Abstract
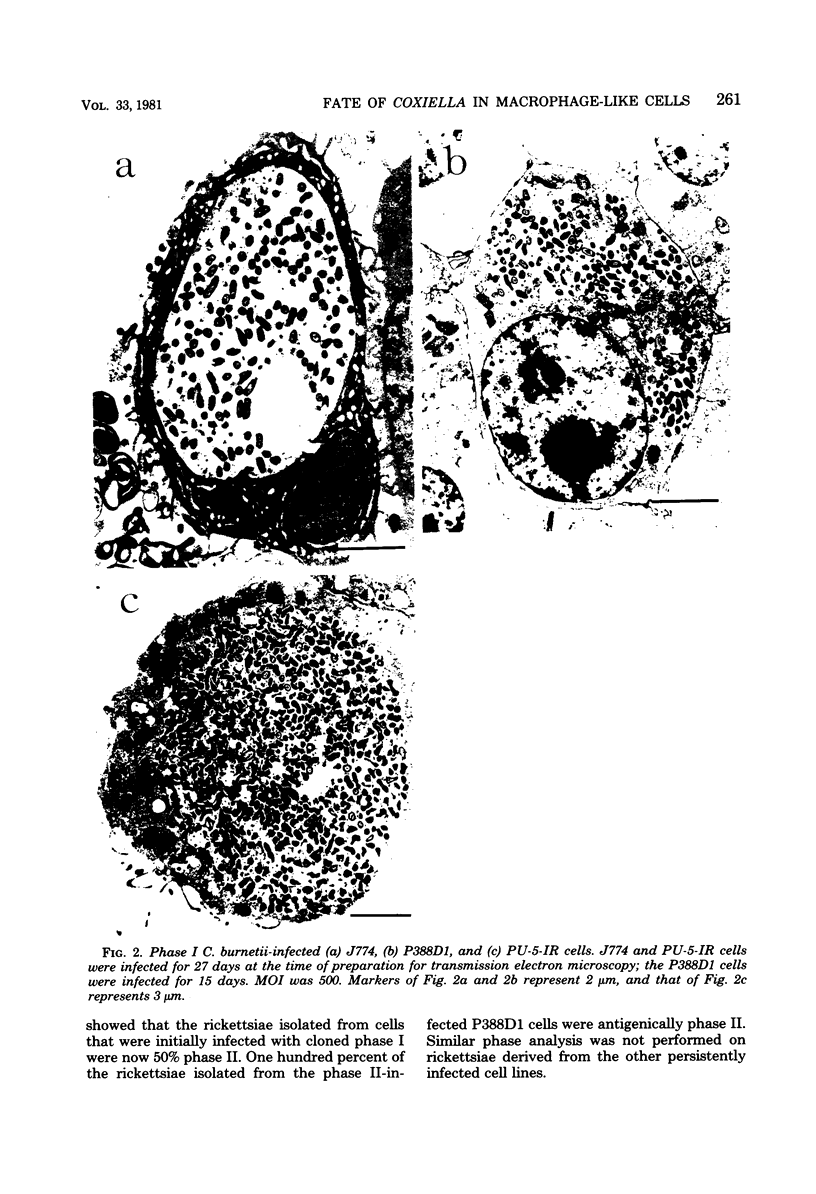
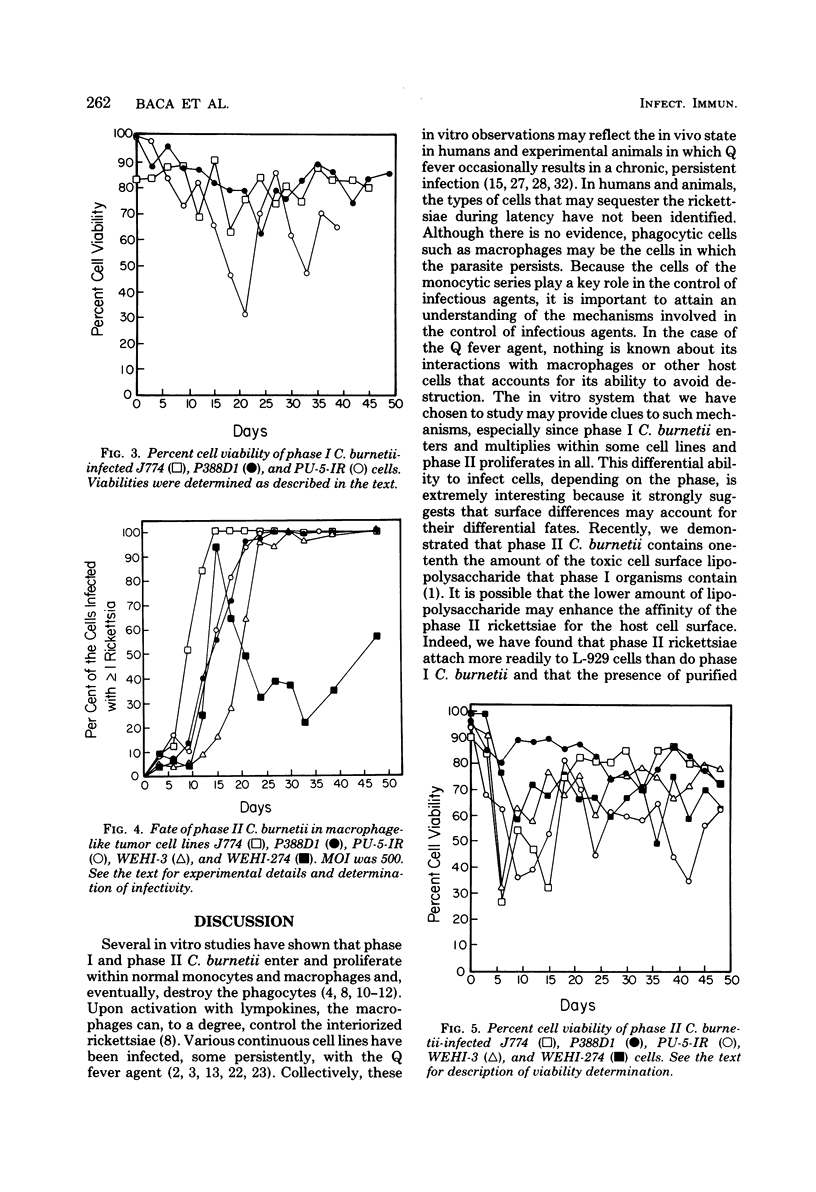
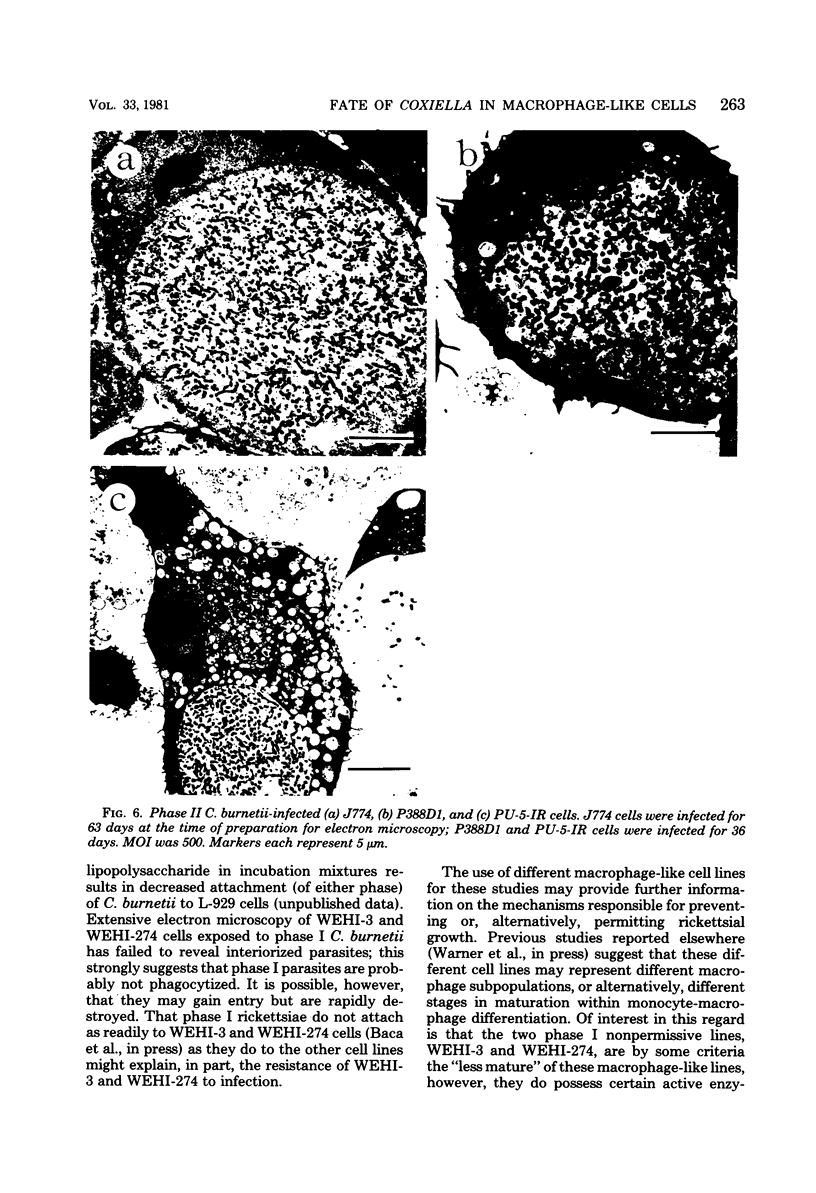
Several macrophage-like tumor cell lines of murine origin were exposed to phase I and phase II Coxiella burnetii, and the subsequent fate of the parasites was determined by electron and bright-field microscopy. Phase I C. burnetii proliferated within and established a persistent infection of P388D1, J774, and PU-5-IR cell lines but not of WEHI-3 and WEHI-274 cell lines. Phase II C. burnetii, however, entered into and persistently infected all five cell lines. The parasites proliferated within vacuoles. Macrophage cell lines persistently infected with phase I and phase II C. burnetii were maintained for over 200 and 100 days, respectively. Within P388D1 cells, the phase I C. burnetii converted, in part, to phase II; phase II organisms remained in the phase II state. The differential fate of the two rickettsial phases after exposure to the WEHI-3 and WEHI-274 cells may be attributable to surface differences such as lipopolysaccharide content.
Full text
PDF

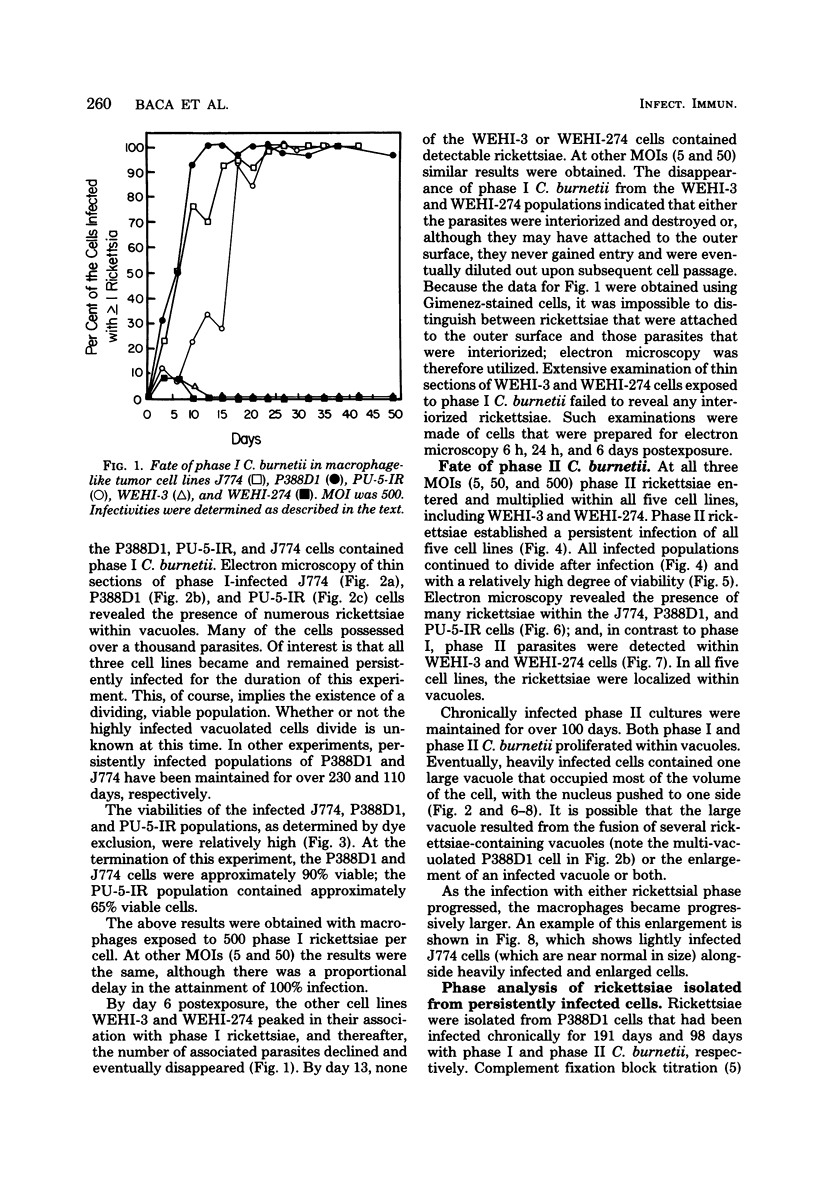


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOZEMAN F. M., HOPPS H. E., DANAUSKAS J. X., JACKSON E. B., SMADEL J. E. Study on the growth of Rickettsiae. I. A tissue culture system for quantitative estimations of Rickettsia tsutsugamushi. J Immunol. 1956 Jun;76(6):475–488. [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs C. M. Phagocytosis of coxiella burneti, phase I and phase II by peritoneal monocytes from normal and immune guinea pigs and mice. Zentralbl Bakteriol Orig. 1968 Apr;206(3):329–343. [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect Immun. 1973 Oct;8(4):631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs D. J., Jerrells T. R. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976 Sep;117(3):996–1003. [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Canonico P. G., Shirey F. G., Walker J. S. Electron microscopic study on the interaction between normal guinea pig peritoneal macrophages and Coxiella burnetii. Infect Immun. 1976 Oct;14(4):1087–1096. doi: 10.1128/iai.14.4.1087-1096.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Shirey F. G., Canonico P. G., Walker J. S. Fat of Coxiella burnetti in macrophages from immune guinea pigs. Infect Immun. 1977 Feb;15(2):601–607. doi: 10.1128/iai.15.2.601-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Walker J. S. Interaction between Coxiella burnetii and guinea pig peritoneal macrophages. Infect Immun. 1976 Aug;14(2):416–421. doi: 10.1128/iai.14.2.416-421.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordová N., Burton P. R., Downs C. M., Paretsky D., Kovácová E. The interaction of Coxiella burnetti phase I and phase II in Earle's cells. Can J Microbiol. 1970 Feb;16(2):125–133. doi: 10.1139/m70-021. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Kávai M., Laczkó J., Csaba B. Functional heterogeneity of macrophages. Immunology. 1979 Apr;36(4):729–732. [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S. Macrophage nomenclature: where are we going? J Reticuloendothel Soc. 1980 Feb;27(2):223–245. [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS A. N., DOWNS C. M. Study on the growth of Coxiella burnetii in the L strain mouse fibroblast and the chick fibroblast. J Bacteriol. 1959 Feb;77(2):194–204. doi: 10.1128/jb.77.2.194-204.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- SCHAECHTER M., BOZEMAN F. M., SMADEL J. E. Study on the growth of Rickettsiae. II. Morphologic observations of living Rickettsiae in tissue culture cells. Virology. 1957 Feb;3(1):160–172. doi: 10.1016/0042-6822(57)90030-2. [DOI] [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- SYRUCEK L., SOBESLAVSKY O., GUTVIRTH I. Isolation of Coxiella burneti from human placentas. J Hyg Epidemiol Microbiol Immunol. 1958;2(1):29–35. [PubMed] [Google Scholar]
- Silberman R., Fiset P. Method for counting Rickettsiae and Chlamydiae in purified suspensions. J Bacteriol. 1968 Jan;95(1):259–261. doi: 10.1128/jb.95.1.259-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck W. P., Howitt G., Turnberg L. A., Fox H., Longson M., Matthews M. B., Das Gupta R. Chronic Q fever. Q J Med. 1976 Apr;45(178):193–217. [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Wilson H. G., Neilson G. H., Galea E. G., Stafford G., O'Brien M. F. Q fever endocarditis in Queensland. Circulation. 1976 Apr;53(4):680–684. doi: 10.1161/01.cir.53.4.680. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Fiset P., Ormsbee R. A. Interaction of rickettsiae and phagocytic host cells. V. Phagocytic and opsonic interactions of phase 1 and phase 2 Coxiella burneti with normal and immune human leukocytes and antibodies. J Immunol. 1967 Oct;99(4):669–674. [PubMed] [Google Scholar]