Abstract
Background
Opportunistic pulmonary infection with Nocardia species is rare in humans, and only a few studies have radiologically analyzed patients with pulmonary nocardiosis using high-resolution computed tomography (HRCT).
Methods
We retrospectively reviewed the medical records of patients with pulmonary nocardiosis at our hospital between April 2006 and December 2011 to assess HRCT and clinical findings. We also searched the medical literature for pulmonary nocardiosis reported in Japan between 2002 and 2011 for comparison.
Results
We identified seven patients at our institution and 33 reported infections in Japan. Four of our patients were immunocompetent, whereas the other three had impaired cellular immunity due to type 2 diabetes mellitus or having been inappropriately treated with steroid. Thoracic HRCT revealed no zonal predominance, but tropism for distribution from the middle to the peripheral area, and radiological findings of nodules, cavitation, mass, consolidations, bronchial wall thickening, septal line thickening and ground glass opacity (GGO) were evident. The main HRCT finding in our study comprised nodules (n=5, 71.4%) <30 mm and four patients had multiple nodules as described in other reports. Furthermore, we discovered a crazy paving appearance (CPA) around nodules, cavities, masses or consolidations in five patients (71.4%).
Conclusions
Multiple nodules distributed from the middle to the peripheral area on HRCT might reflect pulmonary nocardiosis, and CPA seemed to be a worth paying attention to the diagnosis.
KEY WORDS : Crazy paving appearance, multiple nodules, lung infections, opportunistic pathogen, pulmonary nocardiosis
Introduction
Nocardia is a rare opportunistic pathogen that particularly affects immunocompromised patients, and only a few reports have described high resolution computed tomography (HRCT) manifestations in a case series of pulmonary infection (1-7). Although HRCT plays an important role in the diagnosis of pulmonary nocardiosis, variations in HRCT findings have not correlated with clinical diagnosis. Here, we describe the radiological features of pulmonary nocardiosis with respect to pulmonary nodules. Impaired cell-mediated immunity would increase the prevalence of infection (8-10), however, there was no study to examine the status of cumulative and/or daily dose of steroid treatment in patients with pulmonary nocardiosis. The aim of this study is to assess the HRCT findings in pulmonary nocardiosis reported in Japan together with examine the effects of steroid treatment in individual patients.
Methods
Data sources
We conducted a retrospective review of the medical records at our hospital between April 2006 and December 2011 and identified 16 consecutive patients with pulmonary nocardiosis. We analyzed the clinical and radiological findings of these patients. The inclusion criteria were the presence of Nocardia spp. in sputum or bronchoscopic samples from the respiratory tract. Nine of the 16 patients were excluded from this study because CT images were missing, leaving a total of 7 patients (male:female, 3:4; average (mean ± S.D) age at the diagnosis, 67.6±15.2 years). The species of four Nocardia strains isolated from the seven patients were determined by 16S ribosomal DNA sequencing (11). We also searched the medical literature to find reports of pulmonary nocardiosis in Japan between 2002 and 2011 to compare our findings. We identified 33 patients (average (mean ± S.D). age at diagnosis, 59.2±15.2 y; male:female, 2:1) who were evaluated by HRCT. Pulmonary radiologists with >15 years of experience who were blinded to the clinical findings of the patients independently reviewed the images, and decisions images were interpreted by consensus. The definition of the interpretations followed the Glossary of Terms for Thoracic Imaging proposed by the Fleischner Society (11). We then evaluated correlations between radiological and clinical findings in our patients, and compared the radiological findings with those of published reports. This retrospective study was approved by the Ethical Board of Kyorin University (Mitaka, Tokyo, Japan).
Statistical analysis
Data were statistically analyzed using Pearson’s chi-square test or the Mann-Whitney test and SPSS version 19. A P value of <0.05 in paired two-sided tests was judged to represent statistical significance.
Results
Clinical features of our and reported patients
Table 1 shows the clinical demographics of our seven patients and Table 2 (12-30) summarizes the clinical findings in these and published reports of 33 patients Japan (Table 2) (31-43). The following Nocardia strains were identified in four of our patients: N. farcinica (Patients 1 and 3); N. abscessus (Patient 2); and N. asiatica (Patient 7). Those in the published cases were N. asteroides (n=8, 24.2%) and N. farcinica (n=8, 24.2%), followed by N. otitidiscaviarum (n=3, 9.0%) (Table 3). Underlying disease affected 57.1% (n=4) of our patients and 72.7% (n=24) of reported patients (Table 2). Two of our patients (Patients 3 and 4; 28.6%), were treated with steroid, one (Patient 7; 14.3%) was treated with steroid plus an immunosuppressive drug, and four (Patients 1, 2, 5, and 6; 57.1%) were not treated compared with 10 (30.3%) 7 (21.2%), and 15 (45.5%) previously reported cases, respectively. The total cumulative dose administered to our three patients with a history of steroid therapy ranged from 3,000 to 8,270 mg and two patients had used steroids within 1 year (Patients 3 and 7; 15 and 35 mg/day, respectively; Table 1). The clinical manifestations in our and reported patients were, respectively, productive cough (n=6, 85.7%; n=10, 30.3%), pyrexia (n=6, 85.7%; n=12, 36.4%), sputum (n=0, 0%; n=4, 12.1%), and dyspnea (n=3, 42.9%; n=3, 9%). Seven (21.2%) reported patients had abscesses, whereas ours had none (Table 2).
Table 1. Clinical demographics of patients (n=7).
| Patient No. | Species | Sex | Age | Current use of steroid/cumulative dose | Diagnostic method | Prognosis |
|---|---|---|---|---|---|---|
| 1 | Nocardia farcinica | F | 78 | -/- | Sputum | Alive |
| 2 | Nocardia abscessus | F | 77 | -/- | Sputum | N.A. |
| 3 | Nocardia farcinica | M | 74 | 15 (mg/day)/8,270 (mg) | Sputum | Alive |
| 4 | Nocardia sp. | F | 84 | -/3,000 mg | Sputum | Dead |
| 5 | Nocardia sp. | M | 51 | -/- | Bronchoscopy | Alive |
| 6 | Nocardia sp. | F | 43 | -/- | Sputum | Alive |
| 7 | Nocardia asistica | M | 66 | 35(mg/day)/7,314 (mg) | Bronchoscopy | Alive |
Table 2. Patients’ characteristics and presenting symptoms.
| Our patients | Previously reported cases | |
|---|---|---|
| Total number of patients | 7 | 33 |
| age±SD | 67.6±15.2 | 59.2±15.2 |
| M:F | 3:4 | 22:11 |
| Underlying disease - | 3 | 9 |
| Underlying disease + | 4 | 24 |
| Metabolic & Endocrine | ||
| NIDDM type2 | 1 | 5 |
| Cushing disease | 0 | 1 |
| CVD | ||
| SLE | 0 | 2 |
| MPA | 0 | 2 |
| Lung disease | ||
| IIPs | 2 | 2 |
| HP | 0 | 1 |
| BE | 0 | 2 |
| DPB | 1 | 0 |
| Renal disease | ||
| Post transplantation | 0 | 3 |
| Membranous nephropathy | 0 | 3 |
| Nephrotic syndrome | 0 | 1 |
| Lupus nephritis | 0 | 2 |
| Malignancy | ||
| Malignant lymphoma | 2 | 1 |
| Cardiac disease | ||
| CHF | 1 | 1 |
| AV block (III) | 1 | 0 |
| HOCM | 1 | 0 |
| Miscellaneous | ||
| AIHA | 0 | 1 |
| Drug | ||
| Steroid only | 2 | 10 |
| Steroid + immunosuppressive drug | 1 | 7 |
| Immunosuppressive drug only | 0 | 1 |
| None | 4 | 15 |
| Symptom | ||
| Productive cough | 6 | 10 |
| Pyrexia | 6 | 12 |
| Sputum | 0 | 4 |
| Dyspnea | 3 | 3 |
| Disturbance of consciousness | 0 | 1 |
| Abscess | 0 | 7 |
| General malaise | 0 | 3 |
| Hemoptysis | 0 | 1 |
| Chest pain | 0 | 1 |
| Headache | 0 | 1 |
Table 3. Nocardia species in our and other patients.
| Species | Our patient (n=7) | Published reports (n=33) |
|---|---|---|
| N. sp. | 3 | 8 |
| N. asteroides | 0 | 8 |
| N. farcinica | 2 | 8 |
| N. otitidiscaviarum | 0 | 3 |
| N. nova | 0 | 2 |
| N. beijingensis | 0 | 1 |
| N. cyriacigeorgica | 0 | 1 |
| N. brasiliensis | 0 | 1 |
| N. farcinica + N. cyriacigeorgica | 0 | 1 |
| N. asiatica | 1 | 0 |
| N. abscessus | 1 | 0 |
Radiological findings
Thoracic HRCT showed no zonal predominance in our case series, but predominant distribution from the middle to the peripheral area (Table 4). Figure 1 shows the major representative HRCT findings in our patients (Figure 1). Although various radiological findings such as cavitations, masses, consolidations, bronchial wall and septal line thickening, as well as ground glass opacity, were evident in the present and previous patients (1-5), the main findings in the present study comprised nodules (n=5, 71.4%). Four patients had multiple nodules. We could not obtain detailed HRCT findings with respect to the size and distribution from the reported cases (Table 5), but cavitations were the most prevalent (33.3%), followed by nodules (24.2%). Seven among the 33 reported patients had multiple nodules and one had a solitary nodule, which was a similar trend as the present study. Of note, crazy paving appearance on HRCT were commonly observed in our five cases (71.4%), but statistical significance between the groups with or without CPA was not detected regarding with their clinical findings such as the severity of fever and serum inflammatory markers.
Table 4. Radiological findings from 7 patients in the present study.
| Number of patients (n) | 7 |
|---|---|
| Distribution | |
| Zonal predominance | |
| Upper | 2 (28.6%) |
| Middle | 1 (14.3%) |
| Lower | 2 (28.6%) |
| Random | 2 (28.6%) |
| Anatomical predominance | |
| Central | 0 |
| Middle | 4 (57.1%) |
| Peripheral | 3 (42.9%) |
| Random | 0 |
| Main radiological findings | |
| Nodule (<3 cm) | 5 (71.4%) |
| Cavitation | 1 (14.3%) |
| Mass (≥3 cm) | 0 |
| Consolidation | 1(14.3%) |
| Other radiological findings | |
| Bronchial ectasis | 4 (57.1%) |
| Bronchial wall thickening | 5 (71.4%) |
| Septal line thickening | 6 (85.7%) |
| Centrilobular nodule | 1 (14.3%) |
| Ground glass opacity | 6 (85.7%) |
| Crazy paving like pattern | 5 (71.4%) |
| Pleural effusion | 4 (57.1%) |
Figure 1.
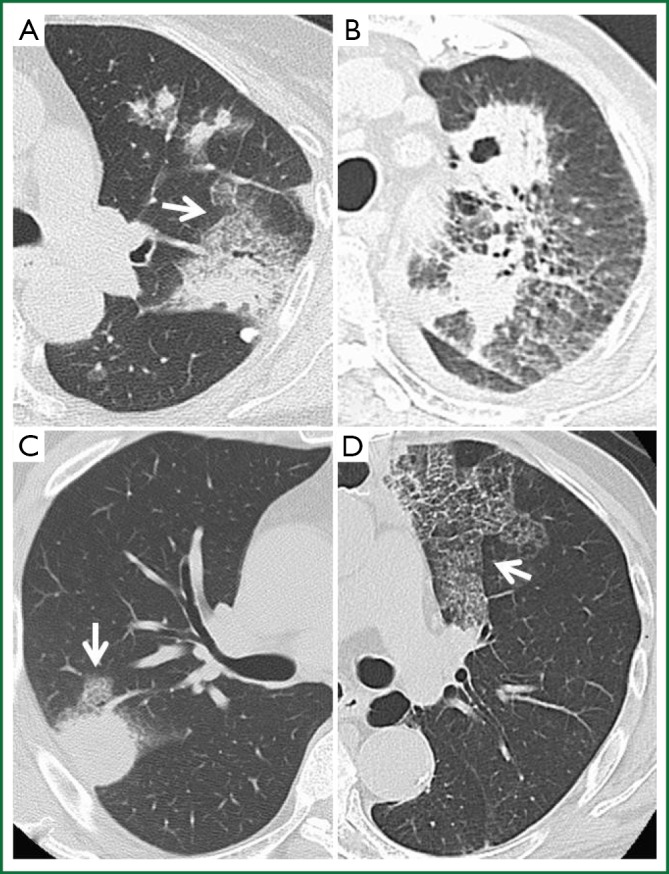
Representative HRCT findings of our patients at diagnosis. A. Air-bronchogram shows consolidation surrounded by CPA (arrow) and scattered nodules with GGO in Patient 1; B. Solitary nodule and cavity measure 27 and 28 mm, respectively, in Patient 3; C. Solitary 28-mm nodule surrounded by CPA (arrow) in Patient 5; D. Septal line thickening with CPA (arrow) in Patient 7.
Table 5. Main radiological findings of our and other patients.
| Our patients (n=7) | Published reports (n=33) | |
|---|---|---|
| Nodule (<3 cm) | 5 (71.4%) | 8 (24.2%) |
| Solitary | 1 | 1 |
| Multiple | 4 | 7 |
| Cavitation | 1 (14.3%) | 11 (33.3%) |
| Solitary | 0 | 3 |
| Multiple | 1 | 8 |
| Mass (≥3 cm) | 0 | 5 (15.1%) |
| Solitary | 0 | 4 |
| Multiple | 0 | 1 |
| Consolidation | 1 (14.3%) | 8 (24.2%) |
| Empyema | 0 | 1 (0.03%) |
Discussion
Various HRCT findings of pulmonary nocardiosis have been reported such as nodules/masses, consolidation/infiltrates, pleural thickening, and cavities (2-4,44). Furthermore, ground glass opacity with superimposed septal thickening and intralobular reticulation was found (45,46), which initially thought to be specific alveolar proteinosis. The total area of lung inflammation on HRCT images and/or the elevation of serum inflammatory markers in the present study seemed irrelevant to the presence of CPA. To the best of our knowledge, HRCT findings of CPA have not been described, which might have been underestimated in pulmonary nocardiosis. Blackmon et al. (4) reported that 57% cases revealed discrete nodules with a tendency for multiple in number, which were also predominant findings in previous reports and in our case series. Oszoyoglu et al. (3) also reported that the most commonly described findings were nodules and cavities. The main HRCT findings in the present study were lung nodules, which tended to be multiple without a zonal preponderance, but were predominantly distributed in the middle to the peripheral area.
Stuck et al. (9) found 12.7% and 8.0% overall rates of infectious complications in steroid-treated and control patients, respectively (relative risk: 1.6), and Klein et al. (8) reported that the rate of infection did not increase in patients administered with <10 mg/day or a cumulative dose of <700 mg of prednisone. Indeed, three of our patients were administered >15 mg/day or a cumulative dose of 3,000 mg of prednisone. The present study is the first to review and examine the effects of daily and cumulative steroid dosing in patients with pulmonary nocardiosis. We also reconfirmed that immunocompetent patients might be infected with Nocardia.
Conclusions
The HRCT findings of pulmonary nocardiosis were diverse and multiple nodules found from the middle to the peripheral area were diagnostic clues. Crazy paving appearance might be a noticeable HRCT findings previously have not been described. Greater understanding and recognition of these findings might result in more rapid and appropriate diagnoses of affected patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Menéndez R, Cordero PJ, Santos M, et al. Pulmonary infection with Nocardia species: a report of 10 cases and review. Eur Respir J 1997;10:1542-6 [DOI] [PubMed] [Google Scholar]
- 2.Hui CH, Au VW, Rowland K, et al. Pulmonary nocardiosis re-visited: experience of 35 patients at diagnosis. Respir Med 2003;97:709-17 [DOI] [PubMed] [Google Scholar]
- 3.Oszoyoglu AA, Kirsch J, Mohammed TL. Pulmonary nocardiosis after lung transplantation: CT findings in 7 patients and review of the literature. J Thorac Imaging 2007;22:143-8 [DOI] [PubMed] [Google Scholar]
- 4.Blackmon KN, Ravenel JG, Gomez JM, et al. Pulmonary nocardiosis: computed tomography features at diagnosis. J Thorac Imaging 2011;26:224-9 [DOI] [PubMed] [Google Scholar]
- 5.Kanne JP, Yandow DR, Mohammed TL, et al. CT findings of pulmonary nocardiosis. AJR Am J Roentgenol 2011;197:W266-72. [DOI] [PubMed] [Google Scholar]
- 6.Lerner PI. Nocardiosis. Clin Infect Dis 1996;22:891-903; quiz 904-5 [DOI] [PubMed] [Google Scholar]
- 7.Shin N, Sugawara Y, Tsukada K, et al. Successful treatment of disseminated Nocardia farcinica infection in a living-donor liver transplantation recipient. Transpl Infect Dis 2006;8:222-5 [DOI] [PubMed] [Google Scholar]
- 8.Joshi N, Caputo GM, Weitekamp MR, et al. Infections in patients with diabetes mellitus. N Engl J Med 1999;341:1906-12 [DOI] [PubMed] [Google Scholar]
- 9.Klein NC, Go CH, Cunha BA. Infections associated with steroid use. Infect Dis Clin North Am 2001;15:423-32, viii. [DOI] [PubMed] [Google Scholar]
- 10.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 1989;11:954-63 [DOI] [PubMed] [Google Scholar]
- 11.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722 [DOI] [PubMed] [Google Scholar]
- 12.Wada H, Sakai N, Matsui Y, et al. A case of pulmonary nocardiosis cured by early sulfamethoxazole-trimethoprim therapy. Nihon Kokyuki Gakkai Zasshi 2007;45:643-7 [PubMed] [Google Scholar]
- 13.Kudo K, Ikegame S, Inoshima I, et al. A case of pulmonary nocardiosis diagnosed five years after hemoptysis. Nihon Kokyuki Gakkai Zasshi 2007;45:952-6 [PubMed] [Google Scholar]
- 14.Takayanagi K, Kimura Y, Kawakami K, et al. A case of pulmonary nocardiosis with Nocardia beijingensis. Kansenshogaku Zasshi 2008;82:43-6 [DOI] [PubMed] [Google Scholar]
- 15.Bandoh C, Amano M, Suzuki M, et al. A case of pulmonary nocardiosis during steroid therapy for asthma. Nihon Kokyuki Gakkai Zasshi 2008;46:1024-8 [PubMed] [Google Scholar]
- 16.Hanibuchi M, Nakataki E, Kawano T, et al. Pulmonary nocardiosis complicated with multiple cerebellar abscess. Nihon Kokyuki Gakkai Zasshi 2008;46:1050-4 [PubMed] [Google Scholar]
- 17.Kitamura A, Sakurai T, Tomita K, et al. A case of primary pulmonary nocardiosis with multiple pulmonary nodules successfully treated with moxifloxacin. Nihon Kokyuki Gakkai Zasshi 2009;47:537-42 [PubMed] [Google Scholar]
- 18.Tanaka T, Kuroki R, Ishida M, et al. A case of pulmonary nocardiosis with simultaneous identification of N. farcinica and N. cyriacigeorgica. Nihon Kokyuki Gakkai Zasshi 2009;47:647-51 [PubMed] [Google Scholar]
- 19.Hoshino T, Okamoto M, Azuma K, et al. A case of Cushing syndrome presenting after pulmonary nocardiosis with pyothorax. Nihon Kokyuki Gakkai Zasshi 2009;47:746-50 [PubMed] [Google Scholar]
- 20.Yamaguchi S, Takayanagi N, Tokunaga D, et al. A case of pulmonary alveolar proteinosis which initially deteriorated rapidly with exacerbation of pulmonary nocardiosis, responded promptly to treatment of the pulmonary nocardiosis. Nihon Kokyuki Gakkai Zasshi 2010;48:580-3 [PubMed] [Google Scholar]
- 21.Hadano Y, Ohmagari N, Suzuki J, et al. A case of pulmonary nocardiosis due to Nocardia cyriacigeorgica with prompt diagnosis by gram stain. Nihon Kokyuki Gakkai Zasshi 2011;49:592-6 [PubMed] [Google Scholar]
- 22.Shimada S, Iwai K.Subcutaneous abscess due to Nocardia farcinica. Kansenshogaku Zasshi 2010;84:206-9 [PubMed] [Google Scholar]
- 23.Iriyama T, Horikoshi E, Sawada S, et al. Case of pulmonary nocardia infection complicated with microscopic polyangiitis during its course. Jpn J Antibiot 2008;61:89-91 [PubMed] [Google Scholar]
- 24.Takagi K, Yamada Y, Masue N, et al. Disseminated nocardiosis presenting as retroperitoneal abscess: a case report. Hinyokika Kiyo 2010;56:691-5 [PubMed] [Google Scholar]
- 25.Shimokubo T, Ashitani J, Ihi T, et al. A case of mixed infection by Nocardia asteroides and Mycobacterium tuberculosis. Nihon Kokyuki Gakkai Zasshi 2002;40:703-7 [PubMed] [Google Scholar]
- 26.Tone A, Matsuo K, Watanabe Y, et al. Pulmonary Nocardia otitidis-caviarum infection in a patient with bronchiectasia. Nihon Naika Gakkai Zasshi 2002;91:3037-9 [DOI] [PubMed] [Google Scholar]
- 27.Morita H, Torii M, Yokoyama T, et al. A case of pulmonary nocardiosis with a polypoid lesion in a bronchus. Nihon Kokyuki Gakkai Zasshi 2004;42:893-6 [PubMed] [Google Scholar]
- 28.Shimokawaji T, Kobayashi H, Kanoh S, et al. Pulmonary nocardiosis complicated with multiple brain abscess. Nihon Kokyuki Gakkai Zasshi 2005;43:375-8 [PubMed] [Google Scholar]
- 29.Enomoto M, Yamasawa H, Sawai T, et al. Pulmonary nocardiosis with bilateral diffuse granular lung shadows in a patient with subcutaneous panniculitic T-cell lymphoma. Intern Med 2002;41:986-9 [DOI] [PubMed] [Google Scholar]
- 30.Hwang JH, Koh WJ, Suh GY, et al. Pulmonary nocardiosis with multiple cavitary nodules in a HIV-negative immunocompromised patient. Intern Med 2004;43:852-4 [DOI] [PubMed] [Google Scholar]
- 31.Matsuda E, Okabe K, Yagi T, et al. Recurrent pulmonary nocardiosis with mediastinal abscess. Nihon Rinsho Geka Gakkai Zasshi 2008;69:1647-50 [Google Scholar]
- 32.Ohta K, Sumi M, Ishikawa S.Bronchial occlusion for intractable pneumothorax associated with pulmonary nocardiosis. Kikanshigaku 2008;30:252-6 [Google Scholar]
- 33.Yoshiwara R, Oyama M, Nakao K, et al. Pulmonary nocardiosis in a hemodialysis patient with rheumatoid arthritis receiving etanercept. Nihon Toseki Igakukai Zasshi 2010;43:341-6 [Google Scholar]
- 34.Aritomi S, Kikukawa Y, Shigematsu Y, et al. Two case of pulmonary nocardiosis. Amakusa Medical journal 2006;20:15-20.
- 35.Kameyama N, Matsuo K, Kudo K.Pulmonary nocardia otitidiscaviarum infection in patient that died seven years after onset. Jpn J Chest Dis 2011;70:180-5 [Google Scholar]
- 36.Kagaya M, Takahashi H, Nakano H, et al. A case of multiple muscular nocardial abscesses in the lower limbs. Nishi Nihon Hifuka 2008;70:286-91 [Google Scholar]
- 37.Nomura Y, Tomita K, Shibata M, et al. A case of multiple subcutaneous and muscular nocardiosis. Jpn J Dermatol 2007;117:1745-51 [Google Scholar]
- 38.Suzuki M, Narazaki M, Oshima S, et al. Pulmonary nocardiosis in a patient with lupus nephritis. Jpn J Chest Dis 2002;61:820-6 [Google Scholar]
- 39.Shibuya Y, Horimi T, Okabayashi T, et al. A case of pulmonary nocardiosis in renal transplant recipient. Japn J Transplant 2003;38:440-2 [Google Scholar]
- 40.Nakada M, Koide M, Saito N, et al. Two cases of cutaneous nocardiosis, definitely diagnosed by isolating as Nocardia otitidiscaviarum and Nocardia brasiliensis. Jpn J Dermatol 2004;58:622-5 [Google Scholar]
- 41.Morikawa H, Hasegawa S, Wada H.Pulmonary nocardiosis in a patient of membranous nephropathy. J Jpn Assoc Chest Surg 2005;19:885-8 [Google Scholar]
- 42.Shibuya Y, Horimi T, Okabayashi T, et al. A Case of Pulmonary nocardiosis in a renal transplant recipient. Japn J Transplant 2003;38:440-2 [Google Scholar]
- 43.Uchida K, Yanagisawa R, Kawamura T, et al. Pulmonary nocardiosis in a renal transplant recipient receiving tacrolimus treated with a combination of trimethoprim-sulfamethoxazole and sparfloxacin. J Med Soc Toho 2005;52:250-4 [Google Scholar]
- 44.Buckley JA, Padhani AR, Kuhlman JE. CT features of pulmonary nocardiosis. J Comput Assist Tomogr 1995;19:726-32 [DOI] [PubMed] [Google Scholar]
- 45.Rossi SE, Erasmus JJ, Volpacchio M, et al. “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2003;23:1509-19 [DOI] [PubMed] [Google Scholar]
- 46.Johkoh T, Itoh H, Müller NL, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 1999;211:155-60 [DOI] [PubMed] [Google Scholar]


