Abstract
The aim of the study was to investigate the inhibitory effects of calcium against intestinal cancer in vitro and in vivo. We first investigated the effects of calcium treatment in HCT116 and HT29 human colon cancer cells. At the concentration range of 0.8-2.4 mM, calcium significantly inhibited cell growth (by 9-29%), attachment (by 12-26%), invasion (by 15-31%), and migration (by 19-61%). An immunofluorescence microscope analysis showed that the treatment with calcium (1.6 mM) for 24 h increased plasma membrane β-catenin but decreased nuclear β-catenin levels in HT29 cells. We then investigated the effect of dietary calcium on intestinal tumorigenesis in ApcMin/+ mice. Mice received dietary treatment starting at 6 weeks of age for the consecutive 8 weeks. The basal control diet contained high-fat (20% mixed lipids by weight) and low-calcium (1.4 mg/g diet) to mimic the average Western diet, while the treatment diet contained an enriched level of calcium (5.2 mg calcium/g diet). The dietary calcium treatment decreased the total number of small intestinal tumors (by 31.4%; P < 0.05). The largest decrease was in tumors which were ≥ 2 mm in diameter, showing a 75.6% inhibition in the small intestinal tumor multiplicity (P < 0.001). Immunohistochemical analysis showed significantly reduced nuclear staining of β-catenin (expressed as nuclear positivity), but increased plasma membrane staining of β-catenin, in the adenomas from the calcium-treated groups in comparison to those from the control group (P < 0.001). These results demonstrate intestinal cancer inhibitory effects of calcium both in human colon cancer cells and ApcMin/+ mice. The decreased β-catenin nuclear localization caused by the calcium treatment may contribute to the inhibitory action.
Keywords: Calcium, human colon cancer cells, ApcMin/+ mice, β-catenin
Introduction
Colorectal cancer is one of the most common cancers in both incidence and mortality among men and women worldwide [1]. The prevention of this disease is, therefore, a significant public health issue. Both environmental and genetic factors are important in the etiology of this type of cancer [1]. Diet is a major environmental factor that affects colorectal cancer risk [2]. Generally, a Western style diet containing high fat, low calcium, and low fiber is considered to be associated with a higher risk for colorectal cancer [1,2]. This suggests that colorectal cancer is preventable by dietary modification.
Calcium is an essential nutrient, and the primary function of calcium is to form the structure of bones and teeth. Calcium is also involved in the cell signaling of the muscular, cardiovascular, endocrine, and nervous systems [3]. Beyond such nutritional functions, disease-preventive activities of calcium, including cancer-preventive activities, also have received attention recently [4,5]. Although the epidemiological data on the relationship between calcium intake and colorectal cancer are inconsistent [6], results from meta-analysis indicated that supplemental calcium was effective for the prevention of adenoma reoccurrence in populations with the history of adenomas [7-9]. For example, in the Calcium Polyp Prevention Trial on subjects with recent histories of colorectal adenomas, calcium supplementation (1,200 mg/day) has been found to decrease the risk of all types of neoplastic and hyperplastic polyps, especially on more advanced colorectal lesions [8,9]. Results from animal studies are not consistent. With the azoxymethane- or 1,2-dimethylhydrazine-induced aberrant crypt foci and colon tumorigenesis rat models, the inhibitory effects of dietary calcium has also been demonstrated in many studies [10-23], though not in some [24-27]. In a study with ApcMin/+ mouse model, half (0.2%) or double (1.2%) the level of calcium in the AIN93G diet (0.5%) did not significantly affect the polyp number or tumor load [28]. In another study with ApcMin/+ mouse, dietary calcium supplementation (1.2%) increased intestinal tumor formation [29]. A noteworthy study was with C57bl/6 mice with a "Western style diet" that mimicks the high fat, low calcium, and low vitamin D features of the American diet [30,31]. The mice developed hyperplasia and dysplasia in the colon [30], and the supplements of calcium and vitamin D prevented development of these lesions [31]. This finding suggests that the inhibitory effects of calcium can be best demonstrated in a diet that mimics human diet with high fat and low calcium contents. Recently, we showed that dietary treatments with calcium effectively inhibited aberrant crypt foci formation in azoxymethane-induced rats and mice maintained on high-fat and low-calcium diet [32].
In normal colonic epithelial cells, β-catenin consists of adherence junction together with transmembrane glycoprotein E-cadherin, thought to be tumor suppressor in the colon, and functions to maintain epithelial cell-cell adhesion and cell integrity [33]. The translocation of β-catenin from the plasma membrane to the nucleus of cells results in an increased transcription of genes involved with cell proliferation, which is a key event in colon carcinogenesis [34,35]. Calcium treatment has been shown to increase E-cadherin levels and to suppress subsequent β-catenin signaling in different cell culture systems [36-39]. It is of important to see if such effects also occur in vivo.
ApcMin/+ mice carry a germline mutation at codon 850 of the mouse homologue of the human Adenomatous Polyposis Coli (APC) gene [40], which is frequently mutated in human colon cancer [41]. ApcMin/+ mice, therefore, have been recognized as a genetically relevant animal model for human colon carcinogenesis and have been widely utilized in various chemoprevention studies [42].
In the present study, we aimed to determine the inhibitory effects of calcium against intestinal cancer in human colon cancer cells and ApcMin/+ mouse model. Further, we investigated the effect of calcium treatment on β-catenin translocation both in vitro and in vivo.
Materials and Methods
Cell culture
HCT116 and HT29 human colon cancer cells were purchased from the Korean Cell Line Bank (Seoul, Korea) and the American Type Culture Collection (Manassas, VA, US), respectively. Cells were maintained in the minimum essential medium (MEM Eagle; Sigma-Aldrich, St, Louis, MO) with 10% fetal bovine serum (FBS; Thermo Scientific, Logan, UT, US) and 100 units/mL penicillin/0.1 mg/mL streptomycin (Welgene Inc., Daegu, Korea) at 37℃ in 95% humidity and 5% CO2. Calcium chloride (Sigma Aldrich, St, Louis, MO, US) was dissolved in ultra pure water (Biosesang Inc., Seongnam, Korea) and used for the treatment of cells.
Cell viability, attachment, invasion, and migration assays
For the cell viability assay, HCT116 and HT29 cells (1 × 104 cells/well) were seeded in 96-well plates. After 24 h, cells were treated with serial concentrations of calcium in serum complete media (containing 10% FBS). At respective time points after treatments, the media were replaced by fresh media containing 0.5 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Aldrich). After 4 h incubation at 37℃, MTT-containing media were removed and the reduced formazan dye was dissolved by adding dimethyl sulfoxide (DMSO) to each well. After gentle mixing, the absorbance was monitored at 540 nm using a plate reader (Bio-Rad Laboratories, Hercules, CA, US).
For the cell attachment assay, HCT116 cells (4 × 104 cells/well) in either calcium-containing or water-containing serum complete media were plated into each well of 96-well plates. After 16 h, the cells were subjected to MTT assay as described above.
For the cell invasion assay, a matrigel-coated chamber (BD Biosciences, Oxford, UK) was used where HCT116 cells (2.5 × 105 cells/well) were loaded in inner chambers containing serum-free media with or without calcium at respective concentrations. Cells were then moved through the extracellular matrix layer at the bottom of the inner chamber toward the outer chamber containing the serum complete media. At 48 h after the treatment, cells in the outer chamber were quantified using Alamar blue (Invitrogen, NY, US). The fluorescence was measured at an excitation of 550 nm and an emission of 590 nm using GENios plate reader (Tecan, Durham, NC, US).
For the scratch wound healing assay, HCT116 and HT29 cells were grown on 30 mm dish or 6-well plate, up to 70-80% confluence, and wounds were made with tip of micropipette. Cells were then maintained in either calcium-containing or water-containing serum complete media for 24 h (in the case of HCT116 cells) and 48 h (in the case of HT29 cells). The width of wound was measured using phase contrast time-lapse microscopy (Carl Zeiss Co., Ltd., Seoul, Korea) with iSolution Lite software.
Breeding and genotyping of ApcMin/+ Mice
The breeding colony was established using male C57BL/6J-ApcMin/+ and female wild-type littermate mice (The Jackson Laboratory, Bar Harbor, ME, US) as founders in the animal facility of the Susan Lehman Cullman Laboratory for Cancer Research (Rutgers,The State University of New Jersey, Piscataway, NJ, US). Genotyping of the pubs produced from the colony was done by routine polymerase chain reaction assays as previously reported [43].
Diet treatment and tissue harvesting
Male and female C57BL/6J-ApcMin/+ mice at the age of 6 weeks received either the control diet containing high-fat (20%; w/w) and low-calcium (1.4 mg/g diet) or the calcium-enriched diet (5.2 mg calcium/g diet; Table 1) [32]. Body weight, food consumption, and fluid consumption were measured weekly. After 8 weeks of treatment, mice were sacrificed by CO2 asphyxiation. Blood was collected via cardiac puncture, and serum was isolated by centrifugation. Since ApcMin/+ mice develop tumors throughout intestinal tract, mainly in the small intestine, the entire intestinal tract was harvested, flushed thoroughly with cold 0.9% saline, cut open longitudinally, and flattened on filter paper to expose tumors in the lumen. The flattened tissues on filter paper were placed on dry ice briefly to aid in scoring the visible tumors [44,45]. Small intestine tissues from male mice were fixed in 10% buffered formalin for 24 h and Swiss rolled for routine pathological processing.
Table 1.
Composition of diet used for the animal study1)
1)Composition is expressed as a percentage by weight.
2)Mixed lipid contains 16% beef tallow, 10% lard, 12% butter fat, 30% hydrogenated soybean oil, 27% corn oil, and 5% peanut oil.
3)Mineral mix contains 8.4% magnesium oxide, 51.5% magnesium sulfate 7H2O, 0.4% chromium potassium sulfate, 0.2% cupric carbonate, 0.7% potassium iodate, 4.2% ferric citrate, 2.5% manganous carbonate, 0.7% sodium selenite, 1.1% zinc carbonate, and 31.7% sucrose.
Immunocytochemistry and immunohistochemistry
For immunocytochemical analysis, HT29 cells on 25 mm square glass coverslips (Corning, Cambridge, MA, US) were fixed in methanol for 30 seconds at 20℃ and blocked by incubation with PBS containing 1% bovine serum albumin (BSA) for 1 h at room temperature. Cells were then incubated with the β-catenin antibody in PBS containing 1% BSA for 1 h at room temperature, washed, incubated with secondary antibodies for 45 minutes at room temperature, washed, and mounted in Vecta Shield (Vector Laboratories, Burlingame, CA). Immnufluorescence microscopic analysis was done at excitation wavelengths of 488 nm (for fluorescein isothiocyanate) and 543 nm (for tetramethyrhodamine isothiocyanate).
For immunohistochemical analysis, embedded tissue blocks were cut serially for at least 30 slides and labeled numerically. Four slides per mouse were stained for haematoxylin and eosin for histopathological evaluation, and the remaining slides were used for immunohistochemistry. A standard avidin-biotin complex method was used as previously described [34,35], and the localization of β-catenin (antibody purchased from Cell Signaling Technology) and E-cadherin (antibody purchased from BD Biosciences, San Jose, CA) were analyzed. Positivity of nuclear staining for β-catenin was counted manually and expressed as the percentage of positive-staining cells in the total number of tumor cells.
Determination of calcium levels
Serum calcium levels were measured in ApcMin/+ mice following the manufacturers' protocol (BioVision, CA, US). In brief, serum sample (or standard), chromogenic reagent, and assay buffer were mixed, incubated for 10 minutes at room temperature protecting from light, and then measured at the absorbance of 575 nm using GENios plate reader (Tecan).
Statistical analysis
SPSS and Excel softwares were used for statistical analysis. For the simple comparisons between the two groups, Student's t-test was used. One-way ANOVA combined with appropriate post-hoc tests was used for the comparisons among multiple groups. Two-way ANOVA was used for assessing whether or not data from two different animal experiments (one with females and the other with males) could be combined. The effects of the two factors, treatment and experiment, as well as the interaction of treatment and experiment, on the response variable, tumor numbers, were analyzed.
Results
Effect of calcium on cell growth, attachment, invasion, and migration in human colon cancer cells
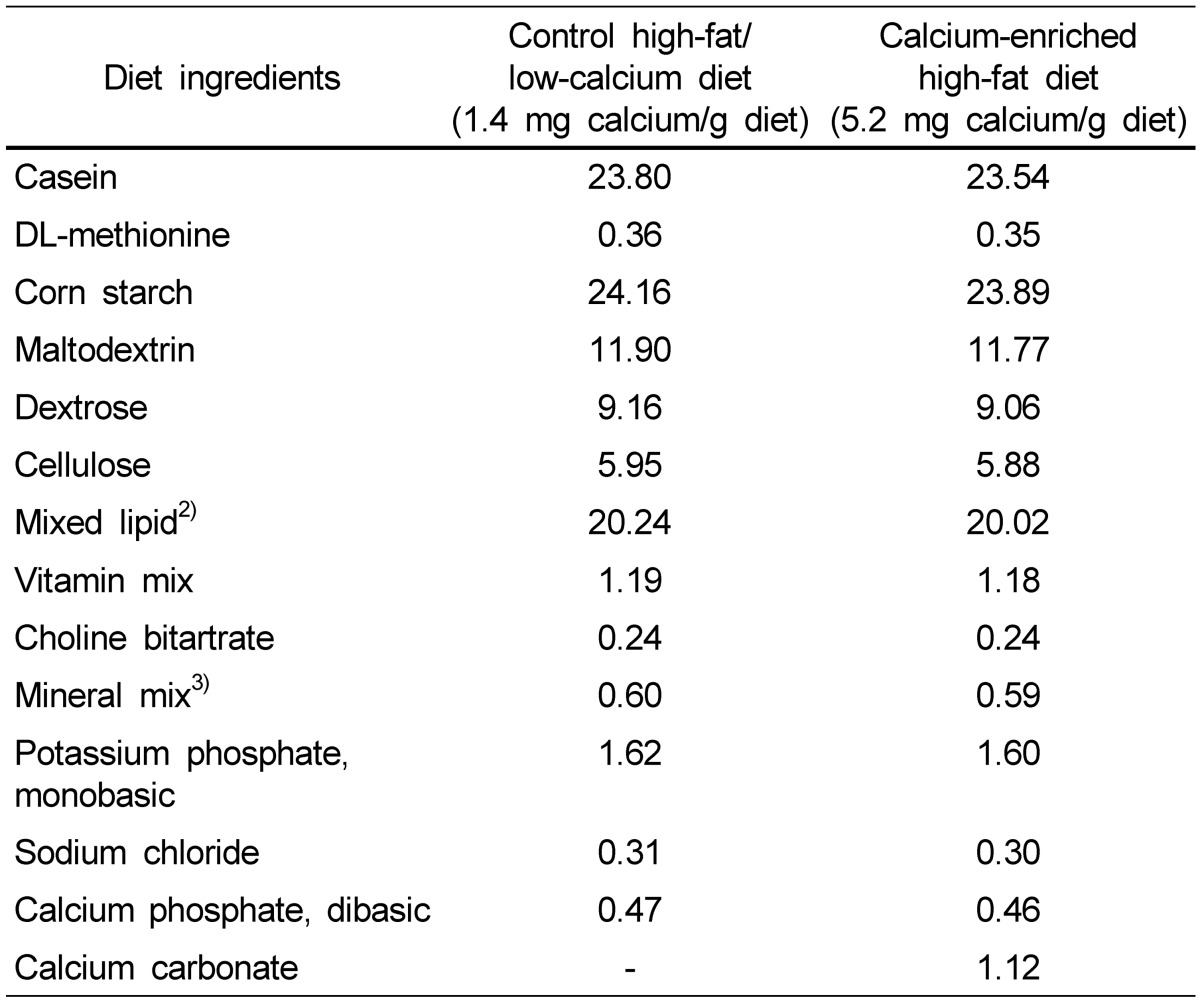
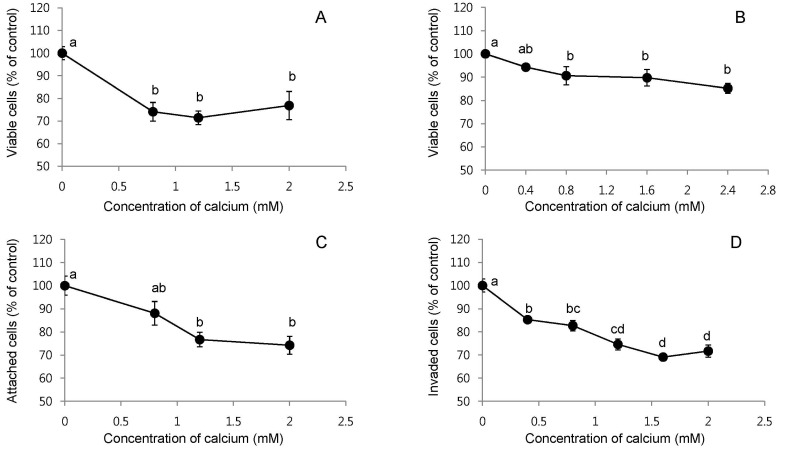
We investigated the effects of calcium treatment on cell growth, attachment, invasion, and migration in HCT116 and HT29 human colon cancer cells. The treatment of HCT116 cells with calcium at 0.8, 1.2, and 2 mM for 72 h inhibited the cell growth by 23-29% (P < 0.001; Fig. 1A). Longer treatment with calcium, such as 96 h, showed slightly greater growth inhibition, but the difference was not significant (data not shown). The treatment of HT29 cells with calcium at 0.8, 1.6, and 2.4 mM for 48 h inhibited the cell growth by 9-15% (P < 0.05; Fig. 1B). Longer treatment with calcium, such as 72 h and 144 h, did not show greater effects than those found at the 48 h time point (data not shown). The calcium treatment of HCT116 cells at 0.8, 1.2 and 2 mM for 16 h decreased the cell attachment on the bottom of the culture plate by 12-26% (P < 0.001; Fig. 1C). In the transwell assay, the treatment of HCT116 cells with calcium at 0.4, 0.8, 1.2, 1.6, 2.0 mM concentration for 48 h decreased cell invasion by 15-31% (P < 0.05; Fig. 1D). In the scratch wound healing assay, the treatment with calcium at 0.8, 1.2, and 2.0 mM for 24 h (in the case of HCT116 cells) and 48 h (in the case of HT29 cells) inhibited cell migration by 19-61% (P < 0.05; Fig. 2A and B). In the calcium-treated cells, dying cells were observed around the edge of wounds, but not in the control cells (Fig. 2C).
Fig. 1.
Effects of the calcium treatment on cell growth, attachment and invasion in human colon cancer cells. Percentage of viable cells after the treatment of HCT116 cells with 0.8, 1.2, and 2.0 mM calcium for 72 h (A) and HT29 cells with 0.4, 0.8, 1.6 and 2.4 mM calcium for 48 h in serum complete medium (B). Percent of attached cells on the bottom of culture plate after the treatment of HCT116 cells with 0.8, 1.2, and 2.0 mM calcium in serum complete medium (C). Percentage of invaded cells to the outer chamber containing serum complete media from the inner chamber containing serum free media after the treatment of HCT116 cells with 0.4, 0.8, 1.2, 1.6 and 2.0 mM calcium for 48 h (D). The values shown are mean ± SE. Different letters (a-d) indicate statistical difference by one-way ANOVA followed by Tukey test (P < 0.05).
Fig. 2.
Effects of the calcium treatment on cell migration in human colon cancer cells. Percentage of wound closure after the treatment of HCT116 and HT29 cells with 0.8, 1.2, and 2.0 mM calcium for 24 h (in the case of HCT116 cells) and 48 h (in the case of HT29 cells) in serum complete medium (A). The values shown are mean ± SE. Different letters (a, b) indicate a statistical difference by one-way ANOVA followed by Tukey test (P < 0.05). Representative pictures of the wound closure in HT29 cells with or without 2.0 mM calcium for 48 h (B). Dying cells were observed around the edge of wound in calcium-treated HT29 cells at the 48 h time points, but not in the control cells (C).
Effect of calcium on the localization of β-catenin in human colon cancer cells
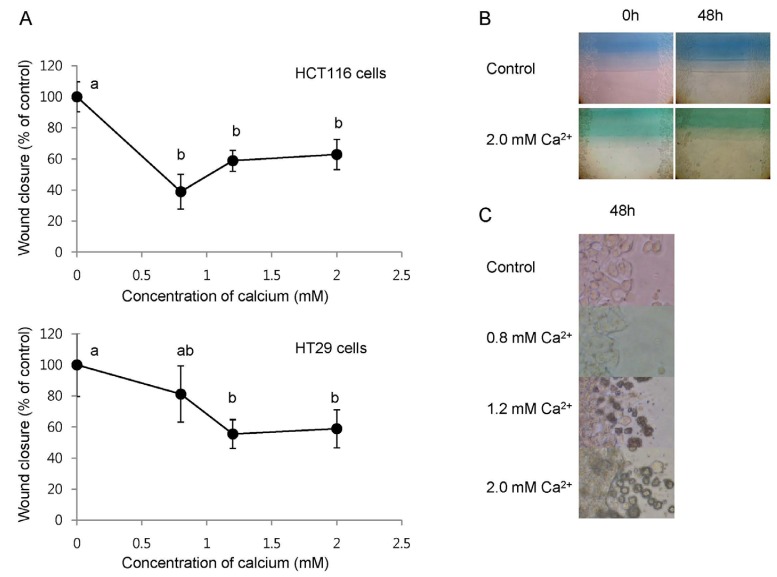
We examined the effects of calcium treatment on the localization of β-catenin in the HT29 cells. As shown in Fig. 3, β-catenin (red) was found in both nucleus (blue) and cytoplasm in the control cells. Treatment of cells with 1.6 mM calcium for 24 h, however, resulted in β-catenin localized predominantly in the plasma membrane and found in the cytoplasm.
Fig. 3.
Effects of the calcium treatment on the localization of β-catenin in HT29 human colon cancer cells. Fluorescence microscopy analyses of cells in the absence (A) or presence of 1.6 mM extracellular calcium for 24 h (B). The yellow boxes in the panels A and B were magnified and shown in the panels C and D, respectively. Red and blue immunofluorescences are due to fluorescein isothiocyanate- and 4V,6-diamidino-2-phenylindole-conjugated secondary antibodies used for the staining of β-catenin and nucleus, respectively. Results are representatives of two independent experiments with consistent results.
Effects of calcium treatment on serum calcium levels and intestinal tumor formation in ApcMin/+ mice
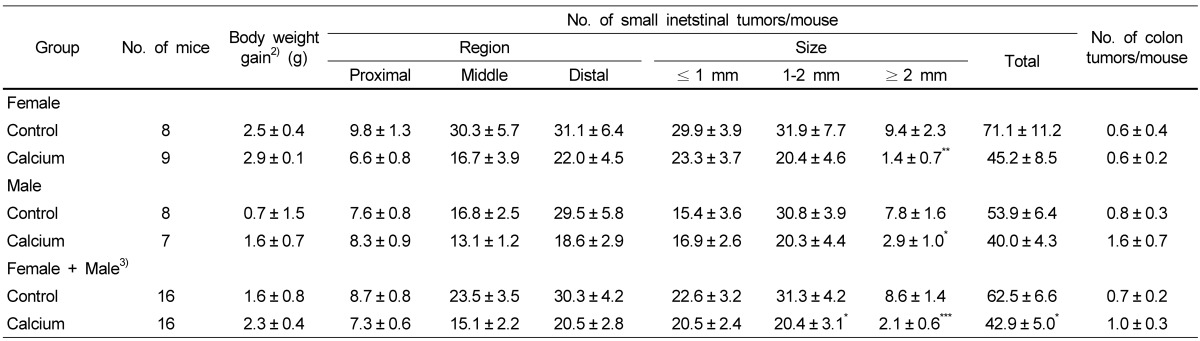
We conducted the animal study using the ApcMin/+ mouse model to determine whether the anti-cancer effects of calcium in vitro can be observed in vivo. Serum calcium levels in the control mice were approximately 1.9 mM (n = 6). As expected, the calcium treatment for 8 weeks did not change serum levels of calcium in mice (2.0 mM in calcium-treated mice, n = 6). As shown in Table 2, the calcium treatment for 8 weeks decreased total number of small intestinal tumors in both female (by 36.4%) and male (by 25.8%) ApcMin/+ mice. The calcium treatment resulted in a more prominent decrease in the number of large-size tumors (≥ 2 mm) than the number of small-size tumors (≤ 1 mm) in the small intestine; the multiplicity of the large-size tumors were decreased by 85.1% (P < 0.01) in female mice and 62.8% (P < 0.05) in male mice. These two datasets from male and female mice were analyzed by two-way ANOVA, and the results indicated that there was no effect on either the gender or interaction between gender and the calcium treatment on the tumor numbers. The male and female data, therefore, were combined and reanalyzed together. Calcium treatment resulted in significantly decreased total number of small intestinal tumors by 31.4% (P < 0.05). The largest decrease was in tumors with ≥ 2 mm in diameter, showing 75.6% inhibition of tumor multiplicity in the small intestine. Small intestinal tissues from male ApcMin/+ mice were subjected to histopathological analysis. As shown in Table 3, histologically identified adenomas containing ≥ 5 crypts (in 4 slides per colon) were 47% fewer in the calcium group compared to those in the control group (3.1 ± 1.0 versus 5.8 ± 1.3; P = 0.06 by one-tailed t-test).
Table 2.
Effect of calcium treatment on intestinal tumor formation in ApcMin/+ mice1)
1)ApcMin/+ mice at the age of 6 weeks were maintained on the control or calcium-enriched diet for 8 weeks. Each value represents mean ± SE.
2)Difference between final and initial body weights.
3)Two datasets from male and female mice were analyzed by two-way ANOVA, and results indicated that there was no effect on either the gender or the interaction between gender and calcium treatment on the tumor numbers. The male and female data were then combined and reanalyzed together.
*P < 0.05, **P < 0.01, ***P < 0.001: statistical difference from the value of control group in the column (two-tailed t-test).
Table 3.
Effect of calcium treatment on β-catenin localization in small intestinal adenomas1)
1)Each value in nuclear positivity represents mean ± SD. Each value in both cytoplasm and plasma membrane expression represents the numbers of adenomas scored to corresponding expression level (% of total numbers of adenomas analyzed per group).
2)Nuclear positivity or cytoplasmic expression of β-catenin in the adenomas from the calcium-treated adenomas differs to the correspondence from the control group by two-tailed t-test or chi-square test, respectively (P < 0.001).
3)Plasma membrane expression of β-catenin in the adenomas from the calcium-treated adenomas differs to that from the control group by chi-square test (P < 0.01).
The treatment with calcium did not influence body weight or the food and fluid intake. No noticeable signs of toxicity were observed in any of the groups.
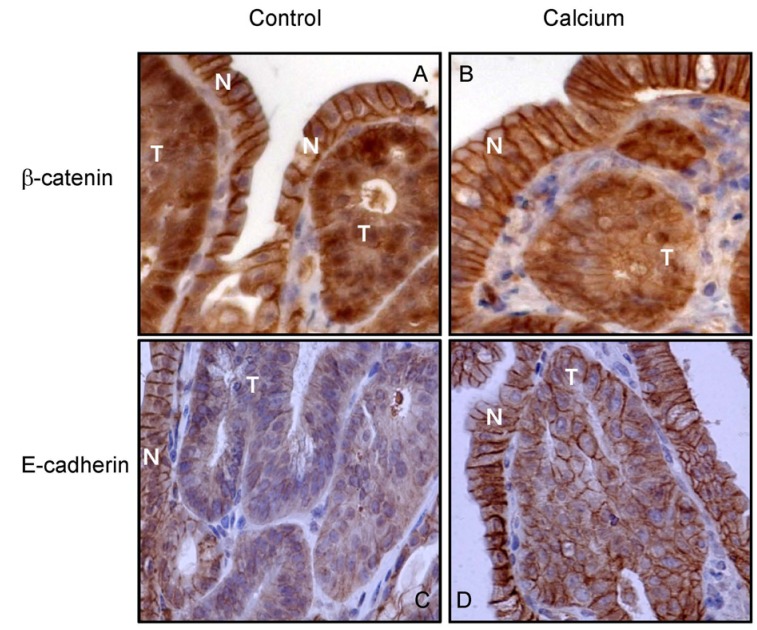
Effects of calcium on the localization of β-catenin and E-cadherin in small intestinal adenomas
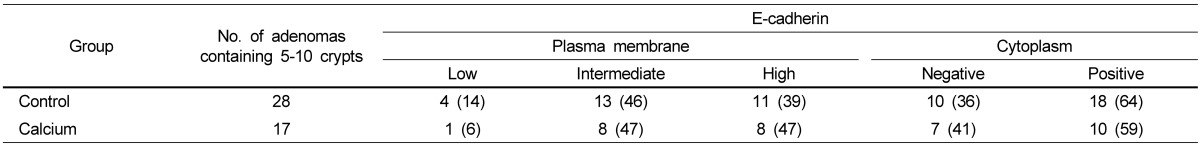
Among histologically identified adenomas, only those containing 5-10 crypts were analyzed for the localization of β-catenin and E-cadherin (Fig. 4). As previously reported [43,45], all adenomas from the control group had enhanced nuclear and cytoplasmic β-catenin staining but reduced membranous staining (Fig. 4A). Significantly reduced nuclear staining of β-catenin, as expressed in nuclear staining positivity, was observed in the adenomas from the groups treated with calcium (by 32.9%) in comparison to those from the control group (Fig. 4B, Table 3). In adenomas from the calcium-treated groups, the intensity of cytoplasmic staining was reduced to low expression levels (in 16 out of 17 adenomas; 94.1%), while membranous staining was increased to high expression levels (in 8 out of 17 adenomas; 47.1%; P < 0.01 by Chi-square test). Small intestinal adenomas from the control group appeared to have low levels of E-cadherin staining compared to normal epithelial cells (Fig. 4C). An increased level of the plasma membrane E-cadherin staining was observed in the adenomas from the calcium-treated groups in comparison to those from the control group (Fig. 4D). However, statistically significant changes in the localization of E-cadherin by calcium treatment were not found (Table 4).
Fig. 4.
Effects of the dietary calcium treatment for 8 weeks on the localization of β-catenin and E-cadherin in the small intestinal adenomas from ApcMin/+ mice. Strong plasma membrane staining of β-catenin was observed in the normal mucosa (indicated as "N") from both untreated control (A) and calcium-treated mice (B). Adenomas (indicated as "T") from the control mice showed strong nuclear and cytoplasmic β-catenin staining (A). Treatment with calcium reduced the level of nuclear staining but increased the level of membrane staining in the adenomas (B). Strong plasma membrane staining of E-cadherin was observed in normal mucosa from both untreated control (C) and calcium-treated mice (D). The treatment with calcium increased the plasma membrane staining levels of E-cadherin in adenomas (D).
Table 4.
Effect of calcium treatment on E-cadherin localization in small intestinal adenomas1)
1)Each value represents the number of adenomas scored to corresponding expression level (% of total number of adenomas analyzed per group).
Discussion
In the present study, we demonstrated the inhibitory effects of calcium against colon cancer both in vitro and in vivo. Previously, extracellular calcium has been shown to inhibit cell growth in CBS human colon cancer cells [36]. Our present results confirmed such a growth-inhibitory effect in other types of human colon cancer cells, HCT116 and HT29 (Fig. 1). Both HCT116 (bearing wild type APC and mutant β-catenin) and HT29 cells (APC-null and bearing wild-type β-catenin) represent aberrant β-catenin signaling, which is similar to the case of ApcMin/+ mice [40]. Based on this consideration, these two cell lines were selected for the present study. We also extended our findings to the inhibitory effects of calcium against cell attachment, invasion, and migration (Figs. 1 and 2). To our knowledge, this is the first study showing such inhibitory effects of calcium in human colon cancer cells. The effective concentration range of calcium was found to be 0.8-2.0 mM in our cell studies (Figs. 1 and 2). Since extracellular level of calcium is approximately 1.2 mM [46], and the serum calcium levels were approximately 2 mM in the control and the calcium-treated ApcMin/+ mice, the inhibitory effects of calcium in our cell studies appear to be physiologically relevant.
We also found that calcium treatment inhibited intestinal tumor formation in the ApcMin/+ mouse model (mainly in the small intestine; Table 2). The control diet was designed to mimic the human diet in the Western countries [32], containing high fat (20% fat; the mixture of corn oil, beef fat, lard, butterfat, soybean oil, and peanut oil) and low calcium (at a level of 1.4 mg/g diet). The calcium level in the control diet (1.4 mg/g diet) corresponds to the estimated average human intake of 600 mg calcium/day in the United States [47,48]. The calcium-enriched diet used in our study contained 5.2 mg calcium/g diet, corresponding to a human intake of 2,200 mg calcium/day, which is below the upper limit of the current Dietary Reference Intake of 2,500 mg calcium/day [49]. Our study has significant public health implications in that the treatment with dietary calcium at a nutritional level effectively inhibited intestinal tumorigenesis in vivo.
Translocation of β-catenin from the plasma membrane to the nucleus of cells is a key event in colon carcinogenesis that results in increased transcription of genes involved with cell proliferation [34,35]. Calcium treatment has been shown to increase E-cadherin levels and suppress subsequent β-catenin signaling in cell culture systems [36-39]; however such effects in vivo have never been reported. In the present study, we found that calcium treatment resulted in decreased nuclear β-catenin levels, but increased plasma membrane β-catenin levels in vitro and in vivo (Figs. 3 and 4, Table 3). To our knowledge, this is the first report that treatments with dietary calcium, at nutritional level, altered the localization of β-catenin in intestinal adenomas in vivo. E-cadherin functions to mediate epithelial cell-cell adhesion by interacting with β-catenin at the plasma membrane [33]. Our results from immunohistochemical analysis suggest that dietary calcium treatment may increase the plasma membrane levels of E-cadherin in vivo (Fig. 4). Calcium sensing receptor (CaSR) has been also shown to be involved in calcium-induced E-cadherin expression, E-cadherin and β-catenin complex, and suppression of subsequent events such as β-catenin signaling in vitro [36-38]. CaSR might be, therefore, involved in the observed alteration of β-catenin localization by the calcium treatment in vivo. Further mechanistic studies on the upstream and downstream events of the altered β-catenin localization by the calcium treatment are needed in the future.
In summary, the present study showed that calcium treatment inhibited not only cell growth, attachment, invasion, and migration in the human colon cancer cell culture system but also intestinal tumor formation in the ApcMin/+ mouse model. We also demonstrated that the dietary calcium treatments altered the localization of β-catenin both in vitro and in vivo. More studies are needed to better understand the mechanisms of the inhibitory action of calcium against colorectal carcinogenesis.
Footnotes
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (No. 2010-0025311).
References
- 1.Boyle P, Levin B. World Cancer Report 2008. Geneva: WHO Press; 2008. pp. 11–55. [Google Scholar]
- 2.McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004;23:6349–6364. doi: 10.1038/sj.onc.1207716. [DOI] [PubMed] [Google Scholar]
- 3.Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. 2007;22:286–296. doi: 10.1177/0115426507022003286. [DOI] [PubMed] [Google Scholar]
- 4.Chia V, Newcomb PA. Calcium and colorectal cancer: some questions remain. Nutr Rev. 2004;62:115–120. doi: 10.1111/j.1753-4887.2004.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 5.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 6.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–219. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 7.Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32:789–803. doi: 10.1016/j.clinthera.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, Greenberg ER Calcium Polyp Prevention Study Group. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 9.Wallace K, Baron JA, Cole BF, Sandler RS, Karagas MR, Beach MA, Haile RW, Burke CA, Pearson LH, Mandel JS, Rothstein R, Snover DC. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. 2004;96:921–925. doi: 10.1093/jnci/djh165. [DOI] [PubMed] [Google Scholar]
- 10.Kenar L, Karayilanoglu T, Aydin A, Serdar M, Kose S, Erbil MK. Protective effects of diets supplemented with omega-3 polyunsaturated fatty acids and calcium against colorectal tumor formation. Dig Dis Sci. 2008;53:2177–2182. doi: 10.1007/s10620-007-0107-8. [DOI] [PubMed] [Google Scholar]
- 11.Belbraouet S, Felden F, Pelletier X, Gastin I, Lambert D, Floquet J, Guéant JL, Debry G. Dietary calcium salts as protective agents and laminin P1 as a biochemical marker in chemically induced colon carcinogenesis in rats. Cancer Detect Prev. 1996;20:294–299. [PubMed] [Google Scholar]
- 12.Ranhotra GS, Gelroth JA, Glaser BK, Schoening P, Brown SE. Cellulose and calcium lower the incidence of chemically-induced colon tumors in rats. Plant Foods Hum Nutr. 1999;54:295–303. doi: 10.1023/a:1008149107282. [DOI] [PubMed] [Google Scholar]
- 13.Pence BC, Dunn DM, Zhao C, Landers M, Wargovich MJ. Chemopreventive effects of calcium but not aspirin supplementation in cholic acid-promoted colon carcinogenesis: correlation with intermediate endpoints. Carcinogenesis. 1995;16:757–765. doi: 10.1093/carcin/16.4.757. [DOI] [PubMed] [Google Scholar]
- 14.Viñas-Salas J, Biendicho-Palau P, Piñol-Felis C, Miguelsanz-Garcia S, Perez-Holanda S. Calcium inhibits colon carcinogenesis in an experimental model in the rat. Eur J Cancer. 1998;34:1941–1945. doi: 10.1016/s0959-8049(98)00197-x. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi C, Oredipe OA, Barth RF, Downie AA, Webb TE. Effects of the experimental chemopreventative agent, glucarate, on intestinal carcinogenesis in rats. Carcinogenesis. 1989;10:1539–1541. doi: 10.1093/carcin/10.8.1539. [DOI] [PubMed] [Google Scholar]
- 16.Wargovich MJ, Allnutt D, Palmer C, Anaya P, Stephens LC. Inhibition of the promotional phase of azoxymethane-induced colon carcinogenesis in the F344 rat by calcium lactate: effect of simulating two human nutrient density levels. Cancer Lett. 1990;53:17–25. doi: 10.1016/0304-3835(90)90005-i. [DOI] [PubMed] [Google Scholar]
- 17.Karkare MR, Clark TD, Glauert HP. Effect of dietary calcium on colon carcinogenesis induced by a single injection of 1,2-dimethylhydrazine in rats. J Nutr. 1991;121:568–577. doi: 10.1093/jn/121.4.568. [DOI] [PubMed] [Google Scholar]
- 18.Adell-Carceller R, Segarra-Soria M, Gibert-Jerez J, Salvador Sanchís JL, Lázaro-Santander R, Escrig-Sos J, Ruiz-Castillo J. Inhibitory effect of calcium on carcinogenesis at the site of colonic anastomosis: an experimental study. Dis Colon Rectum. 1997;40:1376–1381. doi: 10.1007/BF02050826. [DOI] [PubMed] [Google Scholar]
- 19.Pereira MA, Barnes LH, Rassman VL, Kelloff GV, Steele VE. Use of azoxymethane-induced foci of aberrant crypts in rat colon to identify potential cancer chemopreventive agents. Carcinogenesis. 1994;15:1049–1054. doi: 10.1093/carcin/15.5.1049. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Tomotake H, Wan G, Watanabe H, Kato N. Combined effect of dietary calcium and iron on colonic aberrant crypt foci, cell proliferation and apoptosis, and fecal bile acids in 1,2-dimethylhydrazine-treated rats. Oncol Rep. 2001;8:893–897. doi: 10.3892/or.8.4.893. [DOI] [PubMed] [Google Scholar]
- 21.Mølck AM, Poulsen M, Meyer O. The combination of 1alpha,25 (OH2)-vitamin D3, calcium and acetylsalicylic acid affects azoxymethane-induced aberrant crypt foci and colorectal tumours in rats. Cancer Lett. 2002;186:19–28. doi: 10.1016/s0304-3835(02)00285-9. [DOI] [PubMed] [Google Scholar]
- 22.Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, Price R, Gray K, Kelloff GJ. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21:1149–1155. [PubMed] [Google Scholar]
- 23.Li H, Kramer PM, Lubet RA, Steele VE, Kelloff GJ, Pereira MA. Effect of calcium on azoxymethane-induced aberrant crypt foci and cell proliferation in the colon of rats. Cancer Lett. 1998;124:39–46. doi: 10.1016/s0304-3835(97)00453-9. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RL, Tanure JC, Andrianopoulos G. The effect of dietary milk and calcium on experimental colorectal carcinogenesis. Dis Colon Rectum. 1987;30:947–949. doi: 10.1007/BF02554282. [DOI] [PubMed] [Google Scholar]
- 25.Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51:5608–5613. [PubMed] [Google Scholar]
- 26.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123:144–152. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 27.Pence BC, Dunn DM, Zhao C, Patel V, Hunter S, Landers M. Protective effects of calcium from nonfat dried milk against colon carcinogenesis in rats. Nutr Cancer. 1996;25:35–45. doi: 10.1080/01635589609514426. [DOI] [PubMed] [Google Scholar]
- 28.Huerta S, Irwin RW, Heber D, Go VL, Moatamed F, Huerta S, Ou C, Harris DM. Intestinal polyp formation in the Apcmin mouse: effects of levels of dietary calcium and altered vitamin D homeostasis. Dig Dis Sci. 2003;48:870–876. doi: 10.1023/a:1023083025595. [DOI] [PubMed] [Google Scholar]
- 29.Ding S, McEntee MF, Whelan J, Zemel M. Adiposity-related protection of intestinal tumorigenesis: interaction with dietary calcium. Nutr Cancer. 2007;58:153–161. doi: 10.1080/01635580701328248. [DOI] [PubMed] [Google Scholar]
- 30.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 31.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Ju J, Xiao H, Simi B, Hao X, Reddy BS, Huang MT, Newmark H, Yang CS. Effects of combination of calcium and aspirin on azoxymethane-induced aberrant crypt foci formation in the colons of mice and rats. Nutr Cancer. 2008;60:660–665. doi: 10.1080/01635580802290215. [DOI] [PubMed] [Google Scholar]
- 33.Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001;439:725–751. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- 34.Hao X, Frayling IM, Willcocks TC, Han W, Tomlinson IP, Pignatelli MN, Pretlow TP, Talbot IC. Beta-catenin expression and allelic loss at APC in sporadic colorectal carcinogenesis. Virchows Arch. 2002;440:362–366. doi: 10.1007/s00428-001-0570-0. [DOI] [PubMed] [Google Scholar]
- 35.Hao X, Palazzo JP, Ilyas M, Tomlinson I, Talbot IC. Reduced expression of molecules of the cadherin/catenin complex in the transition from colorectal adenoma to carcinoma. Anticancer Res. 1997;17:2241–2247. [PubMed] [Google Scholar]
- 36.Bhagavathula N, Hanosh AW, Nerusu KC, Appelman H, Chakrabarty S, Varani J. Regulation of E-cadherin and beta-catenin by Ca2+ in colon carcinoma is dependent on calcium-sensing receptor expression and function. Int J Cancer. 2007;121:1455–1462. doi: 10.1002/ijc.22858. [DOI] [PubMed] [Google Scholar]
- 37.Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J Biol Chem. 2008;283:3519–3528. doi: 10.1074/jbc.M708318200. [DOI] [PubMed] [Google Scholar]
- 38.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 39.Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006;66:11370–11380. doi: 10.1158/0008-5472.CAN-06-2106. [DOI] [PubMed] [Google Scholar]
- 40.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 41.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 42.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 43.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 44.Sang S, Ju J, Lambert JD, Lin Y, Hong J, Bose M, Wang S, Bai N, He K, Reddy BS, Ho CT, Li F, Yang CS. Wheat bran oil and its fractions inhibit human colon cancer cell growth and intestinal tumorigenesis in Apc(min/+) mice. J Agric Food Chem. 2006;54:9792–9797. doi: 10.1021/jf0620665. [DOI] [PubMed] [Google Scholar]
- 45.Hao X, Sun Y, Yang CS, Bose M, Lambert JD, Ju J, Lu G, Lee MJ, Park S, Husain A, Wang S. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–69. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]
- 46.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 47.Newmark HL, Heaney RP, Lachance PA. Should calcium and vitamin D be added to the current enrichment program for cereal-grain products? Am J Clin Nutr. 2004;80:264–270. doi: 10.1093/ajcn/80.2.264. [DOI] [PubMed] [Google Scholar]
- 48.Newmark HL. Nutrient density: an important and useful tool for laboratory animal studies. Carcinogenesis. 1987;8:871–873. doi: 10.1093/carcin/8.7.871. [DOI] [PubMed] [Google Scholar]
- 49.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, D.C: National Academy Press; 1997. [Google Scholar]