Abstract
Background
Several studies have demonstrated that reduced lung function is a significant risk factor for lung cancer and increased surgical risk in patients with operable stages of lung cancer. The aim of the study was to perform pulmonary function tests and investigate which is a favorable respiratory function test for overall survival between lung cancer stages.
Methods
Lung function tests were performed to lung cancer patients with non-small cell lung cancer of stage I, II, III and IV (241 patients in total). They had the last follow-up consecutively between December 2006 and July 2008. The staging was decided according to the sixth edition of TNM classification of NSCLC. The Forced Expiratory Volume in 1sec (FEV1), Forced Vital Capacity (FVC) and Carbon Monoxide Diffusing Capacity (DLCO) were measured according to American Thoracic Society/European Respiratory Society guidelines. The 6 Minute Walking Test (6MWT) was measured according to the American Thoracic Society.
Results
There was a significant association of the DLCO upon diagnosis and overall survival for stage II (P<0.007) and IV (P<0.003). Furthermore, there was a significant association between 6MWT and overall survival for stage III (P<0.001) and stage IV (P<0.010).
Conclusions
The significance for each lung function test is different among the stages of NSCLC. DLCO and 6MWT upon admission are the most valuable prognostic factors for overall survival of NSCLC.
KEY WORDS : FEV1, FVC, DLCO, 6MWT, NSCLC
Introduction
Lung cancer is the leading cause of cancer mortality worldwide (1). Approximately, 80% to 85% of lung cancer cases are non-small cell lung cancer (NSCLC). Practically, early diagnosis of lung cancer has not been possible, and unfortunately, the majority of patients with lung cancer belong to advanced stages at the time of diagnosis. The relationship between smoking, lung cancer, and airflow obstruction is well recognized (2). Several studies have suggested that airway obstruction based on forced expiratory volume in 1 second (FEV1) reduction, increases lung cancer risk (3). In a large prospective cohort of heavy smokers, it was demonstrated that even a relatively small reduction in FEV1% (predicted) is a significant predictor of increased lung cancer risk (4). Surgical resection remains the treatment of choice for early stage NSCLC, offering the best prospect of long-term survival (5). Nevertheless, many patients have coexisted chronic airflow limitation and/or diffusion impairment, which is associated with an increased risk during surgery (6,7). The American College of Chest Physicians and the European Respiratory Society/European Society of Thoracic Surgeons have recommended measuring lung function and exercise capacity. They provided evidence of cut-off values beyond which the risk of complications is regarded as high, and summarize these recommendations in algorithms. Such algorithms are easy to put into practice and are widely used. However, operability does not rely exclusively on functional data, and there is usually no real threshold beyond which the risk of complications changes radically. Consequently, the British Thoracic Society has recently elaborated an original algorithm based on a tripartite assessment (8-10). There are four lung function tests (LFTs) used in every tertiary center to evaluate such patients. Spirometry and 6 Minute Walking Test (6MWT) is widely available, well standardized and economical. Among the multiple parameters measured, Forced Expiratory Volume in 1 sec (FEV1) and Forced Vital Capacity (FVC) have been included in all the published functional algorithms. Carbon Monoxide Diffusing Capacity (DLCO) evaluates the alveolar-capillary integrity and reflects the surface area and pulmonary capillary blood volume available for gas exchange. A recent study demonstrated that the preoperative DLCO value predicted mortality from non-lung cancer-related causes and in a multivariate analysis only DLCO and not FEV1 was prognostic (11). Split-function study for calculation of predicted postoperative (PPO) is used to evaluate the residual pulmonary function after surgery, through the calculation of PPOFEV1 and PPODLCO, is widely recommended in patients with altered lung function (8-10). Exercise tests are also incorporated in LFTs and are divided in: (I) Formal cardiopulmonary exercise test. The most used and best validated exercise parameter is oxygen consumption (VO2max). In the literature, VO2max% appears to be a strong predictor of post-operative complications, as well as a good predictor of long-term post-operative exercise capacity (12). (II) Low-technology exercise tests. (i) 6 Minute Walking Test (6MWT) which is the most used low-technology test, but the distance walked does not correlate with the VO2max in all (especially if fit) patients. As a result, the 6MWT is not recommended to select patients for lung cancer resection (8-10); (ii) Shuttle Test. In contrast, there is a strong correlation between the distance walked during a shuttle test and VO2max. As a result, the ERS/BTS recommend performing Cardiopulmonary Exercise Testing (CPET) in patients walking <400 m and the BTS considers >400 m as proper function (9,10); (iii) Stair Climbing Test. The stair climbing has also been used as a screening test. The use of stair climbing can be limited by the difficulty in standardizing this test according to the characteristics of the stairs and ceilings. In contrast to patients with advanced NSCLC, factors affecting the decision making are the extent of disease, weight loss and performance status, as these are the significant predictive indicators of median patient survival time after undergoing systemic chemotherapy (13). Patients without substantial systemic manifestations of illness, chemotherapy is known to improve median survival time, when compared with best supportive care alone (14). Good performance status, female sex, age ≤70 years, and cisplatin-based chemotherapy have been known to be predictive of favorable survival rates overall (15). Several third-generation agents are available for treatment of NSCLC, including docetaxel, paclitaxel, gemcitabine, and vinorelbine, and the combination of one of these agents with a platinum compound has been considered the standard treatment option for advanced NSCLC (16-19). In the present study an association of the four basic pulmonary function tests is being presented in correlation with multiple factors of NSCLC patients. Pulmonary function tests are easy to perform by any medical specialty, and provide important findings to the clinician.
Patients and methods
Patients
In total 241 patients diagnosed with primary NSCLC were enrolled from two pulmonary departments which treat lung cancer patients. The patients were divided into four stages according to the TNM staging 6th edition (20). All epidemiological (age, body mass index, obesity, gender and smoking history) and disease characteristics (histology type, performance status and systemic adverse effects due to treatment) of the patients were recorded based on their stage and are presented in Table 1. Data was collected from December 2006 until June 2008. The study was approved by the Investigational Review Boards (IRB) of each hospital and Ethics Committees. Patients enrolled had their respiratory capacity evaluated within 3 months intervals with FEV1, FVC, DLCO and 6MWT, until 3 measurements were completed. Most of the patients were smokers or stopped smoking upon diagnosis of lung cancer, but 21 patients were non-smokers.
Table 1. Patients epidemiological characteristics.
| Stage I [39/241] | Stage II [57/241] | Stage III [83/241] | Stage IV [62/241] | ||
|---|---|---|---|---|---|
| Age (median) | 69.54 | 64.44 | 60.42 | 59.24 | |
| Male/Female | 34/5 | 52/5 | 77/6 | 53/9 | |
| BMI* (median) | 28.05 | 26.92 | 27.35 | 27.20 | |
| Obesity | 20 | 24 | 44 | 30 | |
| PY (median) | 60.53 | 59.74 | 68.61 | 71.44 | |
| Non-smokers (N) | 1 | - | 8 | 12 | |
| Histology | 11 adeno | 18 adeno | 43 adeno | 35 adeno | |
| 28 squamus | 39 squamus | 40 squamus | 27 squamus | ||
| Radiotherapy to the thorax | 15 | 7 | |||
| Bone metastasis | 17 | ||||
| Adrenal gland | 11 | ||||
| Contra lateral | 14 | ||||
| Malignant pleural effusion | 9 | ||||
| More than 1 site | 11 | ||||
| Lobectomy | 30 | 28 | |||
| Bilobectomy | 8 | 22 | |||
| Pneumonectomy | 1 | 7 | |||
*BMI, Body Mass Index.
Methods
Patients were staged with computed tomography (CT) scan of the brain, thorax, abdomen, pelvis and bone scan. Positron emission tomography was used as an additional diagnostic work up for staging, according to the treating physician. The Spirometry test and DLCO were performed according to the American Thoracic Society and European Respiratory Society (ATS/ERS) guidelines (21). The 6MWT was performed following ATS guidelines (22). Patients had their tests every three months when available or with a maximum of 10 days deviation (Figure 1). Regarding the chemotherapy regimens stage II that required adjuvant chemotherapy a doublet containing platinum analog was used (23). In stage III and IV a doublet of platinum analog and docetaxel was used (16). Regarding the 6 MWT patients were instructed to walk at their own pace. Dyspnea was measured using the Borg scale; in addition, oxygen saturation and pulse rate was measured upon initiation and termination of the test. Instructions were also given to the patients to slow down and stop upon adverse effects due to their disease. Obesity was indicated when the Body Mass Index (BMI) was ≥30 kg/m2.
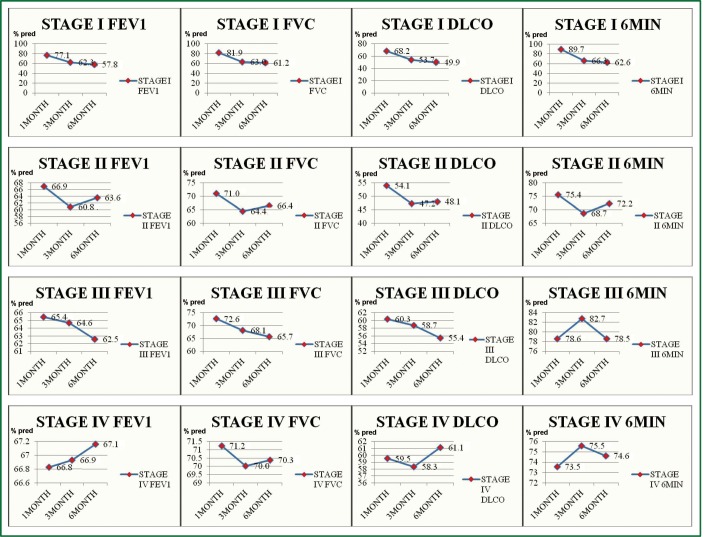
Figure 1.
Forced Exiratory Volume in 1sec (FEV1), Forced Vital Capacity (FVC), Carbon Monoxide Diffusing Capacity (DLCO) and 6 Minute Walking Test (6MWT) measurements for all stages. (In the Vertical Axis the measurement values are presented and in the horizontal axis the time of the measurement) (y axis. % pred; x axis. time of evaluation).
Statistical analysis
Data was initially collected and analyzed in an Excel Microsoft 2007 program. Anthropometric data were expressed as means ±1 SD. Survival data were expressed as median ± standard error of the mean (SEM) and 95% confidence interval (CI). Biostatistical analysis were carried out using the Predictive software analysis program (PASW, IBM, Release 18.0.0 July 30 2009).
All parameters were normally distributed (Kolmogorov-Smirnov test, P>0.05). Overall survival was defined as the interval between the date of pathologic diagnosis and death or last follow-up. Survival function was estimated with Kaplan Mayer survival test and differences among groups of patients with log rank test. Cox regression analysis examined the effect of each variable in respiratory function (FEV1, FVC, DLCO and 6MWT). Null hypothesis was rejected when probability was >5% (Figure 2). A multivariate analysis was also performed to evaluate factors such as age, stage of the disease, smoking status, COPD, obesity etc and to establish the independent prognostic significance of lung function tests.
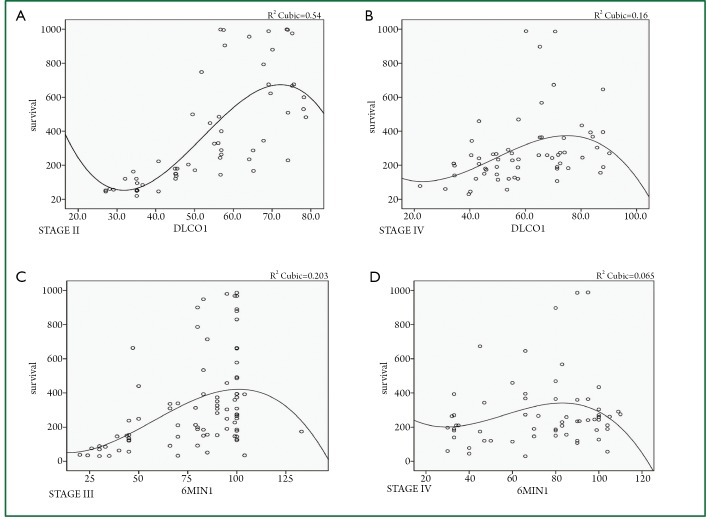
Figure 2.
A. DLCO1 upon diagnosis and overall survival for stage II (P<0.007); B. DLCO1 upon diagnosis and overall survival for IV (P<0.003); C.6MWT1 upon diagnosis and overall survival for stage III (P<0.000); D. 6MWT1 upon diagnosis and overall survival stage IV (P<0.010). (y axis. Days of survival; x axis. pulmonary function test).
Results
All the patients’ epidemiological characteristics are seen in Table 1. In stage I group, the main histology type was squamous (28/39 patients). The mean value upon diagnosis for FEV1, FVC, DLCO and 6MWT was 77.1%, 81.9%, 68.2% and 89.7% (of the predicted), respectively. In stage II group, the main histology type was squamous (39/57 patients). The mean value upon diagnosis for FEV1, FVC, DLCO and 6MWT was 66.9%, 71.0%, 54.1% and 75.4%, respectively. In stage III group, 10 patients were stage IIIA, but due to severe COPD they were not candidates for surgery treatment. The main histology types diagnosed were 43 adenocarcinomas and 40 squamous. Eighteen patients had radiotherapy of the primary site. The mean value upon diagnosis for FEV1, FVC, DLCO and 6MWT was 65.4%, 72.6%, 60.3% and 78.6%, respectively. In stage IV group, the main histology types diagnosed were 35 adenocarcinomas and 27 squamous cell carcinomas. The mean value upon diagnosis for FEV1, FVC, DLCO and 6MWT was 66.8%, 71.2%, 59.5 and 73.5%, respectively. Seven patients of this group had radiotherapy (palliative) to the thorax. In addition, 17 had bone metastasis upon diagnosis. The longest follow up period of patients that underwent radiotherapy of the primary site to the thorax was 9 months and there was no radiotherapy pneumonitis observed.
A significant association of the DLCO upon diagnosis was found with the overall survival for stage II (P<0.007) and IV (P<0.003). Furthermore, there was a significant association between 6MWT and overall survival for stage III (P<0.001) and stage IV (P<0.010). These two observations were made with cox-regression analysis (Table 2, Figures 1,2).
Table 2. Freedman test cox regression.
| FEV1† | FVC╪ | DLCO* | 6MWT§ | Overall survival | |||
|---|---|---|---|---|---|---|---|
| Stage I | <0.001 | <0.001 | <0.001 | <0.001 | Dlco first measurement | NS | |
| 6MWT first measurement | NS | ||||||
| Stage II | <0.001 | <0.001 | <0.001 | <0.001 | Dlco first measurement | <0.007 | |
| 6MWT first measurement | NS | ||||||
| Stage III | <0.002 | <0.001 | <0.001 | <0.001 | Dlco first measurement | NS | |
| 6MWT first measurement | <0.001 | ||||||
| Stage IV | NS | <0.041 | <0.014 | <0.005 | Dlco first measurement | <0.003 | |
| 6MWT first measurement | <0.001 | ||||||
†FEV1, Forced Expiratory Volume in 1sec; ╪FVC, Forced Vital Capacity; *DLCO, Carbon Monoxide Diffusing Capacity; §6MWT, 6 Minute Walking Test.
The multivariate analysis using a general linear model showed that the lung function of the non-small cell lung cancer patients was significantly influenced by the gender (P<0.004), the stage of cancer (P<0.01), the smoking habit (P<0.001), the COPD (P<0.001), the surgery (P<0.033) and the radiotherapy (P<0.01). Factors that were not significant were the adjuvant therapy (P>0.940), the neoadjuvant, therapy (P>0.998), the chemotherapy (P>0.193) and the BMI (P>0.846). The survival for each stage was as follows (CI 95% lower bound/upperbound): (I) Stage I 324/625, (II) Stage II 153/372, (III) Stage III 185/312, (IV) Stage IV 197/272.
Discussion
Respiratory capability has shown to be a significant predictive factor for all early and advance stage lung cancers (6,7,13,24-27). In the general population, reduced respiratory capability has been associated as a predictor of mortality (28). A specific relationship between pulmonary function tests/exercise testing and overall survival has been established either as a preoperative or postoperative risk factor in early NSCLC stages (6,7,13,24). Another factor associated with a positive outcome in surgically resected patients is squamous histology versus non squamous (7). In our study, a positive correlation between histology, pulmonary function tests and overall survival was not observed. In advanced NSCLC, additional predictive factors are: (I) the performance status, (II) the extent of the disease, (III) the presence of malignant pleural effusion, (IV) smoking, (V) low BMI, (VI) weight loss, (VII) hemoglobin level >11 g/dL and (VIII) lactate dehydrogenase levels (13,15,29).
Specifically, smoking history and COPD are well established to cause significant reduction in lung function and were determined as predictive factors of an increased risk of lung cancer. The stage of cancer and whether the patient had undergone surgery also affected lung function and exercise capacity in several studies (4,7,30,31). Additionally, pulmonary function was observed to change after radiotherapy in NSCLC patients with a significant deterioration at 3 months after radiotherapy and no pulmonary recovery at 18 and 36 months (32). In our study the percentage of patients that had radiotherapy was very small for such observation to be made. Other aspects investigated in correlation with lung function tests were quality of life and the efficacy of exercise training in NSCLC patients (33,34). LFTs have been also associated with overall survival in other types of cancer (35). In our study, we presented data where the lung function of the non-small cell lung cancer patients was significantly influenced by the gender, the stage of cancer, the smoking habit, the COPD, surgery and the radiotherapy. In contrast, the BMI and chemotherapy regimen selection did not affect the survival, as in previous studies. However, this was not the endpoint of the study.
Limitations of the study were the lack of molecular testing and genetic testing, and correlation with the multiple variables recorded. However, molecular biomarkers are not always available in every pulmonary center, and are always very expensive. In addition, to date there are no definite data presenting any specific biomarker to be associated with prognosis. Epidermal growth factor (EGFR) mutation is a predictive marker, however these data were not included in the result section and a multivariate analysis was not performed as the number of patients with EGFR mutation was small. Moreover, the study did not include different therapeutic regimes, in order to present data whether any differences exist between platinum doublets with molecular targeting drugs. Finally, the small number of patients included in each stage and the lack of comparison to other populations with underlying pulmonary disease, but without lung cancer.
Spirometry has the advantage of being an objective quantitative measure (21). In surgical candidates, spirometry holds the key of evaluation of whether a patient will undergo surgery, but also reflects a favorable outcome regarding overall survival (7). A correlation of FEV1 of <50% predicted has been associated with increased mortality (27). The 6MWT is also a commonly used objective method, easily repeatable, inexpensive and understandable by patients (7). It is used to assess the functional status of patients with lung disease whether in previous studies assessed patients with lung cancer (36). Until recently, it was used to measure the response to medical intervention in patients with moderate and severe COPD, but also in patients with congestive heart failure and pulmonary hypertension. It has been presented in previous studies that a value of ≥400 m in the 6 MWT at the diagnosis of NSCLC is cut off point for favorable overall survival outcome (36). In our study, such a cut off point was not observed.
Moreover, another factor affecting the prognosis of NSCLC is the tumor size which has been observed in a large study to be positively associated with poor survival (25). It has been observed that in COPD, 6MWT is a better predictor factor for mortality than FEV1 and the reduction is independent of any change in FEV1. The same principle is applicable to lung cancer patients, as the majorities are smokers (2). The spirometry and DLCO measure the respiratory capacity, in contrast to the 6MWT which represents the systemic effects of a disease. An additional explanation to the differentiation of 6MWT is the involvement of cardiopulmonary, nutritional and peripheral muscle status of an individual and in our patient’s cancer burden (37). The walk distance of the 6MWT was found to be shorter in females than in males while increases significantly after rehabilitation (38). An increase of the 6MWT has been also observed after rehabilitation for NSCLC patients (39). Potentially, every observation regarding COPD, can also be observed in lung cancer (2). DLCO is affected by ventilation and perfusion and is associated with gas exchange. It has been identified as an independent prognostic factor for pulmonary complications during surgery and post surgical period (40). In addition, DLCO is positively correlated with cardiac complications. The association of diffusing capacity and cardiac morbidity are not surprising because of the known increase in pulmonary vascular resistance that results from major lung resection (41). The increase of pulmonary vascular resistance and the provoked right heart strain contribute to the relatively high frequency of cardiovascular complications (40). Regarding advance NSCLC, it has been observed that reduced DLCO is also a negative prognostic factor and is associated with poor cardiovascular performance (42). This observation can be explained as advanced stage NSCLC patients are heavy smokers and have a moderate to severe COPD. Patients at these stages usually have poor cardiopulmonary status (28). The 6MWT was found to be strongly associated with stage III and IV due to the additional role as a marker for the clinical status of the patient. Regarding the DLCO correlation with overall survival in stage II, it was attributed to the fact that stage II patients have more extensive surgery and DLCO at this stage represents a predictive factor for mortality. It has been previously presented that DLCO represents gas exchange, which is altered with an extensive surgery in stage II lung cancer. In stage IV, the correlation between DLCO and overall survival attributed to the fact that this group of patients has usually severe COPD and therefore a reduced gas exchange is expected. Nevertheless, VO2max expressed as a percentage of predicted VO2max% which represents the cardiovascular reserve, holds the most significant predictive factor for overall survival (43). It combines pulmonary function, cardiovascular function and clinical status. However, not all the patients can perform cardiopulmonary measurement of VO2max, or this test is not available. In these cases, 6MWT could be a valuable tool for the overall clinical status of a patient. The lung function tests can be used possibly for other cancer types as they are cheap and easily performed (35). Moreover, a correlation should be made first with different doses of chemotherapeutic regimens and targeted therapies. Physicians should always bear in mind that several of these regimens influence the lung parenchyma and might present interstitial fibrotic pattern, therefore present a favorable marker of chemotherapy cytotoxicity (44,45).
In conclusion, although new molecular insights have been added either as prognostic or treatment factors in NSCLC, additional individualization of these patients can be made by using pulmonary function tests. Our study demonstrates that DLCO and 6MWT upon admission are useful prognostic factors for overall survival. Based on our study, the gravity of each lung function test is different among the stages of NSCLC. The 6MWT may be preferred as it is an inexpensive, understandable, easy repeatable test and could be used as an assessment tool of the patient’s clinical status. Additionally, gender, surgery, radiotherapy, smoking habit and underlying respiratory disease (COPD) contribute to complete the profile of NSCLC patient increased mortality risk.
Acknowledgements
PZ, HH and KZ wrote the manuscript. PZ, TK, KP, AK, AR and KZ treated the patients. PZ, KP, and AK performed the pulmonary function tests. TK and OT performed the statistical analysis.CF was the thoracic surgeon that evaluated the patients. AZ assisted in the staging of the patients. NC evaluated the radiologic exams according to RECIST criteria. AR and KZ provided useful insights.
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 2.Petty TL. Are COPD and lung cancer two manifestations of the same disease? Chest 2005;128:1895-7 [DOI] [PubMed] [Google Scholar]
- 3.Nomura A, Stemmermann GN, Chyou PH, et al. Prospective study of pulmonary function and lung cancer. Am Rev Respir Dis 1991;144:307-11 [DOI] [PubMed] [Google Scholar]
- 4.Calabrò E, Randi G, La Vecchia C, et al. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J 2010;35:146-51 [DOI] [PubMed] [Google Scholar]
- 5.Reif MS, Socinski MA, Rivera MP. Evidence-based medicine in the treatment of non-small-cell lung cancer. Clin Chest Med 2000;21:107-20, ix. [DOI] [PubMed] [Google Scholar]
- 6.Win T, Jackson A, Sharples L, et al. Relationship between pulmonary function and lung cancer surgical outcome. Eur Respir J 2005;25:594-9 [DOI] [PubMed] [Google Scholar]
- 7.Birim O, Kappetein AP, van Klaveren RJ, et al. Prognostic factors in non-small cell lung cancer surgery. Eur J Surg Oncol 2006;32:12-23 [DOI] [PubMed] [Google Scholar]
- 8.Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S. [DOI] [PubMed] [Google Scholar]
- 9.Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27 [DOI] [PubMed] [Google Scholar]
- 10.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41 [DOI] [PubMed] [Google Scholar]
- 11.Liptay MJ, Basu S, Hoaglin MC, et al. Diffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancer. J Surg Oncol 2009;100:703-7 [DOI] [PubMed] [Google Scholar]
- 12.Licker M, Schnyder JM, Frey JG, et al. Impact of aerobic exercise capacity and procedure-related factors in lung cancer surgery. Eur Respir J 2011;37:1189-98 [DOI] [PubMed] [Google Scholar]
- 13.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 2002;122:1037-57 [DOI] [PubMed] [Google Scholar]
- 14.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82 [DOI] [PubMed] [Google Scholar]
- 15.Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991;9:1618-26 [DOI] [PubMed] [Google Scholar]
- 16.Zarogoulidis K, Kontakiotis T, Hatziapostolou P, et al. A Phase II study of docetaxel and carboplatin in the treatment of non-small cell lung cancer. Lung Cancer 2001;32:281-7 [DOI] [PubMed] [Google Scholar]
- 17.Gelmon K.The taxoids: paclitaxel and docetaxel. Lancet 1994;344:1267-72 [DOI] [PubMed] [Google Scholar]
- 18.De Petris L, Crinò L, Scagliotti GV, et al. Treatment of advanced non-small cell lung cancer. Ann Oncol 2006;17:ii36-41 [DOI] [PubMed] [Google Scholar]
- 19.Kubota K, Kawahara M, Ogawara M, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol 2008;9:1135-42 [DOI] [PubMed] [Google Scholar]
- 20.Greene FL, Sobin LH. The TNM system: our language for cancer care. J Surg Oncol 2002;80:119-20 [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38 [DOI] [PubMed] [Google Scholar]
- 22.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7 [DOI] [PubMed] [Google Scholar]
- 23.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60 [DOI] [PubMed] [Google Scholar]
- 24.Barnett SA, Rusch VW, Zheng J, et al. Contemporary results of surgical resection of non-small cell lung cancer after induction therapy: a review of 549 consecutive cases. J Thorac Oncol 2011;6:1530-6 [DOI] [PubMed] [Google Scholar]
- 25.Basaki K, Abe Y, Aoki M, et al. Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int J Radiat Oncol Biol Phys 2006;64:449-54 [DOI] [PubMed] [Google Scholar]
- 26.Jeremić B, Miličić B, Milisavljevic S.Clinical prognostic factors in patients with locally advanced (stage III) nonsmall cell lung cancer treated with hyperfractionated radiation therapy with and without concurrent chemotherapy: single-Institution Experience in 600 Patients. Cancer 2011;117:2995-3003 [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Song EM, Sim YS, et al. Forced expiratory volume in one second as a prognostic factor in advanced non-small cell lung cancer. J Thorac Oncol 2011;6:305-9 [DOI] [PubMed] [Google Scholar]
- 28.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 1996;313:711-5; discussion 715-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh WP, Yuan JM, Wang R, et al. Body mass index and smoking-related lung cancer risk in the Singapore Chinese Health Study. Br J Cancer 2010;102:610-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76 [DOI] [PubMed] [Google Scholar]
- 31.Win T, Groves AM, Ritchie AJ, et al. The effect of lung resection on pulmonary function and exercise capacity in lung cancer patients. Respir Care 2007;52:720-6 [PubMed] [Google Scholar]
- 32.Borst GR, De Jaeger K, Belderbos JS, et al. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys 2005;62:639-44 [DOI] [PubMed] [Google Scholar]
- 33.Sarna L, Evangelista L, Tashkin D, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest 2004;125:439-45 [DOI] [PubMed] [Google Scholar]
- 34.Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haugnes HS, Aass N, Fosså SD, et al. Pulmonary function in long-term survivors of testicular cancer. J Clin Oncol 2009;27:2779-86 [DOI] [PubMed] [Google Scholar]
- 36.Kasymjanova G, Correa JA, Kreisman H, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol 2009;4:602-7 [DOI] [PubMed] [Google Scholar]
- 37.Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004;23:28-33 [DOI] [PubMed] [Google Scholar]
- 38.Jay SJ. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 2000;161:1396. [DOI] [PubMed] [Google Scholar]
- 39.Glattki GP, Manika K, Sichletidis L, et al. Pulmonary rehabilitation in non-small cell lung cancer patients after completion of treatment. Am J Clin Oncol 2012;35:120-5 [DOI] [PubMed] [Google Scholar]
- 40.Ferguson MK, Reeder LB, Mick R. Optimizing selection of patients for major lung resection. J Thorac Cardiovasc Surg 1995;109:275-81; discussion 281-3 [DOI] [PubMed] [Google Scholar]
- 41.Nishimura H, Haniuda M, Morimoto M, et al. Cardiopulmonary function after pulmonary lobectomy in patients with lung cancer. Ann Thorac Surg 1993;55:1477-84 [DOI] [PubMed] [Google Scholar]
- 42.Semrau S, Klautke G, Fietkau R.Baseline cardiopulmonary function as an independent prognostic factor for survival of inoperable non-small-cell lung cancer after concurrent chemoradiotherapy: a single-center analysis of 161 cases. Int J Radiat Oncol Biol Phys 2011;79:96-104 [DOI] [PubMed] [Google Scholar]
- 43.Bolliger CT, Perruchoud AP. Functional evaluation of the lung resection candidate. Eur Respir J 1998;11:198-212 [DOI] [PubMed] [Google Scholar]
- 44.Camus P.Interstitial lung disease in patients with non-small-cell lung cancer: causes, mechanisms and management. Br J Cancer 2004;91:S1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camus P, Fanton A, Bonniaud P, et al. Interstitial lung disease induced by drugs and radiation. Respiration 2004;71:301-26 [DOI] [PubMed] [Google Scholar]