Abstract
Purpose
The purpose of this study was to determine the pharmacogenetic effects of complement factor H (CFH) Y402H, LOC387715 and high-temperature requirement factor A1 (HTRA1) genotypes on the treatment of exudative age-related macular degeneration (AMD) by intravitreal bevacizumab injection in a Korean population.
Methods
Seventy-five patients diagnosed with exudative AMD were treated with intravitreal bevacizumab (2.5 mg) monotherapy. All patients received three initial intravitreal bevacizumab injections every four weeks and were then treated "as needed" based on clinical findings, optical coherence tomography and fluorescein angiography during the 12 month follow-up period after the third injection.
Results
The difference in visual acuity improvement among the three genotypes of LOC387715 were statistically significant at six months post-treatment (logarithm of the minimum angle of resolution; TT, 0.346; GT, 0.264; GG, 0.188; p = 0.037). Among the LOC387715 genotypes, the number of additional injections was lower in patients who had the risk T allele (GG, 2.143; GT, 2.000; TT, 1.575; p = 0.064). There was no significant difference between visual acuity and central macular thickness change in the CFH Y402H polymorphism group during the 12 month follow-up period. However, the TC group of CFH Y402H required more additional bevacizumab injections than the TT group (TT, 1.517; TC, 3.363; p = 0.020).
Conclusions
This study demonstrated that different LOC387715/HTRA1 genotypes resulted in different bevacizumab treatment responses on exudative AMD. Patients with the risk allele had an improved treatment response and less need for additional injections. However, patients with the CFH Y402H risk allele needed more additional injections of bevacizumab in order to improve visual acuity. This study illustrates how pharmacogenetic factors may help determine treatment modality and dosing. This could ultimately provide basic data for 'personalized medicine' in AMD.
Keywords: Bevacizumab, Complement factor H Y402H, HTRA1, LOC387715, Macular degeneration
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly population worldwide [1,2]. The Advanced stage AMD is classified into two categories: dry and exudative type. The dry type features a geographic atrophy of the retinal pigment epithelium of the macula and leads to degeneration of photoreceptors. Exudative AMD is typically associated with choroidal neovascularization (CNV) that causes hemorrhage and macular edema, sometimes resulting in sudden profound visual loss [3]. Exudative AMD is now the most common cause of untreatable blindness in the elderly in the Western world, with a prevalence of 11.8% in those greater than 80 years of age [4]. The high prevalence of exudative AMD worldwide has resulted in many studies on AMD risk factors and treatment options in both Caucasian and Asian populations. Aging, smoking, atherosclerosis and obesity are considered environmental risk factors of exudative AMD [5-11]. In addition to environmental risk factors, recent studies have shown that there are genetic risk factors as well [12-17]. Certain single nucleotide polymorphisms (SNP) have been associated with AMD, including the Y402H polymorphism in the complement factor H (CFH) gene [18-25]. Studies have used linkage disequilibrium mapping and case-control associations to determine that chromosome 10q26 has other important susceptible alleles for AMD, such as LOC387715 (rs10490924) and high-temperature requirement factor A1 (HTRA1, rs11200638). These two SNPs are in almost complete linkage disequilibrium and these genetic variations are associated with an increased risk of AMD in Caucasian, Japanese and Chinese populations [26-28]. However, AMD has different disease phenotype and genotype variations in different ethnicities [29] and the relationship between AMD and candidate genes such as CFH, LOC387715/HTRA1 is different among different races. In Asian populations, the association between polymorphism of CFH Y402H and exudative AMD is weaker, while polymorphisms of LOC387715/HTRA1 have greater tendencies to be associated with exudative AMD than in Caucasian populations [30-32]. The present study, which was focused on a Korean population, found that the CFH Y402H polymorphism was only marginally associated with exudative AMD [31] and that the LOC387715 and HTRA1 variants have stronger associations with AMD. These results are consistent with previous reports in Asian populations [32]. In addition to the association between genotypes and disease pathogenesis [33-35], there have been many reports on CFH polymorphism as a predictor of treatment response in Caucasian populations, such as the response to zinc supplements [36], photodynamic therapy (PDT) [37-39], and intravitreal antivascular endothelial growth factor (VEGF) agents such as intravitreal bevacizumab [40] and ranibizumab treatment [41]. Anti-VEGF, which is now a standard treatment for exudative AMD, causes regression of neovascularization and has an anti-inflammatory effect. Unlike the association between CFH Y402H and AMD treatment response, there have been no reports, to the best of our knowledge, about the pharmacogenetic effects of LOC387715/HTRA1 polymorphism on treatment response to bevacizumab in a Caucasian population. There have been two reports on the correlation between PDT and various genetic markers including CFH/HTRA1 [42] and LOC387715 A69S [43] in an Asian population.
The purpose of this study is to determine the pharmacogenetic effect of the CFH Y402H, LOC387715 and HTRA1 genotypes on the treatment of exudative AMD with intravitreal bevacizumab injections in a Korean population.
Materials and Methods
Patient and inclusion criteria
The authors retrospectively reviewed the medical records of 75 eyes from 75 consecutive patients with exudative AMD who were treated only with intravitreal injections of 2.5 mg of bevacizumab between 1 January, 2006, and 31 June, 2008, at the Vitreoretinal Clinic of the Inha University Hospital, Incheon, Korea. The study was performed in accordance with the ethical standards of the Declaration of Helsinki, and data were gathered after obtaining written informed consent. This includes consent for the off-label use of bevacizumab as treatment for exudative AMD. This retrospective study was approved by the institutional review board of Inha University Hospital. Approval for this treatment was also obtained from the National Ministry of Health in South Korea. All patients received follow-up for six months or more, and 54 patients received follow-up for more than 12 months. The potential risks and benefits were discussed with all patients before they received injections, and all patients read and signed informed consent forms. Inclusion criteria were as follows: 1) patients with exudative AMD, defined as the presence of active fluorescein leakage from the lesion as seen on fluorescein angiography, who received anti-VEGF treatment in at least three initial injections every four weeks; and 2) patients who were followed up for a minimum of six months. The exclusion criteria included: 1) patients diagnosed with polypoidal choroidal vasculopathy (PCV) by indocyanine green angiography and treated primarily with PDT; 2) prior macular photocoagulation; 3) a history of subtenon injection of triamcinolone acetonide, or PDT, or anti-VEGF within six months before the intravitreal bevacizumab injection; 4) other intraocular surgery or management during follow-up; 5) the presence of coexisting ocular disease causing macular edema (i.e., diabetic macular edema, retinal vein occlusion, pseudophakic cystoid macular edema, or uveitis); and 6) the presence of comorbid ocular conditions that might affect visual acuity. Thirty-nine patients who underwent PDT more than six months before injection were included in the study. Treatment response was evaluated by comparing the visual acuity (VA) and central macular thickness (CMT) measured by optical coherence tomography (OCT) at baseline with those same variables in the three follow-up periods: immediately after treatment, six months after treatment, and 12 months after treatment.
Clinical examination
The study measured change in VA, change in CMT measured by OCT, and the number of additional intravitreal bevacizumab injections. VA change was evaluated with consistent methods using the Snellen eye examination chart and then converted into a logarithm of the minimal angle of resolution (logMAR) value. AMD characteristics were evaluated in all patients by clinical examination including indirect dilated fundus examination, color fundus photography, OCT, fluorescein angiography, and indocyanine green test, if needed. Greatest linear dimension through fluorescein angiography was calculated by two retinal specialists masked to each genotype.
DNA preparation and genotyping
In previous studies [31,32] the authors collected peripheral venous blood from all patients for extraction of genomic DNA and sequencing of single nucleotide polymorphisms. Genotyping of the SNPs in the CFH Y402H (rs1061170), LOC387715 (rs10490924) and HTRA1 (rs11200638) genes was carried out using Big Dye Terminator cycle sequencing (Applied Biosystems Inc., Foster City, CA, USA) on an automated sequencer (model 3730, Applied Biosystems Inc.). The details of the DNA preparation and genotyping were published previously [31,32].
Intravitreal bevacizumab injection
Intravitreal bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) injections of 2.5 mg/0.1 mL were performed after topical anesthesia with 0.5% proparacaine drops (Alcaine; Alcon Laboratories, Fort Worth, TX, USA) under sterile conditions. All patients received three initial injections every four weeks and were then treated "as needed" based on if there was sign of recurrence. In this study, the sign of recurrence was a significant increase (more than 20%) of central retinal thickness at foveola due to new or residual subretinal fluid in OCT or dye leaks found in fluorescein angiography.
Data analysis
Treatment response was evaluated by comparing VA and CMT at baseline and after three initial injections during the three follow-up periods (immediate, six months, and 12 months after treatment). Baseline demographic and clinical parameters were compared using the Student's t-test and ANOVA test for continuous variables and chi-square tests for categorical variables. VA improvement and CMT change between baseline and follow-up were evaluated among the different genotypes for each gene at each follow-up period by adjusting for the variables (age, hypertension and prior PDT) and using repeated measurement analysis of variance. The study also used paired t-tests and repeated measurement analysis to evaluate whether undergoing PDT more than six months before the start of study affected VA and CMT values at baseline and in each follow-up period. Statistical analyses were performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at p < 0.05.
Results
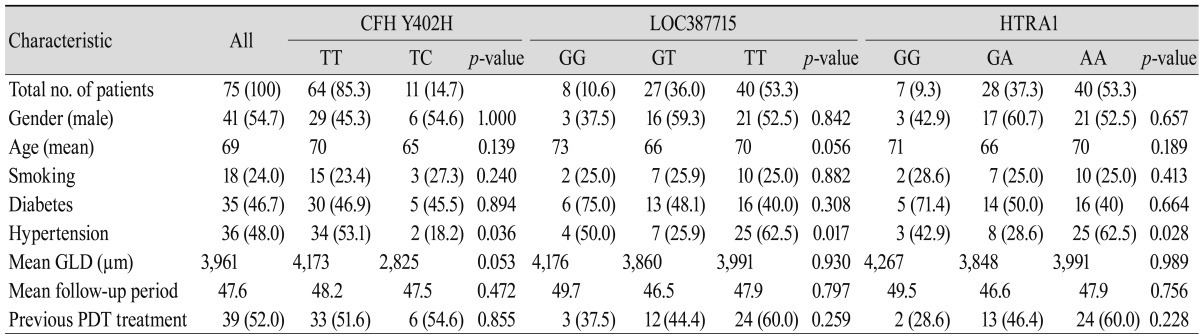
Seventy-five patients who were diagnosed with exudative AMD were enrolled in the study, and all patients were successfully genotyped using the peripheral blood sample. Table 1 shows the demographic and clinical features of exudative AMD in the study population. Patient distribution and baseline evaluation, including prior PDT, were described according to each genotype of the CFH Y402H, LOC387715/HTRA1 genes.
Table 1.
Baseline evaluation and characteristics of age-related macular degeneration for CFH Y402H, LOC387715 and HTRA1 genotypes
Values are presented as number or number (%).
CFH = complement factor H; HTRA1 = high-temperature requirement factor A1; GLD = greatest linear dimension; PDT = photodynamic therapy.
For LOC387715 (rs10490924), eight patients (10.7%) were GG genotype, 27 patients (36.0%) were GT genotype, and 40 patients (53.3%) were TT genotype. The overall frequency of the high risk "T" allele was 71.3%. The LOC387715 GG genotype had the oldest mean age among the three genotypes (p = 0.056). Hypertension was also prevalent, with the highest prevalence in the TT genotypes, followed by the GG, and GT genotypes (p = 0.017). For the HTRA1 (rs11200638) polymorphism, similar patient distributions of LOC3887715 are seen due to its high linkage disequilibrium with LOC387715. Only one patient had different genotypes in LOC387715 (GG) and HTRA1 (GA).
For CFH Y402H (rs1061170), 64 patients (85.3%) were TT genotype, 11 patients (14.7%) were TC genotype, and no patients had the high risk CC genotype. The overall frequency of the high risk "C" allele was 7.3%. The CFH Y402H TT genotype group had an older mean age than the TC group (p = 0.139). The prevalence of hypertension was 53.1% and 18.2% in the TT and TC genotype groups, respectively (p = 0.036).
In both CFH Y402H and LOC387715, the group with the non-risk homozygous allele had a tendency for higher greatest linear dimension, although it was not statistically significant. Results for patients who had PDT more than six months before bevacizumab treatment did not differ significantly from those who had no history of PDT. However, the data showed that the high risk group of LOC387715/HTRA1 included more previous PDT patients compared with other groups.
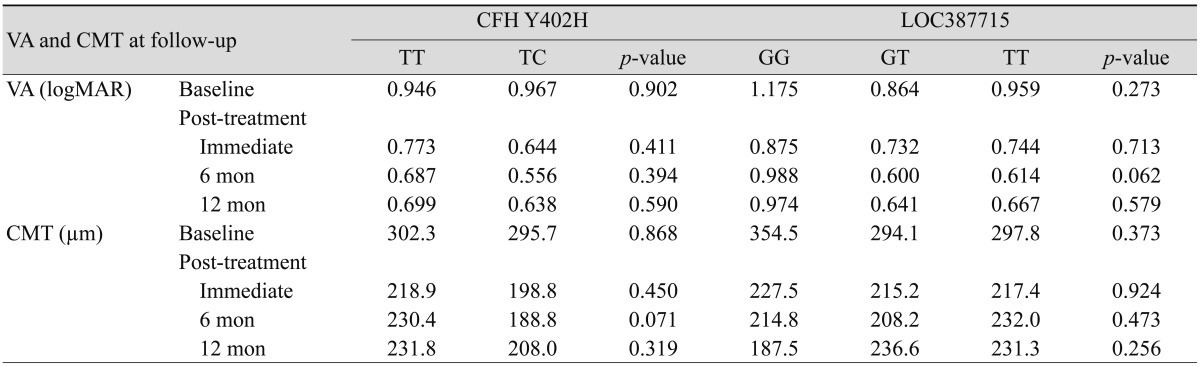
In order to compare the bevacizumab treatment response according to genotype in each candidate gene, baseline VA and CMT were measured and compared with those from the three follow-up periods. Table 2 displays mean VA and CMT of patients at baseline and in the three follow-up periods after the initial three injection treatments for each genotype of candidate genes. Mean pretreatment VA was 1.175 (logMAR) and mean pretreatment CMT was 354.5 µm for the LOC387715 GG genotype (n = 8). In the LOC387715 GT (n = 27) and TT (n = 40) genotype groups, mean pretreatment VA (p = 0.273) and CMT (p = 0.373) were improved when compared to the LOC387715 GG genotype. Mean pretreatment VA was 0.946 (logMAR) and mean pretreatment CMT was 302.3 µm for the Y402H TT genotype (n = 64). For the Y402H TC genotype (n = 11), mean pretreatment VA (p = 0.902) was worse but mean pretreatment CMT was improved than in the Y402H TT group (p = 0.868).
Table 2.
Mean VA and CMT at baseline, immediately post-treatment, and at 6 months and 12 months follow-up
VA = visual acuity; CMT = central macular thickness; CFH = complement factor H; logMAR = logarithm of the minimal angle of resolution.
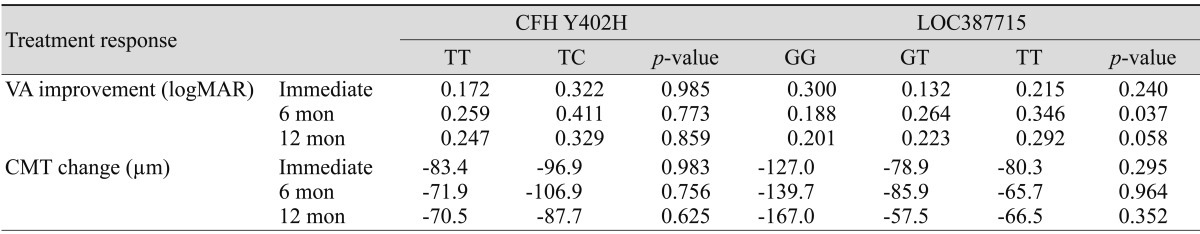
Table 3 shows VA and CMT difference according to genotype. This study examined how the variables affect treatment response in the three follow-up periods, by adjusting the variables and using repeated measured analysis for the three follow-up periods compared with the baseline data (Table 3). The treatment response to intravitreal bevacizumab at six months post-treatment improved as the high risk "T" allele increased; this effect was statistically significant (logMAR; TT, 0.346; GT, 0.264; GG, 0.188; p = 0.037). However, there was no statistically significant change in VA at 12 months after treatment (p = 0.058) among LOC3887715 subgroups (Fig. 1). Changes in CMT during the follow-up period were not significantly different. Both TT and TC genotype groups in CFH Y402H showed the greatest improvement in VA after six months, and the TC group had a more favorable response to treatment although it was not statistically significant (logMAR; TT, 0.259; TC, 0.411; p = 0.773). At 12 months after treatment, both the TT and TC genotype groups had decreased VA compared to VA at six months, but again, this was not statistically significant (p = 0.859). Bonferroni multiple comparison correction showed that there was no significant relationship between CFH Y402H and LOC387715 treatment response.
Table 3.
Change in VA and CMT compared to baseline immediately post-treatment, and at 6 months and 12 months follow-up
CFH = complement factor H; VA = visual acuity; logMAR: logarithm of the minimal angle of resolution; CMT: central macular thickness.
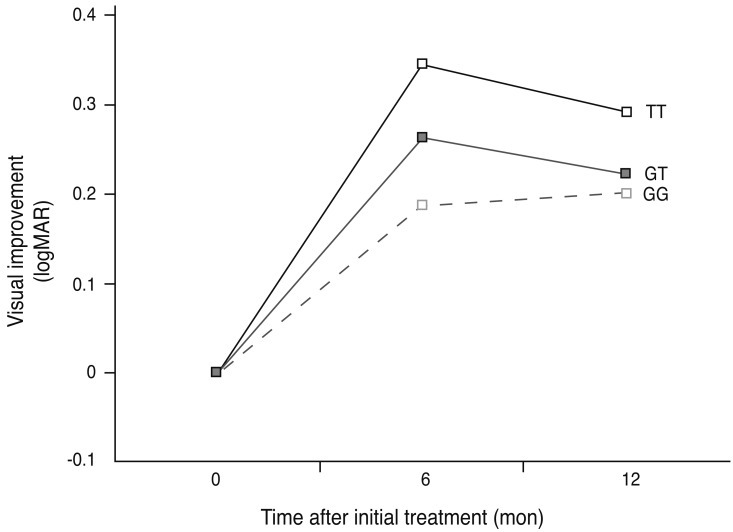
Fig. 1.
Mean logarithm of minimum angular resolution (logMAR) visual improvement in patients with the LOC387715 genotype treated with bevacizumab (black line, TT genotype; grey line, GT genotype; broken line, GG genotype) over a 12 month follow-up period. Visual improvement was greatest in the LOC387715 TT genotype group compared to other groups, at both six months (p = 0.037) and 12 months (p = 0.058) post-treatment.
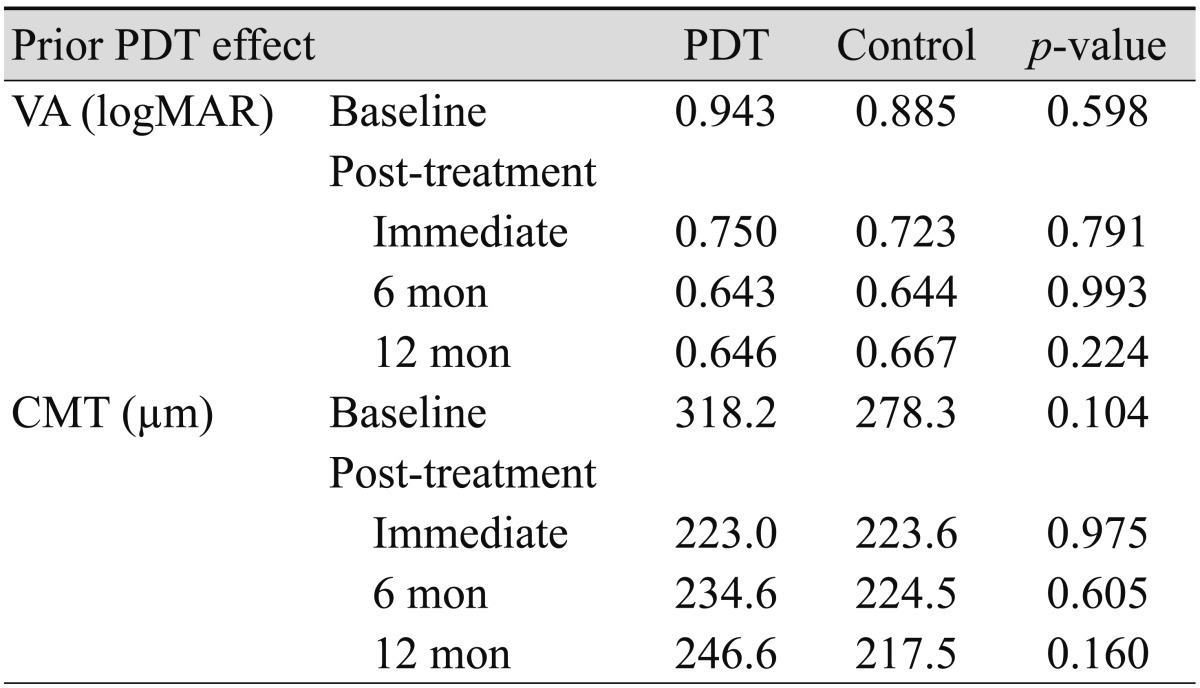
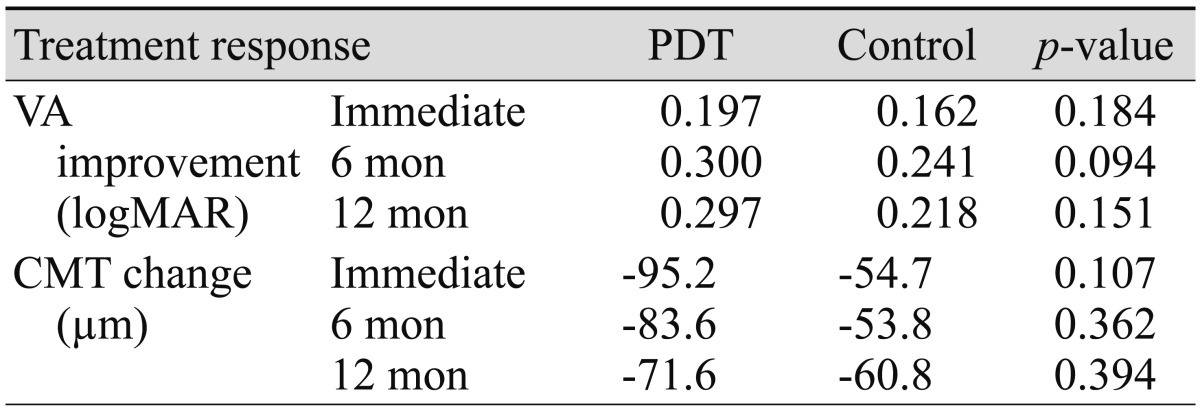
Table 4 shows the comparison study of the effect of prior PDT on mean changes in VA and CMT, which revealed that prior PDT had no statistically significant effect after bevacizumab injection during the total follow-up period. However, when compared to the control, VA in the PDT group tended to show greater improvements in response to treatment during the follow-up period (Table 5).
Table 4.
Comparison of mean VA and CMT at follow-up between patients who underwent previous PDT and the control group
PDT = photodynamic therapy; VA = visual acuity; logMAR = logarithm of the minimal angle of resolution; CMT = central macular thickness.
Table 5.
Change in VA and CMT at follow-up between patients who underwent previous PDT and the control group
PDT = photodynamic therapy; VA = visual acuity; logMAR = logarithm of the minimal angle of resolution; CMT = central macular thickness.
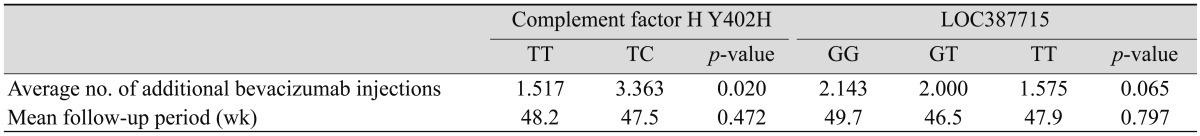
This study analyzed the number of additional intravitreal injections of bevacizumab needed to maintain target VA or disease stability within subgroups of the CFH Y402H and LOC387715 genes (Table 6). The TC genotype group of CFH Y402H required additional bevacizumab injections compared to the TT genotype group (TT, 1.517; TC, 3.363; p = 0.020). In the subgroup of the LOC387715 gene, the number of additional injections tended to be higher in the GG genotype group than in other groups (GG, 2.143; GT, 2.000; TT, 1.575; p = 0.065).
Table 6.
Average number of additional bevacizumab injections and mean follow-up period after initial three intravitreal injections, according to genotype
Discussion
In this retrospective study examining the effects of bevacizumab on exudative age-related macular degeneration in the Korean population, patients with the risk T allele of LOC387715 had better visual outcome and generally required a lower number of additional injections after the initial three injections. Although the function of LOC387715 is unknown [44], the gene is in nearly complete linkage disequilibrium with the HTRA1 gene. The HTRA1 gene is part of the family of heat shock proteins (HSP) [45-47].
Patients with the risk allele of LOC387715/HTRA1 might have a better treatment response because the HTRA1 polymorphism could cause higher sensitivity to oxidative damage. The function of HSP is to protect tissues from various types of damage, especially oxidative stress [48]. The role of HSP is particularly important in the retina, which consumes more oxygen than any other organ in the human body. HSP protects the retinal pigmented epithelium, the photoreceptor, and even the vascular endothelium. However, the HTRA1 polymorphism might cause changes in the function of HSP [47]. Angiogenesis, which is the main pathogenic factor for exudative age-related macular degeneration, is known to be related to oxidative stress [48]. Thus, if there is an alteration in the protective effect of HSP in the high risk allele group, patients might have sensitivity to oxidative damage and the oxidative stress associated with angiogenesis would then cause much more damage to the retina. Accordingly, an anti-VEGF agent would be able to inhibit angiogenesis related to the oxidative stress, which might reduce the activity of exudative AMD in the retina. This study hypothesizes that patients with the risk allele in LOC387715/HTRA1 have a better treatment response due to decreased sensitivity to oxidative damage by anti-VEGF, which reduces the photoreceptor's functional loss and increases the chance of recovery against functional alteration of HSP. Therefore, these patients might have better visual acuity and less need for additional injections for disease stability.
Treatment response based on genotype was variable during different time periods in this study. Visual acuity improved significantly in the risk T allele group during the six-month follow-up period. Similarly, the 12-month results showed a correlation between the T allele and better treatment response, but this correlation was not statistically significant (p = 0.058).
At the end of the entire 12-month follow-up period, 54 of the original 75 patients remained in the study and 21 patients were lost during follow-up. Patients lost during follow-up after six months were those with TT (n = 11) and GT (n = 8) genotypes that had good treatment response. This likely contributed to the less distinguishable differences in treatment response at the end of the study period. It is still evident that patients with the risk T allele in LOC387715 have better visual acuity after the initial treatment with bevacizumab and less need for additional injections. The anti-angiogenic effect of the treatment may eventually reduce stress, which will have a profound impact on patients with the risk T allele.
Patients with the risk C allele in CFH Y402H genotype had visual improvement at the final 12-month follow-up. After the initial three injections of bevacizumab, patients with the risk C allele had better visual acuity. However, during the monthly follow-up after initial treatment, these patients underwent more additional injections "as needed" based on patient's symptoms and clinical findings (TT, 1.517; TC, 3.363 injections; p = 0.020). Finally, patients with the risk C allele had better visual improvement than those with the TT genotype at the 6-month follow-up and at the end of the study period. However, this result might be due to the additional maintenance injections.
Interestingly, in the LOC387715/HTRA1 group, patients with a better response received less additional injections in contrast to patients in the CFH group who received more additional injections and also had a better outcome. In order to explain this, one must consider the pathogenesis of exudative age-related macular degeneration: neovascularization and inflammation. These two factors are also related to the mechanism underlying the treatment response between different time periods. In the immediate post-treatment phase, intravitreal bevacizumab reduces AMD activity, such as angiogenesis, and reduces the macular edema. The immediate post-treatment CMT was reduced to less than 250 µm in all groups. This immediate reduction of macular edema was responsible for improved visual acuity. Thus, bevacizumab seems to effectively increase visual acuity regardless of genotype. However, in the later phase of treatment (six months post-treatment) macular thickness does not seem to be responsible for the change in visual acuity of patients. All patients had CMT within the normal range at six and 12 months post-treatment, but visual acuity varied between different genotype groups. Thus, it appears that the functional status of the photoreceptor is affected by which pathway (inflammatory or metabolic) is responsible for the later phase of the treatment response. With this in mind, CFH inhibits the alternative pathway, which may cause dysregulation of the common pathway in risk allele group and ultimately may cause continued inflammation. Thus, additional bevacizumab, an anti-VEGF agent with anti-inflammatory effects [49,50], is needed to reduce the inflammation. Accordingly, appropriate maintenance therapy of additional bevacizumab results in visual improvement. This may also be applicable to the LOC387715/HTRA1 group, in that immediate post-treatment response did not differ between the various LOC387715/HTRA1 genotypes due to bevacizumab's initial action in reducing macular edema, regardless of genotype. But on follow-up at six months, the risk allele group had improved treatment response.
On the other hand, in studies of the genetic effect of CFH Y402H on Caucasian population, patients with the C allele required additional injections, but treatment still resulted in worse visual acuity [40]. This supports the current study's hypothesis that the altered inflammatory pathway in the CFH polymorphism is responsible for the need for more bevacizumab injections in the risk C allele group. Perhaps if more bevacizumab had been administered after the initial treatment in the Caucasian study, there would have been improved visual outcome at the end of study.
A recent study by Sakurada et al. [43] showed that higher T-allele frequency in LOC387715 A69S was associated with worse visual prognosis after photodynamic therapy for PCV. On the contrary, the present study showed that higher T-allele frequency in LOC387715 was associated with better visual prognosis after intravitreal bevacizumab for CNV. These results show the variable effect of genetic polymorphism on treatment response associated with disease phenotype (PCV vs. CNV) and treatment modality (PDT vs. bevacizumab). Patients with the risk T-allele could have poor visual prognosis when treated for PCV [43] but could have a better prognosis when treated for CNV; also, patients with the risk T-allele could have poor visual prognosis due to PDT but may have an improved visual prognosis on bevacizumab treatment. Based on these results, one could logically use genetic information of each disease phenotype to personalize treatment modality. For instance, in PCV patients with the risk T allele, it might be useful to incorporate bevacizumab or bevacizumab/PDT combination therapy rather than PDT treatment alone.
There are several limitations to the present study: the patient cohort is small, this is a retrospective study, and patient follow-up was incomplete, resulting in insufficient 12-month follow-up results. In fact, the results were not statistically significant; however, genotypically different distributions were observed based on age, diabetes mellitus, and prior PDT in the LOC387715/HTRA1 group. The authors could not rule out that statistical error might be due to small sample size. Therefore, Tables 2 and 3 show the adjusted results by multivariate statistical analysis for not only hypertension (which was statistically significant in the baseline study) but also for age and prior PDT treatment. In addition, patients who were treated with PDT more than six months before the start of bevacizumab treatment were included. These patient groups had an unfavorable response to PDT and, therefore, would be potential candidates for bevacizumab treatment. There was no statistically significant difference in VA or CMT between the prior PDT group and the control during follow-up (Table 4). However, as mentioned previously, statistical error due to limited sample size cannot be ruled out. Many of the patients in the high risk group of the LOC387715/HTRA1 group had previously received PDT. There was also a tendency for enhanced visual improvement over baseline in patients with prior PDT compared to the control. These results imply that the high risk group, which had a better treatment outcome, might be influenced by PDT. It appears that this response is a result of PDT/bevacizumab combination therapy, not bevacizumab alone. Finally, this study was conducted on an Asian population and may not be directly applicable to Caucasian populations.
Despite these limitations, the present study is the first to show statistical relevance between the LOC387715/HTRA1 genes and their pharmacogenetic association to bevacizumab in an Asian population. This study may be a guide to further studies on bevacizumab as well as other anti-VEGF agents in Asian population. Further studies with prospective randomized, controlled trials are needed, and pharmacogenetic studies of ranibizumab, antioxidant supplements and zinc in Asian populations are also recommended. Currently, three monthly initial intravitreal injections of anti-VEGF agent and additional maintenance treatment for disease stability is a widely accepted treatment protocol for exudative AMD. However, results are still inconclusive regarding the recommended treatment interval or type of drug used in maintenance treatment. This report describes a relationship between treatment response and additional injections, although the relationship was not statistically significant. This study could be a guide for future research examining maintenance treatment intervals according to genotyping in exudative AMD patients.
In conclusion, this study showed that different LOC387715/HTRA1 genotypes had different treatment responses when bevacizumab was used to treat exudative AMD. Patients with the risk allele had better treatment responses and less need for additional injections. In addition, patients with the risk allele of CFH Y402H needed additional injections of bevacizumab for better visual improvement. This suggests that shows pharmacogenetic factors could be used to determine treatment modality and dosing and this study may ultimately provide basic data for 'personalized medicine' in AMD.
Acknowledgements
This work was supported by an Inha University research grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.De Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cackett P, Wong TY, Aung T, et al. Smoking, cardiovascular risk factors, and age-related macular degeneration in Asians: the Singapore Malay Eye Study. Am J Ophthalmol. 2008;146:960–967.e1. doi: 10.1016/j.ajo.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Dandekar SS, Jenkins SA, Peto T, et al. Does smoking influence the type of age related macular degeneration causing visual impairment? Br J Ophthalmol. 2006;90:724–727. doi: 10.1136/bjo.2005.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeters A, Magliano DJ, Stevens J, et al. Changes in abdominal obesity and age-related macular degeneration: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 2008;126:1554–1560. doi: 10.1001/archopht.126.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 10.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997;123:199–206. doi: 10.1016/s0002-9394(14)71036-0. [DOI] [PubMed] [Google Scholar]
- 13.Gorin MB. A clinician's view of the molecular genetics of age-related maculopathy. Arch Ophthalmol. 2007;125:21–29. doi: 10.1001/archopht.125.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Mullins RF. Genetic insights into the pathobiology of age-related macular degeneration. Int Ophthalmol Clin. 2007;47:1–14. doi: 10.1097/IIO.0b013e31802bd3e6. [DOI] [PubMed] [Google Scholar]
- 15.Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Hum Mol Genet. 2007;16 Spec No 2:R174–R182. doi: 10.1093/hmg/ddm212. [DOI] [PubMed] [Google Scholar]
- 16.Seddon JM, Cote J, Page WF, et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Hammond CJ, Webster AR, Snieder H, et al. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002;109:730–736. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- 18.Edwards AO, Ritter R, 3rd, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 19.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 20.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conley YP, Jakobsdottir J, Mah T, et al. CFH, ELOVL4, PLEKHA1 and LOC387715 genes and susceptibility to age-related maculopathy: AREDS and CHS cohorts and meta-analyses. Hum Mol Genet. 2006;15:3206–3218. doi: 10.1093/hmg/ddl396. [DOI] [PubMed] [Google Scholar]
- 22.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38:1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 24.Postel EA, Agarwal A, Caldwell J, et al. Complement factor H increases risk for atrophic age-related macular degeneration. Ophthalmology. 2006;113:1504–1507. doi: 10.1016/j.ophtha.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Tuo J, Ning B, Bojanowski CM, et al. Synergic effect of polymorphisms in ERCC6 5’ flanking region and complement factor H on age-related macular degeneration predisposition. Proc Natl Acad Sci U S A. 2006;103:9256–9261. doi: 10.1073/pnas.0603485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida T, DeWan A, Zhang H, et al. HTRA1 promoter polymorphism predisposes Japanese to age-related macular degeneration. Mol Vis. 2007;13:545–548. [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R, Rowland ML, Harris MI. Racial/ethnic differences in age-related maculopathy. Third National Health and Nutrition Examination Survey. Ophthalmology. 1995;102:371–381. doi: 10.1016/s0161-6420(95)31012-3. [DOI] [PubMed] [Google Scholar]
- 30.Gotoh N, Yamada R, Hiratani H, et al. No association between complement factor H gene polymorphism and exudative age-related macular degeneration in Japanese. Hum Genet. 2006;120:139–143. doi: 10.1007/s00439-006-0187-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim NR, Kang JH, Kwon OW, et al. Association between complement factor H gene polymorphisms and neovascular age-related macular degeneration in Koreans. Invest Ophthalmol Vis Sci. 2008;49:2071–2076. doi: 10.1167/iovs.07-1195. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Kim NR, Chin HS. LOC387715/HTRA1 polymorphisms, smoking and combined effects on exudative age-related macular degeneration in a Korean population. Clin Experiment Ophthalmol. 2010;38:698–704. doi: 10.1111/j.1442-9071.2010.02316.x. [DOI] [PubMed] [Google Scholar]
- 33.Andreoli MT, Morrison MA, Kim BJ, et al. Comprehensive analysis of complement factor H and LOC387715/ARMS2/HTRA1 variants with respect to phenotype in advanced age-related macular degeneration. Am J Ophthalmol. 2009;148:869–874. doi: 10.1016/j.ajo.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leveziel N, Puche N, Richard F, et al. Genotypic influences on severity of exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:2620–2625. doi: 10.1167/iovs.09-4423. [DOI] [PubMed] [Google Scholar]
- 35.Seitsonen S, Jarvela I, Meri S, et al. Complement factor H Y402H polymorphism and characteristics of exudative age-related macular degeneration lesions. Acta Ophthalmol. 2008;86:390–394. doi: 10.1111/j.1600-0420.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 36.Klein ML, Francis PJ, Rosner B, et al. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology. 2008;115:1019–1025. doi: 10.1016/j.ophtha.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 37.Brantley MA, Jr, Edelstein SL, King JM, et al. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to photodynamic therapy. Eye (Lond) 2009;23:626–631. doi: 10.1038/eye.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goverdhan SV, Hannan S, Newsom RB, et al. An analysis of the CFH Y402H genotype in AMD patients and controls from the UK, and response to PDT treatment. Eye (Lond) 2008;22:849–854. doi: 10.1038/sj.eye.6702830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seitsonen SP, Jarvela IE, Meri S, et al. The effect of complement factor H Y402H polymorphism on the outcome of photodynamic therapy in age-related macular degeneration. Eur J Ophthalmol. 2007;17:943–949. doi: 10.1177/112067210701700612. [DOI] [PubMed] [Google Scholar]
- 40.Brantley MA, Jr, Fang AM, King JM, et al. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology. 2007;114:2168–2173. doi: 10.1016/j.ophtha.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Lee AY, Raya AK, Kymes SM, et al. Pharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2009;93:610–613. doi: 10.1136/bjo.2008.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuchihashi T, Mori K, Horie-Inoue K, et al. Complement factor H and high-temperature requirement A-1 genotypes and treatment response of age-related macular degeneration. Ophthalmology. 2011;118:93–100. doi: 10.1016/j.ophtha.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Sakurada Y, Kubota T, Imasawa M, et al. Association of LOC387715 A69S genotype with visual prognosis after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2010;30:1616–1621. doi: 10.1097/IAE.0b013e3181e587e3. [DOI] [PubMed] [Google Scholar]
- 44.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 45.De Luca A, De Falco M, De Luca L, et al. Pattern of expression of HtrA1 during mouse development. J Histochem Cytochem. 2004;52:1609–1617. doi: 10.1369/jhc.4A6330.2004. [DOI] [PubMed] [Google Scholar]
- 46.Oka C, Tsujimoto R, Kajikawa M, et al. HtrA1 serine protease inhibits signaling mediated by TGF-beta family proteins. Development. 2004;131:1041–1053. doi: 10.1242/dev.00999. [DOI] [PubMed] [Google Scholar]
- 47.Kaarniranta K, Salminen A, Eskelinen EL, Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways-Implications for age-related macular degeneration (AMD) Ageing Res Rev. 2009;8:128–139. doi: 10.1016/j.arr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Rondanelli M, Melzi d'Eril GV, Anesi A, Ferrari E. Altered oxidative stress in healthy old subjects. Aging (Milano) 1997;9:221–223. doi: 10.1007/BF03340153. [DOI] [PubMed] [Google Scholar]
- 49.Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- 50.Spaide RF. Rationale for combination therapy in age-related macular degeneration. Retina. 2009;29(6 Suppl):S5–S7. doi: 10.1097/IAE.0b013e3181ad237a. [DOI] [PubMed] [Google Scholar]