Abstract
A strain of Synechococcus sp. strain PCC 7942 with no functional Fe superoxide dismutase (SOD), designated sodB−, was characterized by its growth rate, photosynthetic pigments, and cyclic photosynthetic electron transport activity when treated with methyl viologen or norflurazon (NF). In their unstressed conditions, both the sodB− and wild-type strains had similar chlorophyll and carotenoid contents and catalase activity, but the wild type had a faster growth rate and higher cyclic electron transport activity. The sodB− was very sensitive to methyl viologen, indicating a specific role for the FeSOD in protection against superoxide generated in the cytosol. In contrast, the sodB− mutant was less sensitive than the wild type to oxidative stress imposed with NF. This suggests that the FeSOD does not protect the cell from excited singlet-state oxygen generated within the thylakoid membrane. Another up-regulated antioxidant, possibly the MnSOD, may confer protection against NF in the sodB− strain. These results support the hypothesis that different SODs have specific protective functions within the cell.
Oxygenic PET and aerobic respiration evolved during the Precambrian period, improving the efficiency of C metabolism many-fold. The benefits of these new pathways were partially offset, however, by their tendency to form reactive oxygen species that cause oxidative damage to biological molecules. The most significant reactive oxygen species include excited singlet-state oxygen (1O2*), the superoxide ion (O2−), hydrogen peroxide (H2O2), and the highly destructive hydroxyl radical (·OH). O2− and 1O2* mainly occur in the electron-transport chains of PET and respiration (Asada and Takahashi, 1987; Gutteridge and Halliwell, 1990; Shiraishi et al., 1994). They subsequently give rise to H2O2 and ·OH. The reaction of O2− with H2O2 that forms ·OH is often catalyzed by metals, especially Fe2+ (Halliwell and Gutteridge, 1986). As a consequence, Fe- bearing biomolecules, such as metalloenzymes and electron-transport proteins, may be the first sites of O2− damage in cells (Halliwell and Gutteridge, 1986; Kuo et al., 1987; Fridovich, 1989; Gutteridge and Halliwell, 1990; Gardner and Fridovich, 1991). Following oxidative damage to Fe-bearing proteins, freed Fe2+ can adversely react with other cellular components, causing additional damage. O2− may also disrupt Fe-S centers directly in proteins such as Fd, aconitase, succinate dehydrogenase, and PSI (Liochev, 1996). 1O2* occurs in the chlorophyll antennae when excited triplet-state chlorophyll transfers excitation energy to molecular oxygen. 1O2* can give rise to the O2− anion (Asada and Takahashi, 1987; Symons, 1991). This results in a potential for oxidative damage within the thylakoid membrane. In plants, formation of both 1O2* and O2− by PET is favored when normal metabolic pathways are slowed by physiological stress.
Reactive oxygen species are detoxified in cells by a system of antioxidants that co-evolved with PET and respiration. SODs catalyze the conversion of O2− to H2O2. Catalase subsequently oxidizes the H2O2 to molecular oxygen and water. Plant and algal chloroplasts and some bacteria (e.g. Streptococcus spp.) lack catalase and instead use peroxidases that require ascorbate or glutathione as sources of reducing power. In photosynthetic organisms, carotenoid pigments in the light-harvesting antennae quench triplet chlorophyll and 1O2* (Siefermann-Harms, 1987; Koyama, 1991), both of which can produce O2− and lipid peroxyl radicals. Other nonenzymatic reductants, such as tocopherols and tocotrienols, can quench reactive forms of oxygen as well.
The SODs are metalloenzymes that may be separated into three classes, depending on their metal cofactor. MnSODs are found in the cytosol of eubacteria, in the cytosol and thylakoid membrane of cyanobacteria, and in the mitochondrial lumen of eukaryotes. FeSODs are found in the cytosol of eubacteria and cyanobacteria and in the chloroplast stroma of photosynthetic plant cells. FeSODs are not usually found in eukaryotes other than plants. Cu/ZnSODs are present only in eukaryotes and may be found in the cytosol, chloroplast, and mitochondrial intermembrane spaces (Okada et al., 1979; Campbell and Laudenbach, 1995). FeSODs and MnSODs are proposed to have a common prokaryotic origin, whereas Cu/ZnSODs are proposed to have evolved independently in eukaryotes (Bannister et al., 1987; Campbell and Laudenbach, 1995). Despite differing evolutionary histories, the catalytic activities of the different types of SODs are essentially the same. One class of SODs can complement deletion mutations of other classes of SODs within and between species, families, and even kingdoms (Carlioz and Touati, 1986; Laudenbach et al., 1989; Haas and Goebel, 1992; Purdy and Park, 1994; Takeshima et al., 1994). The existence of multiple SODs may result from the fact that the cells of cyanobacteria and eukaryotes are divided into compartments by internal membranes. Since O2− ions are negatively charged and cannot cross a phospholipid bilayer readily, they are effectively trapped within the compartment where they were generated. This may have selected for the evolution of multiple SODs in compartmentalized cells. Indeed, most organisms with compartmentalized cells have SODs in compartments that are likely to generate O2−, such as mitochondria and chloroplasts (for review, see Fridovich, 1989).
We have chosen cyanobacteria as models for the study of SODs. Like eukaryotes, cyanobacteria have compartmentalized cells (cytosol and thylakoid lumen) and multiple SODs (Okada et al., 1979; Herbert et al., 1992; Campbell and Laudenbach, 1995). The photosynthetic apparatus of cyanobacteria is essentially the same as that of algae and plants, but cyanobacteria are easier to genetically manipulate than plants. For example, genes for the enzymatic antioxidants can be specifically inactivated by insertional mutagenesis (Golden, 1988; Porter, 1988). Also, the antioxidant system of cyanobacteria is simpler than that of plants. Genetic overexpression of SODs and other antioxidant enzymes in plants has been attempted with the goal of increasing their stress tolerance (Bowler et al., 1991; Foyer et al., 1994; Rennenberg and Polle, 1994; Allen, 1995; Van Camp et al., 1996). The antioxidant system in plants is quite complex, however, and attempts to create stress tolerance by overexpression of individual antioxidants have met with mixed success (Bowler et al., 1991; Pitcher et al., 1991; Foyer et al., 1994; Van Camp et al., 1996). Genetic improvements of the antioxidant system in plants can be designed more carefully if the specific intracellular roles of different types of antioxidants are better understood by study in simpler systems.
The cyanobacterium Synechococcus sp. strain PCC7942 (hereafter referred to as PCC7942) possesses two SODs. The MnSOD encoded by the sodA gene is thylakoid associated, whereas the FeSOD encoded by the sodB gene is cytosolic (Herbert et al., 1992). We hypothesize that the two SODs have different protective functions within the cyanobacterial cell. The FeSOD protects against O2− formed within the cytosol, whereas the MnSOD protects against O2− formed in the thylakoid membranes or within the thylakoid lumen. A mutant of PCC7942 lacking detectable FeSOD activity was constructed previously and designated sodB− (Laudenbach et al., 1989; Herbert et al., 1992). In this study we compared the response of the wild-type and sodB− strains to damage caused by either O2− formation in the cytosol or by 1O2* formation within the thylakoid membrane. We predicted that, relative to the wild type, the sodB− mutant would be sensitized to damage by cytosolic O2− but not sensitized to damage by 1O2* formed within the thylakoid membranes. We found that the sodB− strain was more sensitive to damage by O2− within the cytosol but was actually partially resistant to damage by 1O2* in the thylakoid membranes.
MATERIALS AND METHODS
Culture and Experimental Conditions
Stock cultures of wild-type and sodB− Synechococcus sp. PCC7942 were grown in 50-mL tubes of BG-11 broth (Sigma) supplemented with 10 mm NaHCO3 and adjusted to pH 8.0 with 5 mm KH2PO4. Cultures were incubated in a water bath at 27°C, sparged with 3% CO2 in air, and illuminated with cool-white fluorescent tubes with a PAR flux of 15 to 35 μmol photons m−2 s−1. Light intensities were measured using a LI-189 quantum sensor (Li-Cor, Lincoln, NE). Dry cell mass, direct cell counts, and A750 were used to monitor cell concentration. We found a high correlation (r = 0.97, n = 25) between A750 and cellular dry mass, confirming the use of absorbance at this wavelength as an indicator of biomass (data not shown). Unless otherwise noted, cultures were diluted to A750 = 0.3 with fresh BG-11 broth without supplemental NaHCO3 prior to experiments. Oxidative stress treatments were imposed in water-jacketed beakers maintained at 27°C in air and illuminated with 100 μmol photons m−2 s−1 from cool-white fluorescent tubes. Samples were stirred continuously during treatments.
Growth Measurements
Rapidly growing cells were centrifuged and resuspended in fresh BG-11 broth to an A750 of 0.1 to 0.3 and illuminated at 30 μmol photons m−2 s−1. Aliquots of 3 mL were removed at approximately 24-h intervals and A750 was measured. Periodically, 10-mL samples were removed, vacuum filtered onto 0.45-μm membrane filters, and dried for 24 h at 100°C to determine dry mass.
Oxidative Stress Induction
O2− generation was catalyzed by adding MV (paraquat) to cultures to obtain a final concentration of 0.1 to 5.0 μm. MV catalyzes the formation of O2− at a variety of electron-transport sites, but in photosynthetic organisms in light the vast majority of O2− is generated at the FA and FB centers of PSI (Fujii et al., 1990; Dodge, 1991). MV is a competitive inhibitor of the PSI cyclic pathway at concentrations of 100 μm (Yu et al., 1993). However, the low concentrations of MV used in our experiments had no effect on the PSI cyclic pathway (Herbert et al., 1995; Martin et al., 1997). 1O2* generation was catalyzed indirectly by adding the carotenoid inhibitor NF to cultures to obtain a final concentration of 0.1 to 5.0 μm. NF inhibits phytoene desaturase and thus blocks synthesis of β-carotene and other carotenoids (Ben-Aziz and Koren, 1974; Kümmel and Grimme, 1975). Since carotenoids (β-carotene in particular) normally quench 1O2* in the chlorophyll antenna, the addition of NF promotes the formation of 1O2* within the thylakoid membrane.
P700 Oxidation Reduction
The photooxidation and dark-reduction kinetics of P700 were measured in intact cells using the broadband A820 change (ΔA820), as described elsewhere (Herbert et al., 1995). The ΔA820 was monitored by reflectance using a modulated detection system (Walz, Effeltrich, Germany) consisting of a PAM 101 control unit and an ED 800T emitter-detector unit. A branched fiber optic cable was used to deliver modulated 820 nm and white actinic light to the sample and to collect reflected 820 nm light. Actinic light (1000 μm photons m−2 s−1) was provided by a tungsten projector lamp (model EJV, General Electric) fitted with three Calflex C heat filters (Balzers, Liechtenstein) and a mechanical shutter (Uniblitz VS25, Vincent Associates, Rochester, NY). Output from the PAM 101 control unit was collected and analyzed with a MacLab/2e data acquisition system using Scope v3.3 software (AD Instruments, Milford, MA) on a Macintosh computer. Samples for ΔA820 measurements were prepared at room temperature by vacuum filtering 10 mL of culture onto 0.45-μm membrane filters (type HA, Millipore). The filter and sample were then placed under an acrylic light guide at the end of the trifurcated fiber optic cable. Electron transport inhibitors (DCMU and/or DBMIB) were added to the samples prior to filtration.
A820 transients were analyzed and interpreted as described by Yu et al. (1993), with the exception that we measured the initial slopes instead of using half-times to determine rates of oxidation and re-reduction of P700. Typical traces of raw data are shown in Figure 1. Inhibitors of electron transport were used to block different inputs of electrons to PSI, as has been done previously (Maxwell and Biggins, 1976; Herbert et al., 1992; Yu et al., 1993). Input from PSII was abolished with 25 μm DCMU. Input from the plastoquinone pool was blocked with 25 μm DBMIB, leaving only “nonspecific reductants” to slowly re-reduce P700. The nature of these nonspecific reductants is uncertain (Maxwell and Biggins, 1976). Recent findings indicate that DBMIB itself may act as a slow electron donor to PSI (Martin et al., 1997).
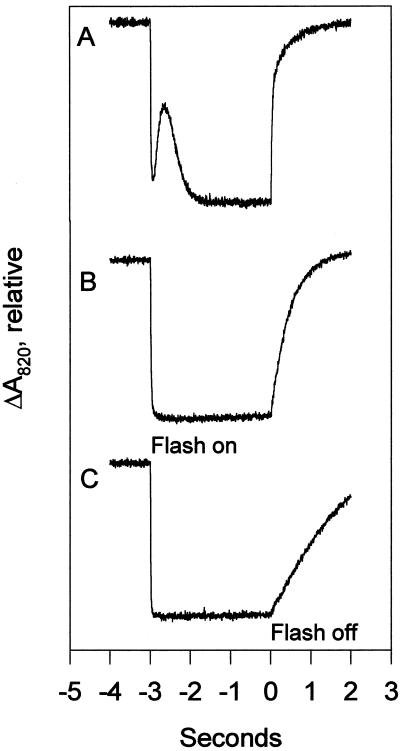
Figure 1.
P700 oxidation-reduction trace. These are typical traces from P700 measurements with and without added inhibitors. Each trace is an average of four measurements. Oxidation occurs when the sample is flashed with actinic light (“flash on”). Re-reduction occurs in the dark when the light is turned off (“flash off”). The difference in absorbance between the oxidized and reduced states is proportional to the amount of P700 present. The initial oxidation rate (“on rate”) is proportional to the efficiency with which the photosynthetic antennae deliver excitation energy to P700. The initial re-reduction rate (“off rate”) is proportional to the rate of electron transfer to P700 from the cyclic and noncyclic electron transport pathways. The transient seen just after the actinic flash in untreated samples (A) is thought to be due to electron input from PSII. In the presence of DCMU (B), the input of electrons from PSII to PSI is blocked, resulting in a lower rate of re-reduction. In the presence of DBMIB (C), electrons from both the cyclic and noncyclic pathways are blocked, resulting in very slow re-reduction. For comparison, in an unstressed wild-type sample, the relative re-reduction rates are: A = 116, B = 79, and C = 14.
Pigment Measurements
Measurements of photosynthetic pigments were made using a DW-2000 scanning spectrophotometer (SLM/Aminco, Urbana, IL). Estimates of chlorophyll a and phycocyanin concentrations were made by measuring A625, A678, and A700 in whole-cell suspensions and by applying the calculations of Myers et al. (1980). Chlorophyll and carotenoids were then extracted in 100% acetone. Chlorophyll a was quantified from A663 using an absorption coefficient of 11.3. The ratio of total carotenoids to chlorophyll a in the extract was estimated by integrating the absorbance at the intervals of 400 to 520 nm and 640 to 690 nm. Relative amounts of specific carotenoids and chlorophyll a were measured by reverse-phase HPLC. A liquid chromatograph (series 4, Perkin-Elmer) was used in conjunction with an LC-95 UV/visible spectrophotometric detector (Perkin-Elmer) and a Supelcosil LC-18-DB 15-cm × 4.6-mm, 3-μm pore-size column (Supelco, Bellefonte, PA). The carrier protocol was described previously (Millie et al., 1990), but we made one minor alteration. The carrier that we used consisted of 90% methanol, 6% acetonitrile, and 4% water for 7 min, followed by 32% methanol, 3% acetonitrile, and 65% acetone for 10 min. Fifteen milliliters of cell suspension (A750 = 0.3) was centrifuged at 1800g for 10 min. The supernatant was discarded and the pellet extracted in 1 mL of methanol for 1 h at −20°C in the dark. Fifty microliters of the extract was injected into a 20-μL sample loop. Carotenoid and chlorophyll peaks were identified at 420 nm using known standards, absorbance spectra, and/or previously published chromatograms. The presence or absence of tocopherols was investigated using the same HPLC system at 290 nm in a 100% methanol carrier with known standards (Lang et al., 1992).
Catalase Assay
Activity of the catalase in wild-type and sodB− strains of PCC 7942 was determined in intact cells by oxygen evolution in response to exogenous H2O2. Cultures were washed and resuspended to an A750 of 0.3 in fresh medium. Aliquots (1.5 mL) of these samples were placed in a DW1 liquid-phase oxygen electrode cuvette (Hansatech, Norfolk, UK) and stirred vigorously at 27°C. Oxygen in the cuvette was decreased to 30% of air-saturated values with a stream of N2 gas, and the cuvette was sealed and allowed to stabilize. A rate-saturating amount of H2O2 was then injected (100 μL of a 30% solution), and the rate of oxygen evolution was measured 10 to 30 s after injection within the linear range of the reaction. The background rate of spontaneous oxygen formation was measured by injecting the same amount of H2O2 into a sample of fresh medium containing no cells. This background rate was subtracted from the rates obtained with cell samples and was typically less than 20% of the rate obtained with cells. The assays were performed in darkness to avoid contributions from photosynthetic oxygen evolution. Since PCC7942 cells are small and a rate-saturating amount of H2O2 was added for the assay, breakage of the cells was not considered necessary to obtain comparable catalase activities of the two strains. Both H2O2 and oxygen diffuse rapidly in and out of cells, and cell breakage introduces the risk of catalase degradation during the assay.
RESULTS
Growth Data
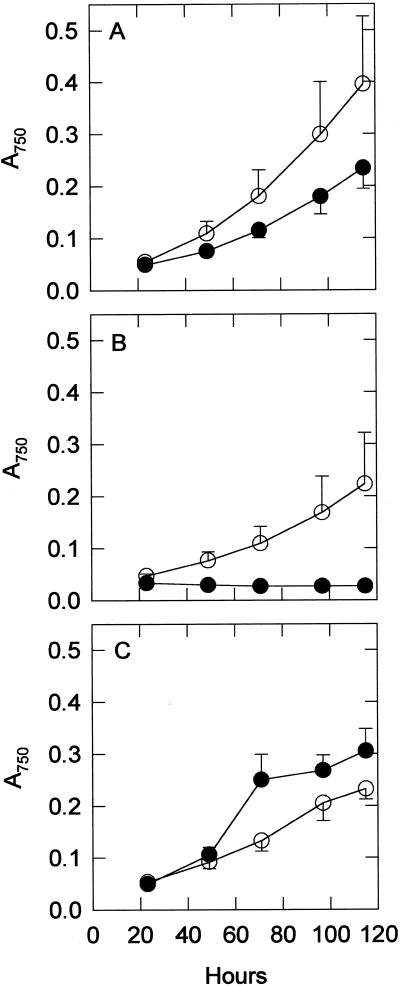
Figure 2 shows the growth rates of both strains with and without oxidative stresses. Without oxidative stress, the wild-type strain grew somewhat faster than the sodB− strain. In the presence of 0.5 μm MV, the growth rate of the wild type was markedly slower, but the sodB− strain did not grow at all. In the presence of 5 μm NF, both strains had lower growth rates, but initially the sodB− strain had a higher growth rate than the wild type. Eventually, both strains died in NF, but the wild type was the first to succumb.
Figure 2.
Growth rates of wild-type (○) and sodB− (•) strains. Growth curves measured at A750 are shown here for control (A), 0.5 μm MV-treated (B), and 5 μm NF-treated (C) groups. Each point is the mean of three samples. Error bars are sds.
Photosynthetic Pigments
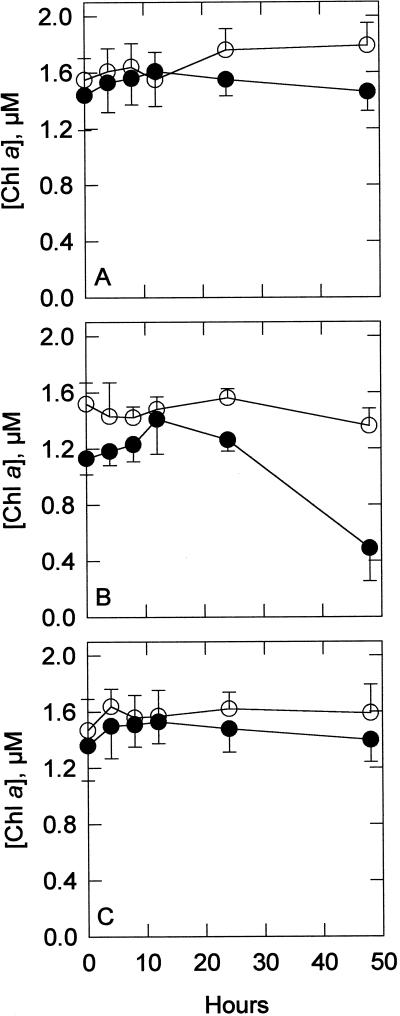
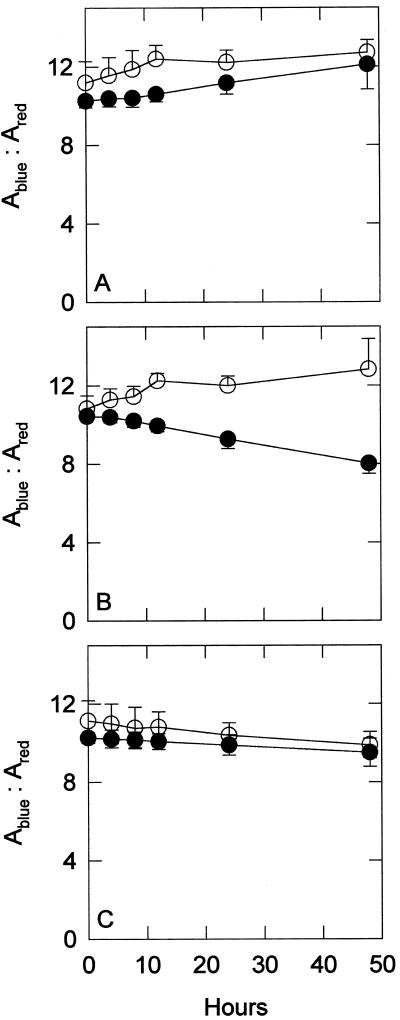
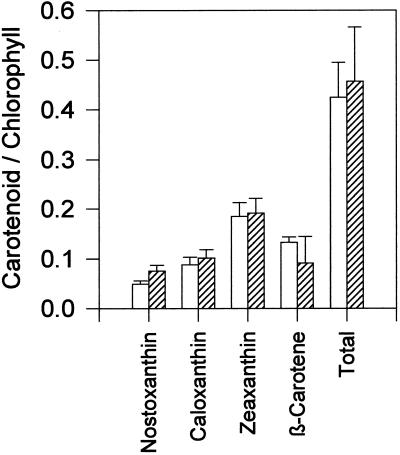
The amount of phycocyanin changed little during all treatments (data not shown). Measurements of chlorophyll a in acetone extracts were consistently lower in the sodB− strain than in the wild type during all treatments, as shown in Figure 3. Measurements of chlorophyll a in whole cells were very similar to the acetone extracts (data not shown). The most marked difference between the two strains was seen during the MV treatments, when the wild type had approximately 3 times more chlorophyll a than the sodB− strain at the end of 48 h. The ratio of total carotenoids to chlorophyll, shown in Figure 4, followed a pattern similar to that seen with total chlorophyll. In the control and NF groups, the ratio remained similar in both strains, increasing slowly in the control and decreasing slowly in the NF treatment. The MV treatment resulted in a larger difference between the two strains at the end of 48 h, with the sodB− strain losing carotenoids. HPLC separation of the pigments, shown in Figure 5, coincides with the spectrophotometry results. Before any treatments, the two strains had approximately the same amount of total carotenoids per unit chlorophyll. Four carotenoids were identified from the HPLC data by absorbance spectra and comparison with previous chromatograms (Masamoto and Furukawa, 1998): nostoxanthin, caloxanthin, zeaxanthin, and β-carotene. As has been shown previously, zeaxanthin and β-carotene were the most abundant carotenoids in this cyanobacterium (Omata and Murata, 1983; Guillard et al., 1985). No evidence of tocopherols was found (data not shown), confirming previous investigations (Powls and Redfearn, 1967).
Figure 3.
Spectrophotometric measurements of acetone-extractable chlorophyll (Chl) a. Chlorophyll was extracted into 100% acetone. Extractable chlorophyll concentrations are shown for wild type (○) and sodB− (•) over 48-h treatment periods for control (A), 0.5 μm MV-treated (B), and 5 μm NF-treated (C) groups. Each point is the mean of five or six samples. Error bars are sds.
Figure 4.
Carotenoid-to-chlorophyll ratios of wild-type (○) and sodB− (•) strains. Chlorophyll and carotenoids were extracted into 100% acetone. The resulting values are the ratios of blue peak absorbance (Ablue) to red peak absorbance (Ared) over 48-h treatment periods for control (A), 0.5 μm MV-treated (B), and 5 μm NF-treated (C) groups (see Methods). Each point is the mean of five or six samples. Error bars are sds. For comparison, purified chlorophyll a from spinach yielded a blue:red ratio of 4.53. Symbols are as in Figure 3.
Figure 5.
Initial relative amounts of chlorophyll and carotenoids as measured by reverse-phase HPLC. All values are ratios of carotenoid to chlorophyll a peak areas (milliabsorbance units s−1) measured by A420. Values are means ± sd; n = 4. Open bars, Wild type; hatched bars, sodB−.
P700 Oxidation Rate
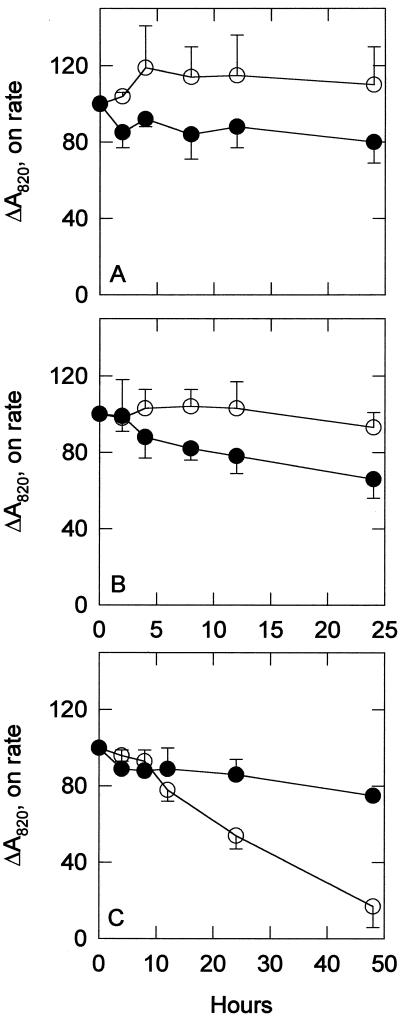
The rates of P700 oxidation, and thus the efficiency of excitation energy transfer from the photosynthetic antennae to P700, are shown in Figure 6. DBMIB (25 μm) was added to slow competing re-reduction of P700 and to ensure the maximum oxidation rate. In the wild-type control, the oxidation rate initially increased during the first 4 h and then decreased to a steady rate that was higher than the initial rate. The sodB− control oxidation rate decreased slightly during the 24-h period. During treatments with MV, the wild-type oxidation rate remained constant throughout most of the treatment, with a slight decrease by the end of 24 h. The sodB− oxidation rate with MV showed only a slight decrease as well but was lower than the rate in the wild type. A much more dramatic difference was seen in the NF treatments. The wild-type oxidation rate decreased almost linearly to less than 20% of the original rate after 48 h. During the same duration, the sodB− rate decreased to only about 70% of the original rate.
Figure 6.
P700 oxidation rate. The ΔA820 over the initial 10 to 30 ms after the onset of actinic light was used to calculate the initial rate of P700 oxidation in control (A), 0.5 μm MV-treated (B), and 5 μm NF-treated (C) cultures. ○, Wild-type strain; •, sodB− strain. Each point represents the mean of three to eight samples and all data are percent of initial values. Error bars are sds. DBMIB (25 μm) was added to all samples to prevent re-reduction by the cyclic and noncyclic pathways.
P700 Activity
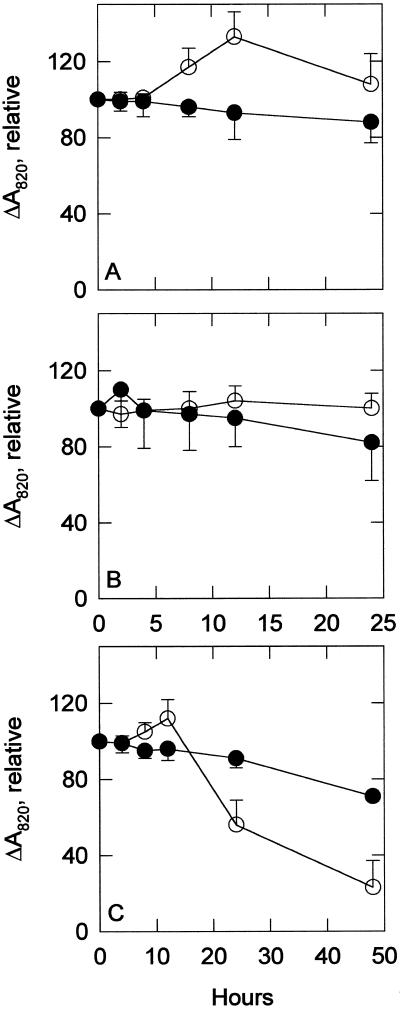
The data of Figure 7 show that the extent of P700 oxidation remained approximately the same in both strains during the control and the 0.5 μm MV treatments. However, the sodB− strain had slightly lower values and was more inhibited by MV than the wild type. When treated with 5 μm NF, the wild-type strain showed a marked decrease in P700 oxidation extent after 12 h, whereas the sodB− strain showed a steadier and much slower decrease. To ensure that the P700 was fully oxidized when measuring the extent of P700 oxidation, saturating actinic light was used and the samples were treated with 25 μm each of DCMU and DBMIB.
Figure 7.
P700 oxidation extent. ΔA820 was used to measure the relative amount of photooxidizable P700 in control (A), 0.5 μm MV-treated (B), and 5 μm NF-treated (C) cultures. ○, Wild-type strain; •, sodB− strain. All data are percent of starting values. Values are means of three to eight samples. Error bars are sds. DCMU and DBMIB (25 μm each) were added to ensure complete oxidation of P700.
PSI Electron Transport
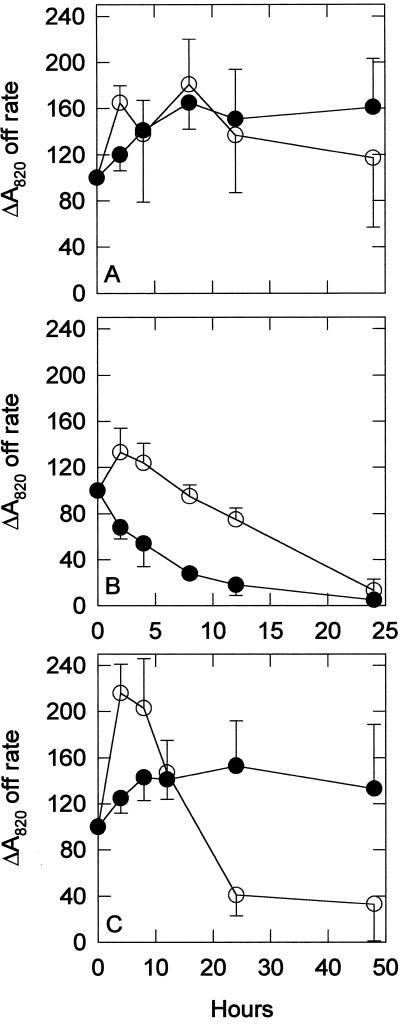
The rate of P700 re-reduction, shown in Figure 8, was measured in both strains in the presence of 25 μm DCMU. This rate is an indicator of PSI-driven cyclic electron transport (Herbert et al., 1995). The wild-type control group initially showed an increase in cyclic electron transport, as indicated by the increased rate of re-reduction. After 12 h, cyclic electron transport activity stabilized at a rate of almost 160% of the initial rate. The sodB− control group had relatively constant cyclic electron transport activity throughout the time course. Both strains exhibited decreased cyclic activity when treated with MV, but the sodB− strain lost activity twice as fast as the wild type. In the NF treatment the re-reduction rate in the wild-type strain increased during the first 4 h and then decreased drastically after 12 h. The re-reduction rate in the sodB− strain increased to a lesser degree during the first 8 h and then remained fairly constant at a rate of approximately 130% of the starting rate.
Figure 8.
P700 re-reduction rate with DCMU. The ΔA820 over the initial 50 to 300 ms after the removal of actinic light was used to calculate the initial rate of P700 re-reduction in control (A), 0.5 μm MV-treated (B), and 5 μm NF-treated (C) cultures. ○, Wild-type strain; •, sodB− strain. Each point represents the mean of three to eight samples and all data are percent of initial values. Error bars are sds. The addition of 25 μm DCMU blocks input from PSII. The data shown represent cyclic electron transport.
Catalase Activity
Activity of the single catalase known in PCC7942 (Mutsuda et al., 1996) was measured by oxygen evolution in response to H2O2 in whole cells. The rates obtained from wild-type and sodB− cells were similar. Wild-type cultures diluted to an A750 of 0.3 produced oxygen at a maximum rate of 0.28 ± 0.09 μmol min−1 mL−1 of sample in response to exogenous H2O2. sodB− cultures measured in the same way produced oxygen at a maximum rate of 0.30 ± 0.10 μmol min−1 mL−1 of sample (values are means ± sample sd). The sample size for both numbers was seven. Each of these seven samples was a separate culture that was assayed two or three times to get an average value. Values from any one culture were typically very repeatable.
DISCUSSION
Loss of cytosolic FeSOD activity in the sodB− mutant sensitized it to oxidative stress imposed with MV and light. Inhibition of growth, loss of photosynthetic pigments, and inhibition of electron transport caused by MV were all much more severe in the sodB− strain than in the wild type. MV catalyzes O2− formation from electron transport at the FA/FB centers of PSI, close to the cytosolic compartment. The sensitivity of the sodB− strain to MV suggests that the FeSOD has a specific role in protecting cellular components from O2− formed in the cytosol. Surprisingly, the sodB− mutant was not sensitized to oxidative stress imposed with the carotenoid synthesis inhibitor NF. In fact, the sodB− strain was partially resistant to NF in comparison with the wild type, exhibiting less inhibition of growth, a similar loss of photosynthetic pigments, and less inhibition of electron transport. NF inhibits the synthesis of colored carotenoid pigments at the phytoene desaturase step and thus promotes formation of 1O2* in the chlorophyll antennae. The resistance of the sodB− strain to NF indicates that the cytosolic FeSOD plays no role in protecting cell components from damage by 1O2* formed within the thylakoid membranes. A different antioxidant that is up-regulated to compensate for the lack of FeSOD may account for NF resistance in the sodB− strain.
MV
The earliest target of MV inhibition in both strains was electron transport into P700 (Fig. 8). Without DCMU, PSII is the major source of these electrons. With DCMU added, the major sources of these electrons are the two cyclic paths of photosynthetic electron transport present in cyanobacteria (Herbert et al., 1992, 1995; Yu et al., 1993). The rate of this cyclic input to P700 declines rapidly in the wild type when treated with MV and much more rapidly in the sodB− strain. The sodB− strain of PCC7942 was shown previously to be sensitive to MV, and the primary site of damage to PET was observed to be in the cyclic electron transport path between the FA/FB centers of PSI and Cyt f (Herbert et al., 1992; Samson et al., 1994). The present study confirms and extends those previous results. Inhibition of the two cyclic paths in cyanobacteria is consistent with oxidative damage to the FA/FB centers of PSI or to Fd. The FA/FB centers of PSI are known to be an early site of oxidative damage to this photosystem (Fujii et al., 1990; Dodge, 1991). They and Fd also contain Fe-S centers, which are known to be disrupted by O2− (Liochev, 1996).
In contrast to P700 re-reduction, chlorophyll concentration, the rate of P700 oxidation by the light-harvesting antenna of PSI, and the quantity of photooxidizable P700 decreased only slightly in both the sodB− strain and the wild type during the first 24 h of MV treatment (Figs. 3, 6, 7, and 8). The P700 reaction center and its chlorophyll antenna are both within the thylakoid membrane and may not be fully exposed to O2− and subsequent ·OH formed by MV. This inaccessibility may account for the fact that the rate and extent of P700 oxidation are not sensitized to MV in the sodB− strain. Alternatively, these targets may not be strongly sensitized to MV because the membrane-associated MnSOD or an unknown lipophilic antioxidant protects them to an equal extent as in the wild type.
It should be noted that MV is a competitive inhibitor of PSI cyclic electron transport at 100 μm (Yu et al., 1993), and such inhibition of the cyclic path could account for inhibition of P700 re-reduction. However, the 0.5 μm MV used in our experiments did not inhibit re-reduction of P700 (data not shown).
CO2 and Irradiance Side Effects
When the cells were transferred from growth conditions (30 μm photons m−2 s−1, 3% CO2) to the stress chambers (100 μm photons m−2 s−1, ambient CO2), the wild type exhibited an increase in both the amount of photooxidizable P700 and in the P700 oxidation rate. Under the same conditions, the sodB− strain was less variable for both parameters. Previous research has shown that cyclic electron transport increases in response to increased light intensity (Herbert et al., 1995). In addition, the transfer from high to low concentrations of CO2 would activate inorganic carbon-concentrating mechanisms (for review, see Kaplan et al., 1991) that require the cyclic pathway (Ogawa, 1991).
NF
The most interesting result from this study was that the sodB− strain is at least partially resistant to the effects of NF. Unlike MV, NF caused a decrease in the rate and extent of P700 oxidation. Both effects would result from oxidation of chlorophyll in PSI by the formation of 1O2* within the thylakoid membrane. When treated with NF, the wild type shows a decreased rate of P700 photooxidation (Fig. 6C), a loss of photooxidizable P700 (Fig. 7C), decreased cyclic PET (Fig. 8C), and a low growth rate (Fig. 2C). The effects of NF on PSI activity in the wild type are consistent with oxidative damage at or near the reaction center. Loss of photooxidizable P700 represents damage to the reaction center itself. Decreased oxidation rate suggests an interruption of the delivery of excitation to the reaction center. Figure 3C shows that oxidative loss of total chlorophyll did not coincide with the decrease of the oxidation rate, so the interruption must occur close to P700.
The NF effects observed in wild-type PCC7942 were much less marked in the sodB− strain. One explanation of this would be that the phytoene desaturase of the sodB− strain is resistant to inhibition by NF, as has been observed in other organisms (Windhövel et al., 1994). The data in Figure 4A do not support this hypothesis, however. With NF treatment, the ratio of total carotenoids to chlorophyll a declined in both the wild-type and sodB− strains, as would be expected if carotenoid synthesis was inhibited. This ratio declines equally in both strains and is a little lower to begin with in the sodB− strain (Figs. 4C and 5).
The Cause of NF Resistance in the sodB−Strain
Since NF-resistant phytoene desaturase does not appear to be the cause of the NF resistance, what is? PCC7942 has a relatively simple antioxidant system consisting of FeSOD and MnSOD (Laudenbach et al., 1989), catalase (Mutsuda et al., 1996), and β-carotene. The ascorbate peroxidase and glutathione systems found in plants have not been found in PCC7942 (Takeda et al., 1994). Also, unlike other cyanobacteria and higher organisms, PCC7942 lacks the lipid-soluble antioxidant α-tocopherol (Powls and Redfearn, 1967). Both the wild-type and sodB− strains have similar catalase activities and carotenoid complements. Increased expression of the MnSOD in the sodB− strain has been reported, however (Herbert et al., 1992). Presumably, the MnSOD activity is increased to compensate for the lack of FeSOD. We propose that the sodB− strain resists the effects of NF because of overexpression of MnSOD, which detoxifies the O2− formed within the thylakoid as a product of 1O2*. Work in progress to obtain a sodA− deletion mutant will confirm this putative role of MnSOD in PCC4972 and further define the different intracellular roles of antioxidants in this organism.
ACKNOWLEDGMENT
This paper is dedicated to the late Prof. David E. Laudenbach, whose leadership of this work was cut short by his untimely death.
Abbreviations:
- DBMIB
2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone
- MV
methyl viologen
- NF
norflurazon
- PET
photosynthetic electron transport
- SOD
O2− dismutase
Footnotes
This work was supported by the U.S. Department of Agriculture Seed Grant (no. 93-37311-9445), the National Science Foundation Experimental Program to Stimulate Competitive Research in Idaho, and University of Idaho Seed Grants to S.K.H.
LITERATURE CITED
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition, Amsterdam, The Netherlands: Elsevier Science Publishers; 1987. pp. 227–287. [Google Scholar]
- Bannister JV, Bannister WH, Rotilio G. Aspects of the structure, function and applications of O2− dismutase. CRC Crit Rev Biochem. 1987;22:111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Ben-Aziz A, Koren E. Interference in carotenogenesis as a mechanism of action of the pyridazinone herbicide Sandoz 6706. Plant Physiol. 1974;54:916–920. doi: 10.1104/pp.54.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inzé D. Manganese O2− dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J. 1991;10:1723–1732. doi: 10.1002/j.1460-2075.1991.tb07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WS, Laudenbach DE. Characterization of four O2− dismutase genes from a filamentous cyanobacterium. J Bacteriol. 1995;177:964–972. doi: 10.1128/jb.177.4.964-972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A, Touati D. Isolation of O2− dismutase mutants in Escherichia coli: is O2− dismutase necessary for aerobic life? EMBO J. 1986;5:625–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge AD (1991) Photosynthesis. In RC Kirkwood, ed, Target Sites for Herbicide Action. Plenum Press, New York, pp 1–23
- Foyer C, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Fridovich I. Superoxide dismutases, an adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- Fujii T, Yokoyama E, Inoue K, Sakurai H. The sites of electron donation of photosystem I to methyl viologen. Biochim Biophys Acta. 1990;1015:41–48. [Google Scholar]
- Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991;266:1478–1483. [PubMed] [Google Scholar]
- Golden SS. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. Methods Enzymol. 1988;167:714–727. doi: 10.1016/0076-6879(88)67083-2. [DOI] [PubMed] [Google Scholar]
- Guillard RRL, Murphy LS, Foss P, Liaaen-Jensen S. Synechococcus spp. as likely zeaxanthin-dominant ultraphytoplankton in the North Atlantic. Limnol Oceanogr. 1985;30:412–414. [Google Scholar]
- Gutteridge JMC, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Haas A, Goebel W. Cloning of a O2− dismutase gene from Listeria ivanovii by functional complementation in Escherichia coli and characterization of the gene product. Mol Gen Genet. 1992;231:313–322. doi: 10.1007/BF00279805. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986;246:501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Herbert SK, Martin RE, Fork DC. Light adaptation of cyclic electron transport through photosystem I in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth Res. 1995;46:277–285. doi: 10.1007/BF00020441. [DOI] [PubMed] [Google Scholar]
- Herbert SK, Samson G, Fork DC, Laudenbach DE. Characterization of damage to photosystems I and II in a cyanobacterium lacking detectable iron O2− dismutase activity. Proc Natl Acad Sci USA. 1992;89:8716–8720. doi: 10.1073/pnas.89.18.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Schwarz R, Lieman-Hurwitz J, Reinhold L. Physiological and molecular aspects of the inorganic carbon-concentrating mechanism in cyanobacteria. Plant Physiol. 1991;97:851–855. doi: 10.1104/pp.97.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y. Structures and functions of carotenoids in photosynthetic systems. J Photochem Photobiol B Biol. 1991;9:265–280. [Google Scholar]
- Kümmel HW, Grimme LH. The inhibition of carotenoid biosynthesis in green algae by Sandoz H 6706: accumulation of phytoene and phytofluene in Chlorella fusca. Z Naturforsch. 1975;30c:333–336. [Google Scholar]
- Kuo CF, Mashino T, Fridovich I. α,β-Dihydroxyisovalerate dehydratase, a O2−-sensitive enzyme. J Biol Chem. 1987;262:4724–4727. [PubMed] [Google Scholar]
- Lang JK, Schillaci M, Irvin B. Vitamin E. In: De Leenheer AP, Lambert WE, Nelis HJ, editors. Modern Chromatographic Analysis of Vitamins. Inc, New York: Marcel Dekker; 1992. pp. 153–195. [Google Scholar]
- Laudenbach DE, Trick CG, Straus NA. Cloning and characterization of an Anacystis nidulans R2 O2− dismutase gene. Mol Gen Genet. 1989;216:455–461. doi: 10.1007/BF00334390. [DOI] [PubMed] [Google Scholar]
- Liochev SI. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radical Res. 1996;25:369–384. doi: 10.3109/10715769609149059. [DOI] [PubMed] [Google Scholar]
- Martin RE, Thomas DJ, Tucker DE, Herbert SK. The effects of photooxidative stress on photosystem I measured in vivo in Chlamydomonas. Plant Cell Environ. 1997;20:1451–1461. [Google Scholar]
- Masamoto K, Furukawa K (1998) Accumulation of zeaxanthin in cells of the cyanobacterium Synechococcus sp. strain PCC 7942 grown under high irradiance. J Plant Physiol (in press)
- Maxwell PC, Biggins J. Biochemistry. 1976;15:3975–3981. doi: 10.1021/bi00663a011. [DOI] [PubMed] [Google Scholar]
- Millie DF, Ingram DA, Dianigri CP. Pigment and photosynthetic responses of Oscillatoria agardhii (Cyanophyta) to photo flux density and spectral quality. J Phycol. 1990;26:660–666. [Google Scholar]
- Mutsuda M, Ishikawa T, Shigeoka S. The catalase-peroxidase of Synechococcus PCC 7942: purification, nucleotide sequence analysis and expression in Escherichia coli. Biochem J. 1996;316:251–257. doi: 10.1042/bj3160251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Graham J-R, Wang RT. Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol. 1980;66:1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. Proc Natl Acad Sci USA. 1991;88:4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Kanematsu S, Asada K. Intracellular distribution of manganese and ferric O2− dismutases in blue-green algae. FEBS Lett. 1979;103:106–110. [Google Scholar]
- Omata T, Murata N. Isolation and characterization of the cytoplasmic membranes from the blue-green alga (cyanobacterium) Anacystis nidulans. Plant Cell Physiol. 1983;24:1101–1112. [Google Scholar]
- Pitcher LH, Brennan E, Hurley A, Dunsmuir P, Tepperman JM, Zilinskas BA. Overproduction of petunia chloroplastic copper/zinc O2− dismutase does not confer ozone tolerance in transgenic tobacco. Plant Physiol. 1991;97:452–455. doi: 10.1104/pp.97.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RD. DNA transformation. In: Packer L, Glazer AN, editors. Cyanobacteria, Vol 167. San Diego, CA: Academic Press; 1988. pp. 703–712. [DOI] [PubMed] [Google Scholar]
- Powls R, Redfearn ER. The tocopherols of the blue-green algae. Biochem J. 1967;104:24c–26c. doi: 10.1042/bj1040024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy D, Park SF. Cloning, nucleotide sequence and characterization of a gene encoding O2− dismutase from Campylobacter jejuni and Campylobacter coli. Microbiology. 1994;140:1203–1208. doi: 10.1099/13500872-140-5-1203. [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Polle A. Protection from oxidative stress in transgenic plants. Biochem Soc Trans. 1994;22:936–940. doi: 10.1042/bst0220936. [DOI] [PubMed] [Google Scholar]
- Samson G, Herbert SK, Fork DC, Laudenbach DE. Acclimation of the photosynthetic apparatus to growth irradiance in a mutant strain of Synechococcus lacking iron O2− dismutase. Plant Physiol. 1994;105:287–294. doi: 10.1104/pp.105.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi T, Takahashi M-a, Asada K. Generation of O2− anion radicals and hydroxyl radicals in chloroplast thylakoids. In: Asada K, Yoshikawa T, editors. Frontiers of Reactive Oxygen Species in Biology and Medicine. Amsterdam, The Netherlands: Excerpta Medica; 1994. pp. 31–32. [Google Scholar]
- Siefermann-Harms D. The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plant. 1987;69:561–568. [Google Scholar]
- Symons MCR (1991) Free radicals in biological systems. In IE Dreosti, ed, Trace Elements, Micronutrients and Free Radicals. Humana Press, Totowa, NJ, pp 1–24
- Takeda T, Ishikawa T, Shigeoka S. The H2O2-scavenging system and tolerance system to H2O2 in algae. In: Asada K, Yoshikawa T, editors. Frontiers of Reactive Oxygen Species in Biology and Medicine. Amsterdam, The Netherlands: Excerpta Medica; 1994. pp. 143–146. [Google Scholar]
- Takeshima Y, Takatsugu N, Sugiura M, Hagiwara H. High-level expression of human O2− dismutase in the cyanobacterium Anacystis nidulans 6301. Proc Natl Acad Sci USA. 1994;91:9685–9689. doi: 10.1073/pnas.91.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W, Capiau K, Van Montagu M, Inzé D, Slooten L. Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-O2− dismutase in chloroplasts. Plant Physiol. 1996;112:1703–1714. doi: 10.1104/pp.112.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhövel U, Geiges B, Sandmann G, Böger P. Expression of Erwinia uredova phytoene desaturase in Synechococcus PCC7942 leading to herbicide resistance against a bleaching herbicide. Plant Physiol. 1994;104:119–125. doi: 10.1104/pp.104.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhao J, Mühlenhoff U, Bryant D, Golbeck JH. PsaE is required for in vivo cyclic electron flow around photosystem I in the cyanobacterium Synechococcus sp. PCC 7002. Plant Physiol. 1993;103:171–180. doi: 10.1104/pp.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]