to grade IV bone marrow suppression. As his general condition gradually improved, the patient received a third cycle of the IP regimen but at decreased concentrations, which may have affected treatment efficacy. Although multi-modal treatment with surgery, RT and chemotherapy results in excellent local control, local recurrence and distant metastases ultimately developed, possibly owing to the characteristics of MCC or inadequate treatment. Therefore, the role of adjuvant postoperative chemotherapy and/or RT in MCC remains to be determined in a future controlled trial.
Since 2001, PET-CT has rapidly replaced standalone PET (25,26). The diagnostic capability of PET-CT in the staging of cancer is improved compared with that of CT alone or PET alone (27) as it enables more accurate assignment of tumour stage and, to a lesser extent, definition of the lymph-node stage. In the present study, identifying the primary tumour aided the determination of the appropriate treatment and was essential for prognosis (27). The patient was followed-up for 15 months by PET-CT following the initial treatment and we identified that adding a PET-CT examination to the diagnostic regimen improved sensitivity in determining the primary tumour and metastases. To summarise, although MCC may be suggested by immunohistochemistry and electron microscopic features, caution should be exercised in making this diagnosis in the absence of a known primary skin tumour. Multimodal treatment with surgery, RT and chemotherapy provides excellent local control, however, local recurrence and distant metastases commonly develop in patients with metastatic MCC in the absence of a primary site. Finally, metastatic MCC in the absence of a primary site is a highly malignant disease and the role of adjuvant postoperative RT and/or chemotherapy in MCC remains to be determined in a controlled trial.
Acknowledgments
The authors thank the doctors responsible for the care of the patient in the present case study and an anonymous referee for his/her helpful comments, which considerably improved the quality of this manuscript.
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110. [PubMed] [Google Scholar]
- 2.Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8:153–158. [PubMed] [Google Scholar]
- 3.Meyer-Pannwitt U, Kummerfeldt K, Boubaris P, Caselitz J. Merkel cell tumour or neuroendocrine skin carcinoma. Langenbecks Arch Chir. 1997;382:349–358. doi: 10.1007/s004230050079. (In German) [DOI] [PubMed] [Google Scholar]
- 4.Poulsen M. Merkel-cell carcinoma of the skin. Lancet Oncol. 2004;5:593–599. doi: 10.1016/S1470-2045(04)01593-1. [DOI] [PubMed] [Google Scholar]
- 5.Haag ML, Glass LF, Fenske NA. Merkel cell carcinoma: diagnosis and treatment. Dermatol Surg. 1995;21:669–683. doi: 10.1111/j.1524-4725.1995.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 6.Deneve JL, Messina JL, Marzban SS, et al. Merkel cell carcinoma of unknown primary origin. Ann Surg Oncol. 2012;19:2360–2366. doi: 10.1245/s10434-011-2213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gollard R, Weber R, Kosty MP, Greenway HT, Massullo V, Humberson C. Merkel cell carcinoma: Review of 22 cases with surgical, pathologic and therapeutic considerations. Cancer. 2000;88:1842–1851. [PubMed] [Google Scholar]
- 8.O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma: Comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929–933. [PubMed] [Google Scholar]
- 9.Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK. Merkel cell carcinoma: Prognosis and management. Arch Surg. 1991;126:1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]
- 10.Savage P, Constenla D, Fisher C, Thomas JM, Gore ME. The natural history and management of Merkel cell carcinoma of the skin: A review of 22 patients treated at the Royal Marsden Hospital. Clin Oncol (R Coll Radiol) 1997;9:164–167. doi: 10.1016/s0936-6555(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 11.Pectasides D, Pectasides M, Psyrri A, Koumarianou A, Xiros N, Pectasides E, et al. Cisplatin-based chemotherapy for merkel cell carcinoma of the skin. Cancer Invest. 2006;24:780–785. doi: 10.1080/07357900601062354. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan GP, Bookbinder MJ, Hood DR, Bridgers SL. Merkel cell tumour of the skin. Hum Pathol. 1988;19:615–616. doi: 10.1016/s0046-8177(88)80219-3. [DOI] [PubMed] [Google Scholar]
- 13.Pilotti S, Rilke F, Bartoli C, Grisotti A. Clinicopathologic correlations of cutaneous neuroendocrine Merkel cell carcinoma. J Clin Oncol. 1988;16:1863–1873. doi: 10.1200/JCO.1988.6.12.1863. [DOI] [PubMed] [Google Scholar]
- 14.Eusebi V, Capella C, Cossu A, Rosai J. Neuroendocrine carcinoma within lymph nodes in the absence of a primary tumour with special reference to Merkel cell carcinoma. Am J Surg Pathol. 1992;16:658–666. doi: 10.1097/00000478-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rice RD, Chonkich GD, Thompson KS, Chase DR. Merkel cell carcinoma of the head and neck. Five new cases with literature review. Arch Otolaryngol Head Neck Surg. 1993;119:782–786. doi: 10.1001/archotol.1993.01880190078016. [DOI] [PubMed] [Google Scholar]
- 16.Yang GC, Schneck MJ, Hayden RE, Gupta PK. Merkel cell tumour-like neuroendocrine carcinoma associated with the submandibular gland. Acta Cytol. 1994;38:742–746. [PubMed] [Google Scholar]
- 17.Straka JA, Straka MB. A review of Merkel cell carcinoma with emphasis on lymph node disease in the absence of a primary site. Am J Otolaryngol. 1997;18:55–56. doi: 10.1016/s0196-0709(97)90050-8. [DOI] [PubMed] [Google Scholar]
- 18.Hitchcock CL, Bland KI, Laney RG, III, Franzini D, Harris B, Copeland EM., III Neuroendocrine (Merkel cell) carcinoma of the skin: Its natural history, diagnosis and treatment. Ann Surg. 1988;207:201–207. doi: 10.1097/00000658-198802000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor 1 and Cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med. 2001;125:228–231. doi: 10.5858/2001-125-0228-IFTTFA. [DOI] [PubMed] [Google Scholar]
- 20.Leonard JH, Ramsay JR, Kearsley JH, Birrell GW. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32:1401–1407. doi: 10.1016/0360-3016(94)00610-W. [DOI] [PubMed] [Google Scholar]
- 21.Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002;20:588–598. doi: 10.1200/JCO.2002.20.2.588. [DOI] [PubMed] [Google Scholar]
- 22.Ott MJ, Tanabe KK, Gadd MA, Stark P, Smith BL, Finkelstein DM, et al. Multimodality management of Merkel cell carcinoma. Arch Surg. 1999;134:388–393. doi: 10.1001/archsurg.134.4.388. [DOI] [PubMed] [Google Scholar]
- 23.Allen PG, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 24.Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, Gilchrist J. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: Case series and review of 204 cases. J Clin Oncol. 2000;18:2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 25.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 26.von Schulthess GK. Cost considerations regarding an integrated CT-PET system. Eur Radiol. 2000;10(Suppl 3):S377–S380. doi: 10.1007/pl00014098. [DOI] [PubMed] [Google Scholar]
- 27.Lardinois D, Weder W, Hany T, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission-tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 28.Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526–533. doi: 10.1148/radiol.2292021598. [DOI] [PubMed] [Google Scholar]