Abstract
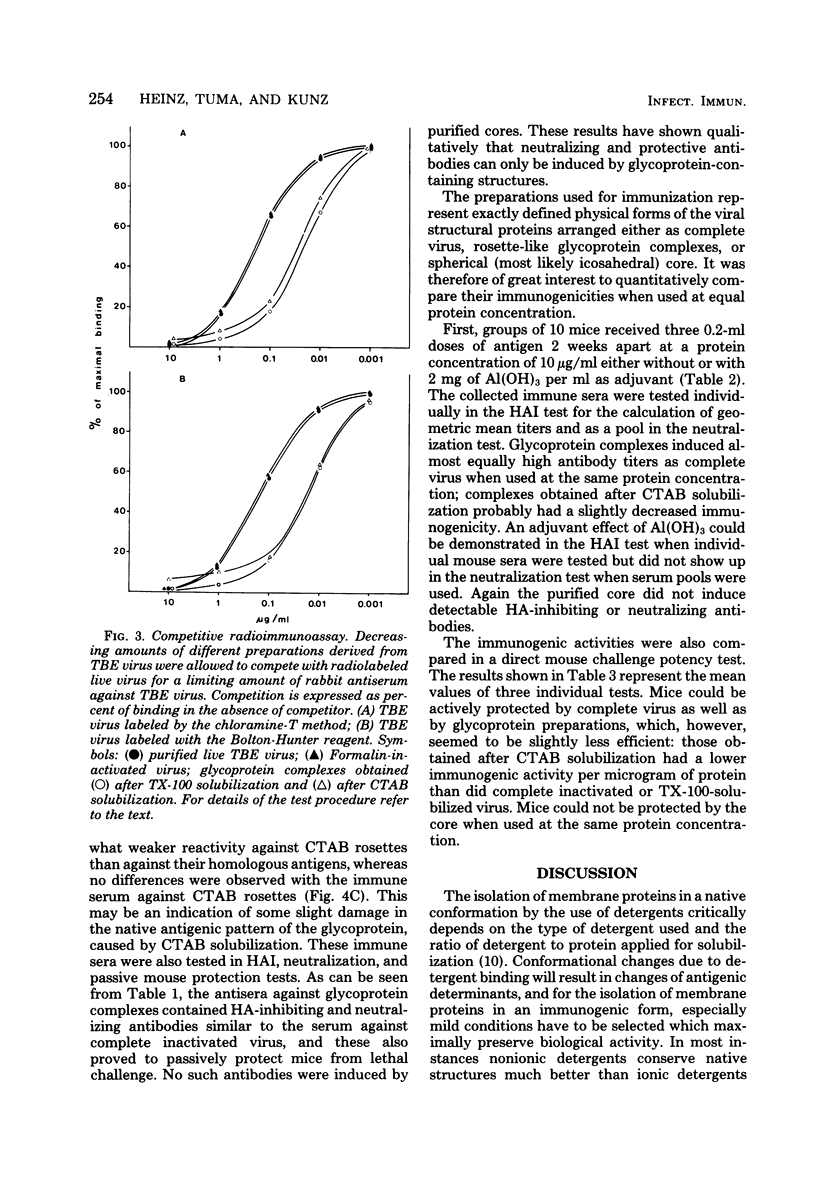
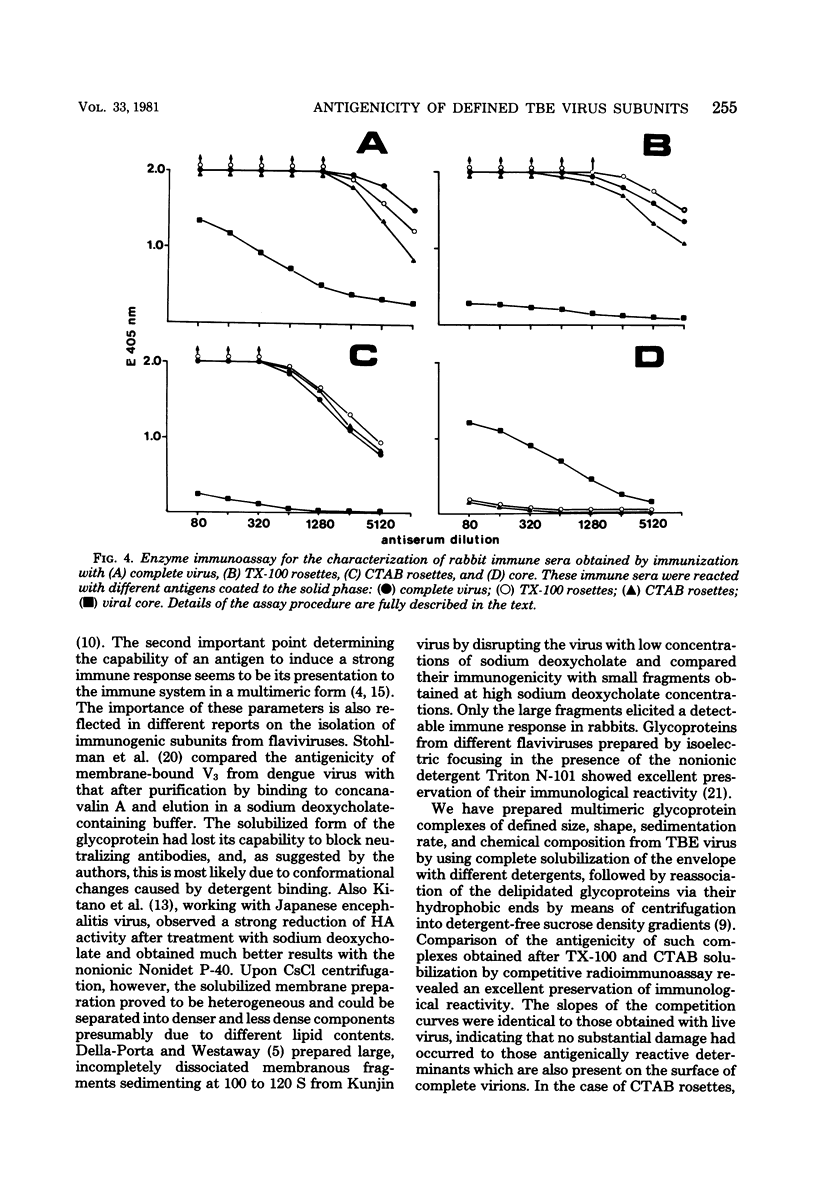
Polymeric, delipidated glycoprotein complexes of defined size and composition were prepared from tick-borne encephalitis virus by solubilization with Triton X-100 or cetyltrimethylammonium bromide, followed by centrifugation into detergent-free sucrose density gradients. The antigenic reactivities and immunogenicities of these complexes were compared with those of complete inactivated virus. These glycoprotein preparations induced hemagglutination-inhibiting and neutralizing antibodies which proved to be protective in passive mouse protection tests and monospecifically reacted only with the viral envelope and not with the internal core. In a competitive radioimmunoassay the glycoprotein complexes revealed about 10-fold higher antigenicity than whole virus when tested at equal protein concentrations. The important implications of these results with respect to antigen quantification in vaccines are discussed. As shown in the mouse challenge potency test, glycoprotein complexes prepared after Triton X-100 solubilization actively protected mice almost as well as did complete inactivated virus at the same protein concentration, whereas those prepared after cetyltrimethylammonium bromide solubilization had a somewhat lower protective activity per microgram of protein.
Full text
PDF


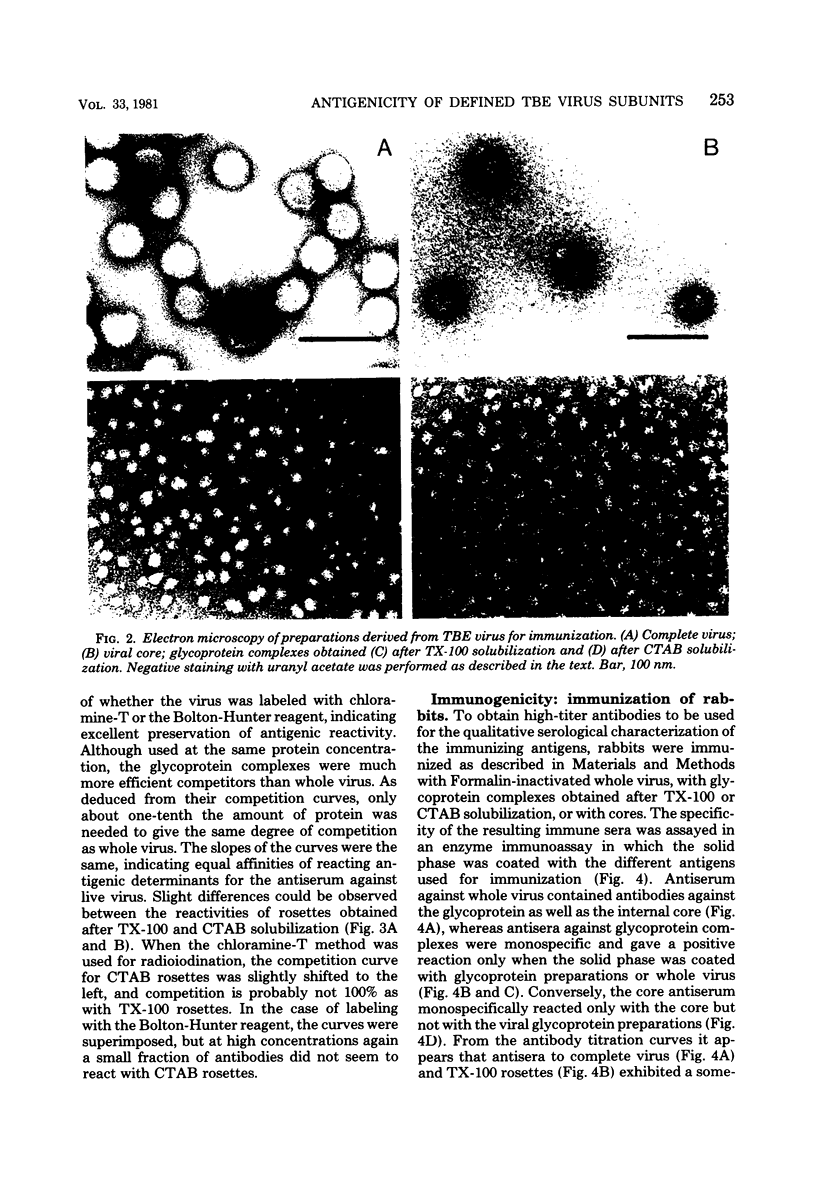

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Porta A. J., Westaway E. G. Immune response in rabbits to virion and nonvirion antigens of the Flavivirus kunjin. Infect Immun. 1977 Mar;15(3):874–882. doi: 10.1128/iai.15.3.874-882.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Kunz C. Formation of polymeric glycoprotein complexes from a flavivirus: tick-borne encephalitis virus. J Gen Virol. 1980 Jul;49(1):125–132. doi: 10.1099/0022-1317-49-1-125. [DOI] [PubMed] [Google Scholar]
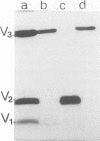
- Heinz F. X., Kunz C. Isolation of dimeric glycoprotein subunits from tick-borne encephalitis virus. Intervirology. 1980;13(3):169–177. doi: 10.1159/000149122. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Kunz C. Protease treatment and chemical crosslinking of a flavivirus: tick borne encephalitis virus. Arch Virol. 1979;60(3-4):207–216. doi: 10.1007/BF01317492. [DOI] [PubMed] [Google Scholar]
- Heinz F., Kunz C. Dissociation of tick-borne encephalitis virus by Triton X-100 and cetyltrimethylammonium bromide. Acta Virol. 1979 May;23(3):189–197. [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Helenius A., von Bonsdorff C. H. Semlike Forest virus membrane proteins. Preparation and characterization of spike complexes soluble in detergent-free medium. Biochim Biophys Acta. 1976 Jul 15;436(4):895–899. doi: 10.1016/0005-2736(76)90421-1. [DOI] [PubMed] [Google Scholar]
- Kitano T., Suzuki K., Yamaguchi T. Morphological, chemical, and biological characterization of Japanese encephalitis virus virion and its hemagglutinin. J Virol. 1974 Sep;14(3):631–639. doi: 10.1128/jvi.14.3.631-639.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Morein B., Helenius A., Simons K., Pettersson R., Käriäinen L., Schirrmacher V. Effective subunit vaccines against an enveloped animal virus. Nature. 1978 Dec 14;276(5689):715–718. doi: 10.1038/276715a0. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Simons K., Helenius A., Leonard K., Sarvas M., Gething M. J. Formation of protein micelles from amphiphilic membrane proteins. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5306–5310. doi: 10.1073/pnas.75.11.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Eylar O. R., Wisseman C. L., Jr Isolation of the dengue virus envelope glycoprotein from membranes of infected cells by concanavalin A affinity chromatography. J Virol. 1976 Apr;18(1):132–140. doi: 10.1128/jvi.18.1.132-140.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent D. W. Antigenic characterization of flavivirus structural proteins separated by isoelectric focusing. J Virol. 1977 Jun;22(3):608–618. doi: 10.1128/jvi.22.3.608-618.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]