Abstract
Several studies revealed that low calcium intake is related to high prevalence of cardiovascular diseases such as hypertension. The prevalence of hypertension is high in Koreans along with their low dietary calcium consumption. Thus, the aim of this study was to evaluate the status of calcium intake between the hypertension and normotension groups and to investigate the correlation between dietary calcium intake and blood pressure, blood lipid parameters, and blood/urine oxidative stress indices. A total of 166 adult subjects participated in this study and were assigned to one of two study groups: a hypertension group (n = 83) who had 140 mmHg or higher in systolic blood pressure (SBP) or 90 mmHg or higher in diastolic blood pressure (DBP), and an age- and sex-matched normotension group (n = 83, 120 mmHg or less SBP and 80 mmHg or less DBP). The hypertension group consumed 360.5 mg calcium per day, which was lower than that of the normotension group (429.9 mg) but not showing significant difference. In the hypertension group, DBP had a significant negative correlation with plant calcium (P < 0.01) after adjusting for age, gender, body mass index (BMI), and energy intake. In the normotension group, total calcium and animal calcium intake were significantly and positively correlated with serum triglycerides. No significant relationship was found between calcium intake and blood/urine oxidative stress indices in both groups. Overall, these data suggest reconsideration of food sources for calcium consumption in management of the blood pressure or blood lipid profiles in both hypertensive and normotensive subjects.
Keywords: Dietary calcium intake, hypertension, oxidative stress indices, plant calcium
Introduction
The relationship between calcium intake and cardiovascular diseases (CVD) has been explored through numerous molecular, animal, and human studies for several decades. An epidemiological study showed that residents who consumed hard water which was rich in calcium and magnesium had a lower mortality rate caused by circulatory diseases than those who had consumed soft water [1]. This finding suggested that the intake of calcium might be related to cardiovascular diseases. Also, several systematic reviews revealed that calcium supplementation reduced systolic blood pressure (SBP), particularly among hypertensive subjects but not among normotensive subjects [2-6]. A reduction of serum total cholesterol has been reported in hypercholesterolemic subjects given 1 g of calcium per day for 8 weeks [7,8]. More recent studies showed that 1 g/day of calcium supplementation for one year increased the level of HDL-cholesterol and decreased the level of LDL-cholesterol [9,10].
In addition, some observational studies reported that low calcium intake was associated with increased risk of CVD. The Iowa Women Health Study found that Caucasian women aged 55-69 years with low calcium intake (< 696 mg/day) had a high mortality rate due to cardiovascular heart disease (RR 1.58, 95% CI 1.02-2.50) compared to those with high calcium intake (> 1,425 mg/day) [11]. Also, Wang et al. [12] found a higher rate of hypertension (RR 1.12, 95% CI 1.05-1.20) among women aged over 45 years consuming low calcium (< 558 mg/day) compared to those with high calcium consumption (1,679 mg/day). Other cohort studies reported the association between low calcium intake and increased risk of hypertension [13,14]. Meanwhile, several studies reported that calcium intake had an association with oxidative stress and suggested that calcium supplementation might increase the lipid oxidation [15,16]. As the pathogenesis of hypertension is often associated with oxidative stress, the relationships among calcium intake, blood pressure, and oxidative stress status should be investigated.
Traditionally, the Korean diet has been highly dependent on plant based foods, such as cereals, pulses, roots and various vegetables, now known for having many healthy benefits. Despite many healthy benefits of plant oriented foods, limited consumption in dairy and animal foods in Korean diet relative to western diet has been concerned for insufficient calcium. According to the recent Korean National Health and Nutrition Examination Survey (KNHANES) 2010, the mean daily calcium intake of Korean adults aged 19 and older was 535.2 mg, which was 75.6% of the Korean recommended level. Also, 64.5% of these adults consumed calcium under estimated average requirement (EAR) [17]. This national data indicate that the average calcium intake of Korean adults is within the range of high CVD related mortality risk or high prevalence of hypertension as reported by the previous researches [11,12].
Furthermore, the prevalence of hypertension in Korean adults (≥ 20 years) is 28.9% (30.1% for males, 27.7% for females) [17]. This is slightly lower than the prevalence of hypertension for Americans (31.4%-32.1% for males, 32.8% for females) [18]. But considering the significant difference in the prevalence of overweight and obesity between US adults (BMI ≥ 25; males 72.8%, females 63.0%) and Korean adults (BMI ≥ 25; males 36.5%, females 26.4%), the prevalence of hypertension in Korea seems to be very high. Based on these concerns, a study to investigate the calcium intake status among Korean adults with or without hypertension and its relation to blood pressure and CVD risk-related parameters is needed.
Therefore, this study was carried out for the following two aims: first, to evaluate the calcium intake status between the hypertension and normotension groups; and second, to investigate the correlation between dietary calcium intake and blood pressure, blood lipid parameters, and blood/urine oxidative stress indices in the hypertension and normotension groups.
Subjects and Methods
Participants
Initially, Korean adults were recruited for this study. Study participants agreed to provide their personal information regarding the purpose and the procedures of the project. Blood pressure was measured twice in seated posture by using a mercury sphygmomanometer, and the two measurements were averaged for systolic and diastolic pressures. Eighty-three subjects with 140 mmHg or higher in SBP or 90 mmHg or higher in diastolic blood pressure (DBP) were assigned to a hypertension group according to JNC-VII guidelines [19]. Participants with 120 mmHg or less SBP and 80 mmHg or less DBP were assigned to a normotension group. Each group was consisted of a similar percentage of subjects in age and gender (42 hypertensive men vs. 41 normotensive men). This study was approved by the College of Medicine, Chung-Ang University Institutional Review Board (IRB), and written informed consent was obtained from each subjects.
Anthropometric measurements
Height was measured using a standard stadiometer following study protocols, and weight in kilograms was measured on a calibrated Inbody (Biospace, Seoul, Korea) system. Measurements were repeated at least two times for each subject and BMI was calculated as weight in kilograms divided by height in meters, squared.
Dietary intake survey
The dietary intake survey was conducted by a 24-hour recall method for 3 days. Study subjects in each group were interviewed by trained research staff to examine the types and amounts of all foods that the subjects had consumed in the previous 3 days. Food models and photographs were used to assist in estimating serving sizes of foods. Dietary intake of calcium and other nutrients were analyzed by using Can-Pro 3.0 (The Korean Nutrition Society, Seoul, Korea).
Collection of blood and urine samples
The subjects fasted overnight after the dietary intake survey, and then 15 ml of venous blood was collected - half volume in an evacuated blood collection tube and the other half in an EDTA-coated blood collection tube. In the evacuated blood collection tube, serum was separated by centrifugation at × 400 g for 15 minutes. Blood samples collected in the EDTA-coated blood collection tube were stored at -20℃ until a test was performed. Spot urine samples were collected and aliquoted for analysis of multiple indices and kept in a freezer at -20℃.
Analysis of biochemical indices
Serum triglyceride, total cholesterol, glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) were analyzed by using a commercial kit based on enzymatic principles, and the content of HDL-cholesterol was analyzed by the colorimetric method after separating LDL and VLDL by using a dextran sulfate-Mg2+ sedimentation [20]. Serum LDL-cholesterol was calculated by the Friedewald formula [21] by utilizing triglyceride, total cholesterol, and HDL-cholesterol values.
Glutathione (GSH) in red blood cells was analyzed by using a modification of van Klaveren's method [22]. After centrifugation of the blood samples at × 2,500 g and 4℃, red blood cells were hemolyzed by adding Millipore grade water. Tricholoroacetic acid (TCA) was added to the hemolyzed sample, and centrifuged at × 10,000 g for 5 minutes. Supernatants were subjected to reaction buffer (0.248 mg/ml NADPH in 143 mM sodium phosphate, 6.3 mM Na4-EDTA at pH 7.5, 6 mM DTNB) with glutathione disulfide reductase (5 U/ml) for 20 minutes at 35℃ and the absorbance was measured in 412 nm by spectrophotometer (UVIKON, Kontron Inc., Milan, Italy). The amount of GSH in red blood cells was indicated as µmol/g hemoglobin (Hb). Glutathione peroxidase (GPx) activity in the red blood cells was analyzed by using methods by Aydin et al. [23]. Regarding GPx, absorbance was measured in 340 nm for 3 minutes using a spectrophotometer (UVIKON, Kontron Inc., Milan, Italy) by adding 8.8 mmol/l H2O2 after 50 mM Tris buffer (pH 7.6), 1 mM Na2EDTA, 2 mM NADPH, 4 mM sodium azide, and 1 unit/ml glutathione reductase, which were reacted with the 20 ul hemolyzed sample at 37℃ for 5 minutes. GPx activity in red blood cells was indicated as U/g Hb.
Urinary lipid peroxidation was analyzed according to the manual of Tagesson et al. [24]. Briefly, 50 µl urine samples were mixed with 23 mM thiobarbituric acid reagent, 0.5 M phosphoric acid 300 µl and then heated at 95℃ for one hour and then cooled down. After adding 100 µl methanol, samples were subjected to HPLC (501 Waters, Messachusetts, USA) for thiobarbituric acid reactive substance (TBARS) analysis. Urinary malondialdehyde (MDA) concentration in urine aliquot was indicated as µmol/g creatinine (Cr).
Statistical analysis
Statistical analyses including the mean and standard deviation values of all results were performed using the SAS program (version 9.1; SAS Institute Inc, Cary, NC, USA). Student's unpaired t-test was used to analyze the differences between the hypertension group and the normotension group and between men and women. Pearson's partial correlation coefficient was used to determine an association between variables and to verify significance. All statistical tests were two-sided, and value of P < 0.05 was considered statistically significant.
Results
General characteristics
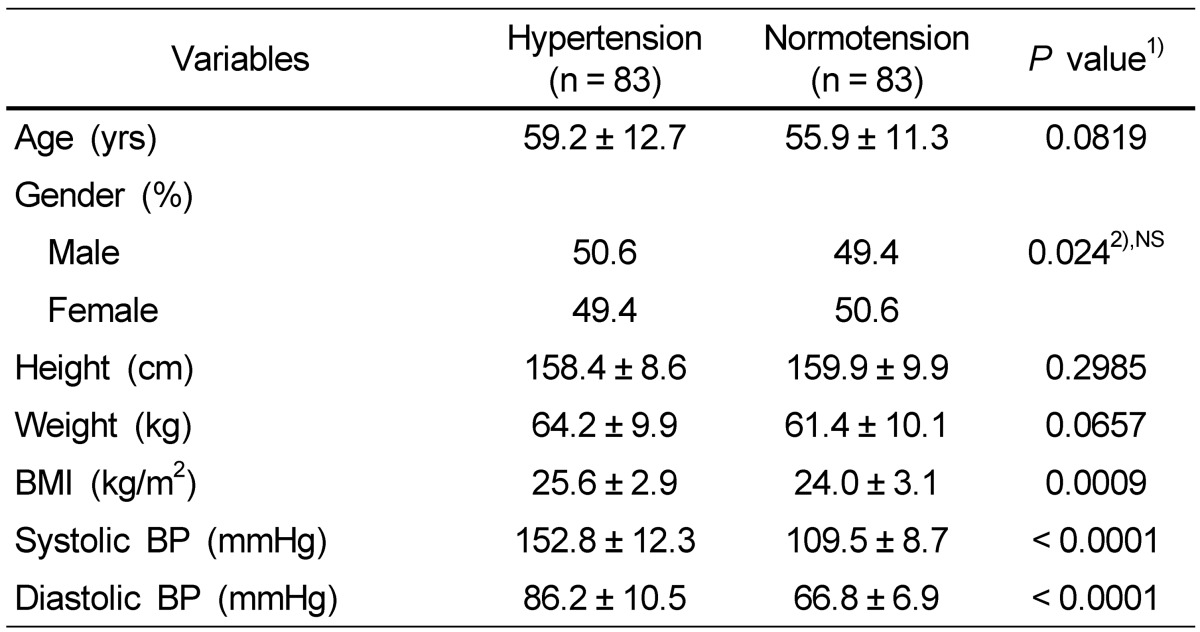
The general characteristics of the hypertension group and the normotension group are presented in Table 1. There were no significant differences in age, height, and weight between the hypertension and normotension groups; however, the hypertension group had significantly higher BMI (P < 0.001), SBP (P < 0.001), and DBP (P < 0.001) than the normotension group.
Table 1.
Anthropometric measurements of the subjects
Data are presented as mean ± SD.
NS, not significant.
1)Significance between hypertensive and normotensive subjects.
2)F-value by χ2-test.
Energy, selective nutrients, and calcium intakes
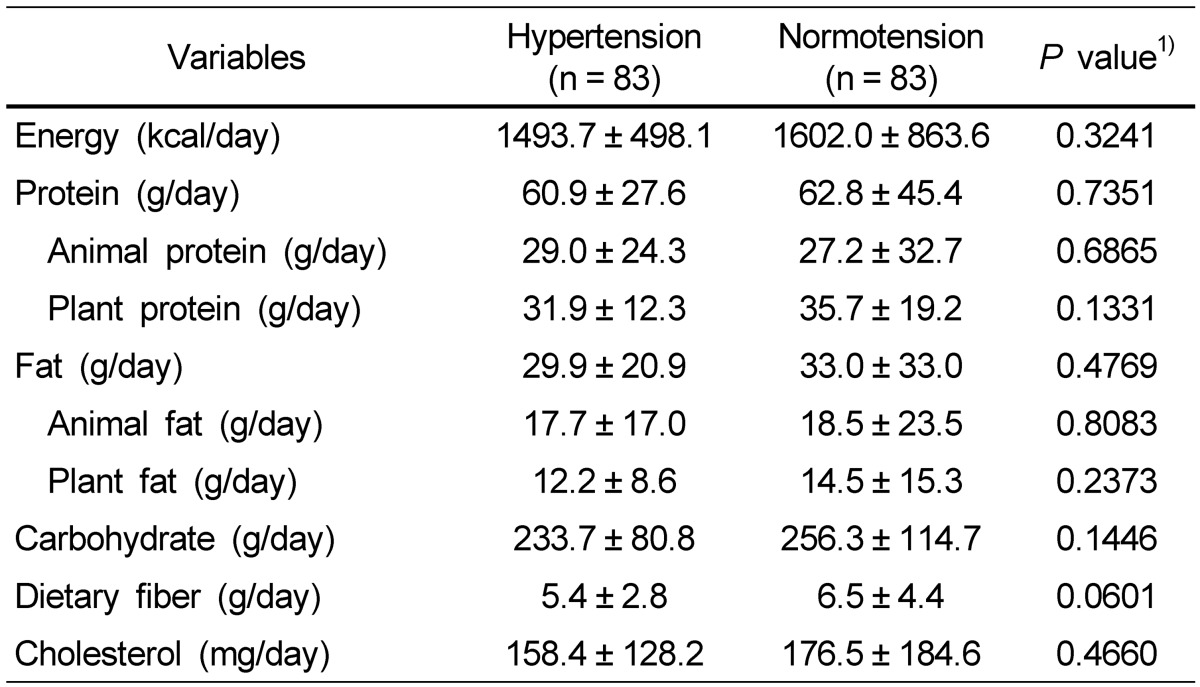
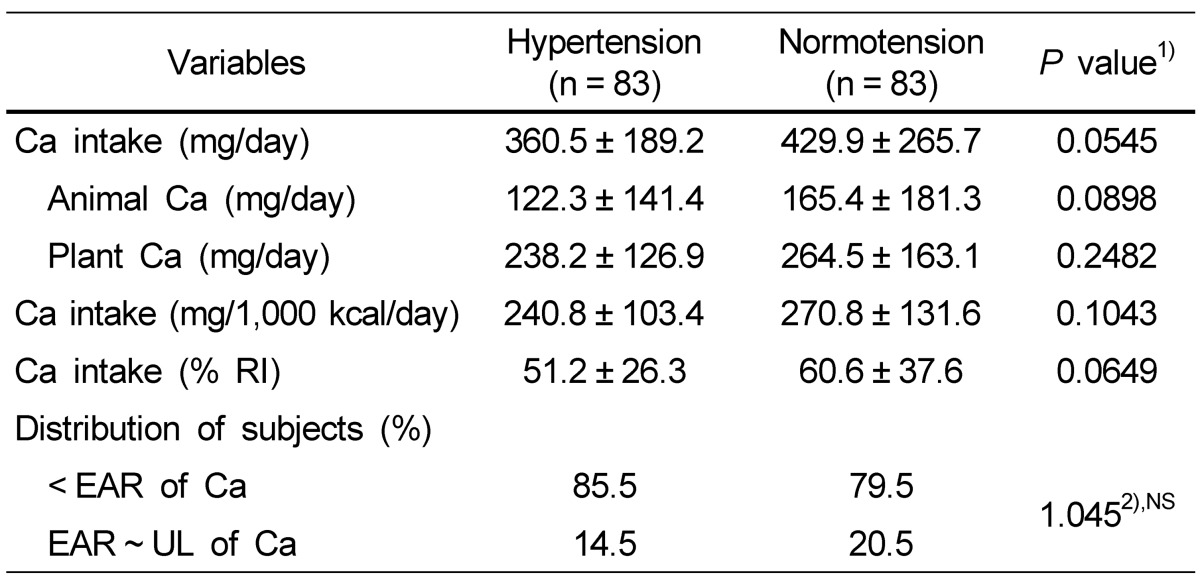
Energy and selective nutrient intake of the subjects is shown in Table 2. The average daily energy intake was 1,493.7 kcal for the hypertension group and 1,602.0 kcal for the normotension group without showing a significant difference between two groups. The hypertension group consumed 360.5 mg calcium per day, which was lower than that of the normotension group (429.9 mg), but there was no significant difference. The percentage of people who consumed calcium less than EAR for Koreans was higher in the hypertension group (85.5%) compared with that in the normotension group (79.5%) but with no statistical difference between the two groups. Also, the mean daily intake of animal calcium and plant calcium, the calcium intake per 1,000 kcal, and the calcium percentage for Recommended Intake (RI) tended to be lower in the hypertension group (Table 3).
Table 2.
Daily energy and nutrient intakes of the subjects
Data are presented as mean ± SD.
1)Significance between hypertensive and normotensive subjects.
2)F-value by χ2-test.
Table 3.
Daily calcium intake status of the subjects
Data are presented as mean ± SD.
NS, not significant; EAR, estimated average requirement; UL, tolerable upper intake level.
1)Significance between hypertensive and normotensive subjects.
2)F-value by χ2-test.
Food group intake status
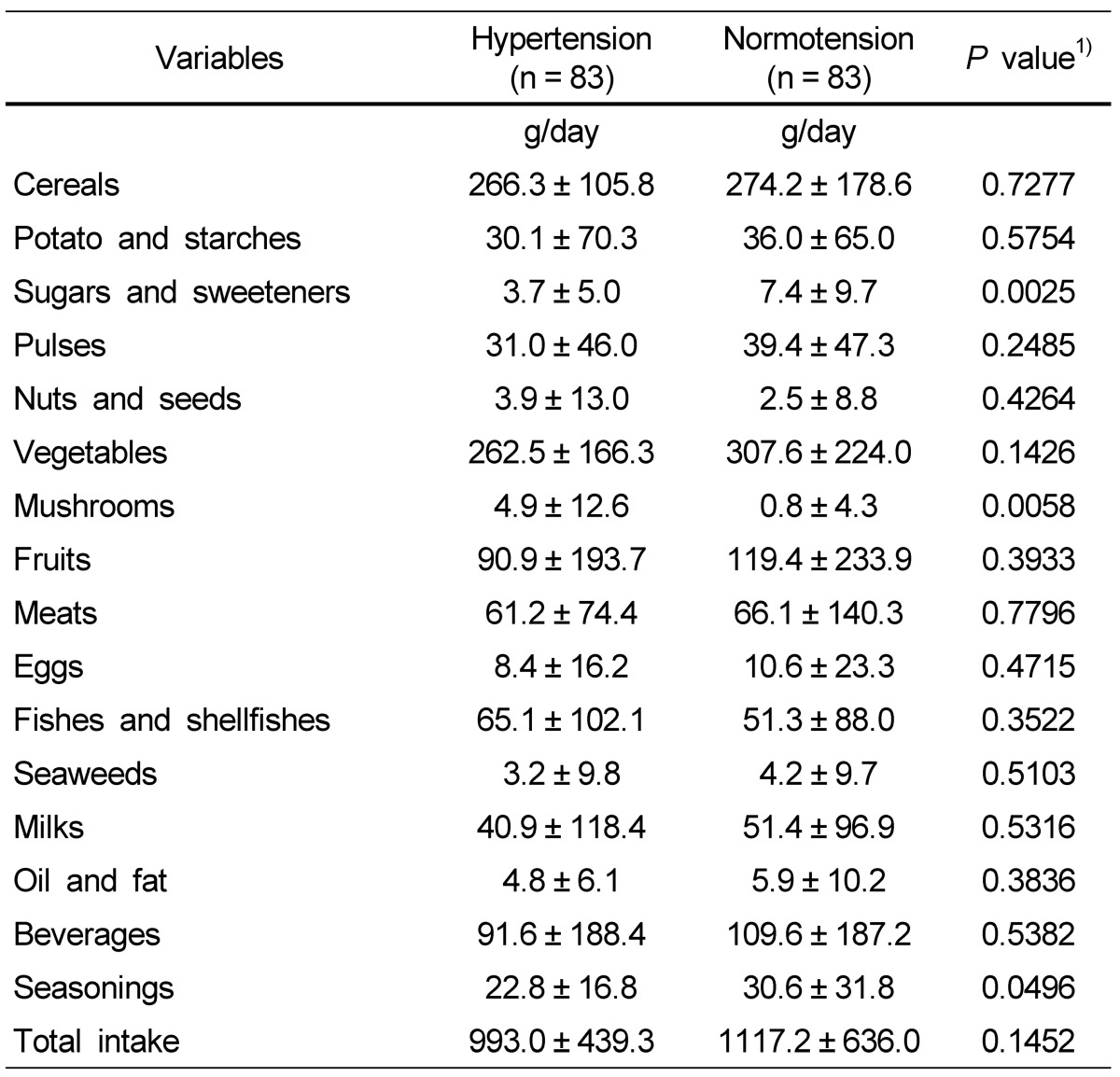
Food intake from different food groups is shown in Table 4. The total daily food intake was 993.0 g for the hypertension group and 1,117.2 g for the normotension group, not showing a significant difference. The intakes of sugar/sweeteners and seasonings were significantly higher in the normotension group, while the intake of mushrooms was higher in the hypertension group. Except for these three food groups, no significant difference was found in consumption of the other food groups between the hypertension and normotension groups.
Table 4.
Daily food intakes from each food group of the subjects
Data are presented as mean ± SD.
1)Significance between hypertensive and normotensive subjects.
Biochemical indices in blood and urine
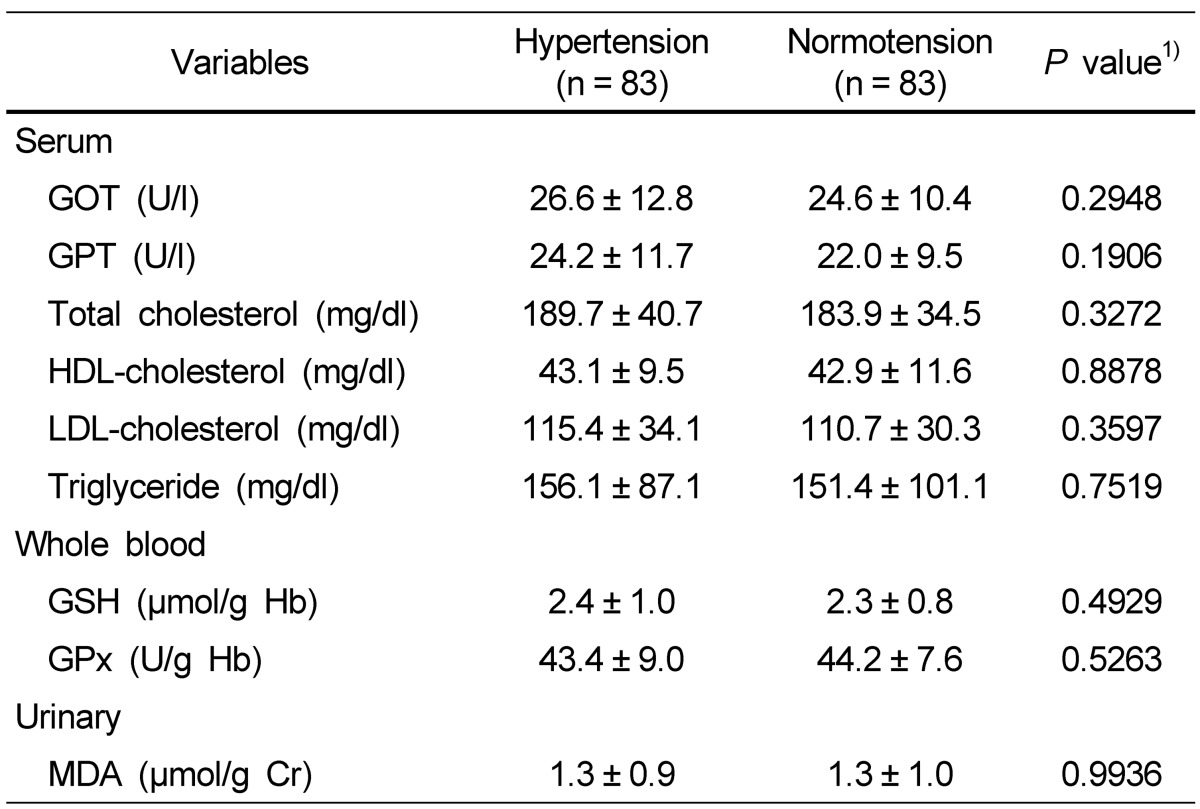
Biochemical indices in blood and urine of the subjects are described in Table 5. No significant difference was found in blood and urine biochemical indices between the hypertension and normotension groups.
Table 5.
Biochemical indices in blood and urine of the subjects
Data are presented as mean ± SD.
NS, not significant.
1)Significance between hypertensive and normotensive subjects.
Correlation between calcium intake, and blood pressure and biochemical indices
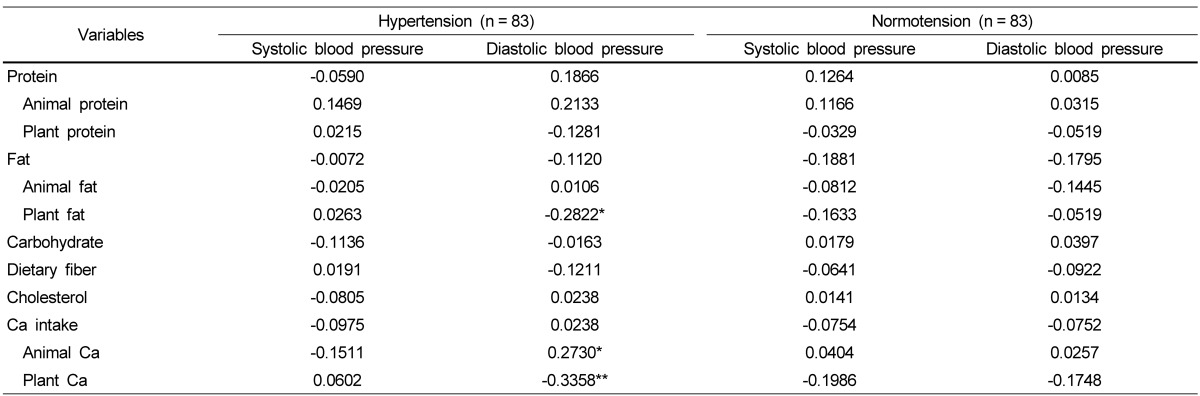
Table 6 shows the correlation between blood pressure and nutrient intake in both groups after adjusting for age, gender, BMI, and energy intake. In the hypertension group, DBP had a significant negative correlation with plant fat (P < 0.05) and plant calcium (P < 0.01). In the normotension group, however, no significant correlation was found between blood pressure and nutrient intake.
Table 6.
Correlations between blood pressure and nutrient intakes adjusted for age, sex, BMI, and energy intake of the subjects
Variables data are presented as Pearson's correlation coefficient.
*P < 0.05; **P < 0.01.
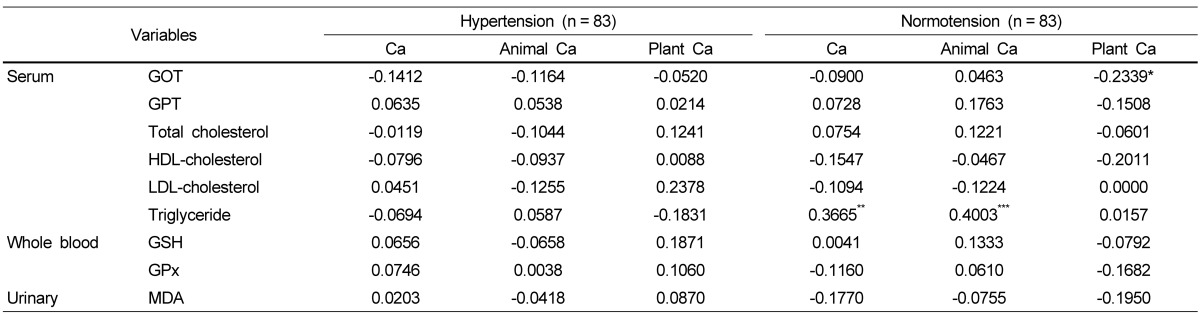
The correlations between biochemical indices and nutrient intake after adjusting for age, gender, BMI, and energy intake are shown in Table 7. No significant difference was found between biochemical indices and nutrient intake in the hypertension group, but in the normotension group, serum triglyceride was significantly and positively correlated with total calcium intake and animal calcium intake.
Table 7.
Correlations between calcium intake and blood parameters adjusted for age, sex, BMI, and energy intake of the subjects
Variables data are presented as Pearson's correlation coefficient.
*P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The present study examined the calcium intake status and the relation between dietary calcium intake and blood pressure, blood lipid profiles, and blood/urine oxidative stress indices in Korean adults with or without hypertension. The major finding was that the calcium intake of both groups was low and was mainly derived from plant foods. Also, the plant food-derived calcium intake was significantly and negatively correlated with DBP in the hypertension group but not in the normotension group.
Dietary calcium intake of the hypertension group tended to be lower than that of the normotension group, and the calcium intake level of both groups is considered quite insufficient. There are two possible reasons for the low calcium intake of the subjects. First, the overall food intake was low, which means a low daily energy intake. The amount of energy intake in both the hypertension and normotension groups was just 1,493.7 kcal and 1,602.0 kcal, respectively. Additionally, calcium intake relative to energy intake of 1,000 kcal within both hypertension and normotension groups was still below the recommended intake [25]. The second possible reason for low calcium intake in study subjects was the low intake of main food sources for calcium, particularly milk and dairy products. According to the KNHANES [17], even though the top ranked food source for calcium intake in Koreans was milk, the average amount of milk consumed by Korean adults was 74.0 g per day, which corresponds to only one third of a small carton (~200 ml) of milk. In the current study, the milk consumption of the study subjects was even less, showing 40.9 g for the hypertension group and 51.4 g for the normotension group. Considering the fact that 100 g of milk contains about 100 mg of calcium, the subjects consumed only 50 mg of calcium from their daily milk consumption. In addition to milk, small bony fishes such as anchovies are an important food source for supplying calcium in the average Korean diet, but the portion size is very small in relative to other side-dishes typically found in the Korean diet and therefore may not play a significant role in increasing calcium consumption. Also, the low intake of calcium derived from animal-based foods, such as milk and small bony fish, compared with relatively high intake of calcium derived from plant foods, which have low bioavailability, in both the hypertension and normotension groups may be indicative of the overall poor calcium status in these study subjects.
Many epidemiological studies have reported results showing the relationship between calcium intake and blood pressure [2,26,27]. For instance, the amount of calcium intake was negatively correlated with high blood pressure, many hypertension patients had a low calcium intake in their diet [2,27], and the supplementation of calcium-rich foods decreased blood pressure [26]. These findings indicate that calcium consumption is beneficial for prevention and treatment of hypertension. In the present study, diastolic blood pressure was significantly and negatively correlated with plant calcium consumption in the hypertension subjects but not in the normotension subjects. Some possible explanations for the association of calcium intake with reducing the risk of hypertension have been suggested by several experimental studies [28-34]. Calcium is involved in regulating blood pressure by controlling vascular smooth muscle cell contractility and thus modulating peripheral vascular resistance [28-31]. In addition, extracellular ionized calcium inhibits renin secretion by interacting with the calcium-sensing receptor [32-34]. Another possible explanation offered by the current study is that calcium intake of the study subjects was more highly dependent on the consumption of plant-based foods than animal-based foods. So far, many studies have mainly focused on the consumption of dairy products for investigating the relationship between calcium and blood pressure [35], but for the subjects whose frequent food source is plant-based, studies may need a different approach to investigate the relationship between the consumption of calcium derived from plant-based food and blood pressure. Based on the reports that lowering diastolic blood pressure by 2 mmHg is associated with 17% reduction in the incidence of hypertension, 6% decrease in the risk of cardiac diseases [36], and reduced ventricular function [37], a follow-up study is warranted as our results show a correlation between calcium derived from plant foods and blood pressure. In addition, the plant calcium intake of the normotension group had no association with their blood pressure, showing a difference from the hypertensive group. Therefore, further studies are also needed to investigate the plant calcium's effect on lowering blood pressure with different blood pressure status.
Calcium consumption is also related to the lipoprotein metabolism and affects the level of blood lipid profiles [38-42]. Two main mechanisms of lowering blood lipids by calcium have been suggested. Dietary calcium engages the formation of intestinal soap with intestinal fatty acids. Particularly saturated fatty acids [40,42] and calcium also increase the excretion of blood lipids through binding with bile acids and consequently lower the synthesis of LDL-cholesterol [41,43]. While such a beneficial effect of calcium is reported in subjects who consumed more than 1,000 mg of calcium, the daily calcium intake of our current study subjects including both the hypertension and normotension groups was very low. In this study the level of total cholesterol and LDL-cholesterol had no significant association with the calcium intake of the hypertension and normotension groups.
Lorenzen et al. [43] reported that calcium intake from dairy products lowers the triglyceride contents in chylomicron and leads to an increase in chylomicron clearance and a decrease in fat absorption. On the other hand, a study of van Meijl et al. [42] argued that the increase in chylomicron clearance was not only from the calcium consumption and that the effect of calcium consumption should be differentiated from the consumption of dairy products. Yu et al. [44] reported that when the average daily calcium intake was above 422 mg, the consumption of dairy products and animal food-based calcium was positively associated with serum LDL cholesterol. In the current study, total calcium consumption and the calcium consumption derived from animal based-foods in the normotensive group were positively associated with blood triglycerides. One possible explanation for the positive relationship between calcium consumption and serum triglycerides is the tendency of the subjects to have high calcium intake from animal-based foods. Our present study identified a significant relationship between calcium and blood pressure and blood lipids in the context of daily calcium intake rather than calcium fortification or high calcium supplements. As this study is one of few studies investigating the role of daily calcium intake in the prevalence of hypertension and other metabolic diseases, further research is needed in a large and diverse population.
In spite of the beneficial effects of calcium, which lowers the blood pressure and the level of blood lipids, several studies raised the concern about calcium over-supplementation [45-46]. Supplementation of high amounts of calcium leads to the increase of cellular calcium ions. These over-produced calcium ions generate free radicals and inflammatory mediators, and continuously cause cellular damage [15]. Also, daily supplementation of 900 mg of calcium as a type of dairy product (3 servings per day) for overweight women for 12 weeks increased the biomarkers of lipids oxidation [16]. In the current study, since the level of calcium intake was very low, this may lead to subtle or no alterations in biomarkers of lipid oxidation in blood and urine samples of the hypertension and normotension groups.
This study has several limitations: 1) the size of study subjects in both the hypertension and normotension groups was not large enough to have statistical power for the results of several serum biomarkers, 2) the level of blood pressure in the hypertension group (152.8/86.2 mmHg, diagnosed as phase I hypertension) was relatively mild, making it difficult to find the differences in several measurements between the hypertension and normotension groups, and 3) limited methodology was used to investigate the calcium intake of subjects as we carried out the 24 hour recall but not food frequency records.
In conclusion, the daily calcium intake of hypertension patients tended to be lower than that of normotensive subjects. Also, relative to animal-based foods, plant-based foods were high contributors to calcium sources for both hypertension and normotension subjects. In the hypertension subjects, the intake of plant food-derived calcium was negatively correlated with the DBP, while animal food-derived calcium was positively correlated with DPB and in the healthy subjects, total calcium intake was positively correlated with serum triglycerides. However, there was no significant finding to show the relationship between calcium and lipid oxidation. Overall, these data suggest the importance of adequate calcium consumption and implicate to reconsider plant based foods as good calcium source for hypertension patients. Further investigation is necessary to identify the role of calcium derived from plant foods in populations with high dependency on plant-based food.
Acknowledgment
We would like to express our appreciation to Glenn Hawes for assistance to manuscript preparation.
References
- 1.Schroeder HA. Relations between hardness of water and death rates from certain chronic and degenerative diseases in the United States. J Chronic Dis. 1960;12:586–591. doi: 10.1016/0021-9681(60)90002-3. [DOI] [PubMed] [Google Scholar]
- 2.Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated metaanalysis of randomized controlled trials. Am J Hypertens. 1999;12:84–92. doi: 10.1016/s0895-7061(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 3.Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: a meta-analysis of randomized clinical trials. Ann Intern Med. 1996;124:825–831. doi: 10.7326/0003-4819-124-9-199605010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bucher HC, Cook RJ, Guyatt GH, Lang JD, Cook DJ, Hatala R, Hunt DL. Effects of dietary calcium supplementation on blood pressure. A meta-analysis of randomized controlled trials. JAMA. 1996;275:1016–1022. doi: 10.1001/jama.1996.03530370054031. [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Siani A, Strazzullo P. Oral calcium supplementation and blood pressure: an overview of randomized controlled trials. J Hypertens. 1989;7:941–946. doi: 10.1097/00004872-198912000-00003. [DOI] [PubMed] [Google Scholar]
- 6.van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–580. doi: 10.1038/sj.jhh.1002038. [DOI] [PubMed] [Google Scholar]
- 7.Carlson LA, Olsson AG, Orö L, Rössner S. Effects of oral calcium upon serum cholesterol and triglycerides in patients with hyperlipidemia. Atherosclerosis. 1971;14:391–400. doi: 10.1016/0021-9150(71)90067-0. [DOI] [PubMed] [Google Scholar]
- 8.Karanja N, Morris CD, Illingworth DR, McCarron DA. Plasma lipids and hypertension: response to calcium supplementation. Am J Clin Nutr. 1987;45:60–65. doi: 10.1093/ajcn/45.1.60. [DOI] [PubMed] [Google Scholar]
- 9.De Bacquer D, De Henauw S, De Backer G, Kornitzer M. Epidemiological evidence for an association between serum calcium and serum lipids. Atherosclerosis. 1994;108:193–200. doi: 10.1016/0021-9150(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 10.Reid IR, Mason B, Horne A, Ames R, Clearwater J, Bava U, Orr-Walker B, Wu F, Evans MC, Gamble GD. Effects of calcium supplementation on serum lipid concentrations in normal older women: a randomized controlled trial. Am J Med. 2002;112:343–347. doi: 10.1016/s0002-9343(01)01138-x. [DOI] [PubMed] [Google Scholar]
- 11.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149:151–161. doi: 10.1093/oxfordjournals.aje.a009781. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 13.Ascherio A, Rimm EB, Giovannucci EL, Colditz GA, Rosner B, Willett WC, Sacks F, Stampfer MJ. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86:1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer JH, Li L, Dwyer KM, Curtin LR, Feinleib M. Dietary calcium, alcohol, and incidence of treated hypertension in the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1996;144:828–838. doi: 10.1093/oxfordjournals.aje.a009017. [DOI] [PubMed] [Google Scholar]
- 15.Dada LA, Sznajder JI. Mitochondrial Ca2+ and ROS take center stage to orchestrate TNF-alpha-mediated inflammatory responses. J Clin Invest. 2011;121:1683–1685. doi: 10.1172/JCI57748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teegarden D, White KM, Lyle RM, Zemel MB, Van Loan MD, Matkovic V, Craig BA, Schoeller DA. Calcium and dairy product modulation of lipid utilization and energy expenditure. Obesity (Silver Spring) 2008;16:1566–1572. doi: 10.1038/oby.2008.232. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1) Seoul: Ministry of Health and Welfare; 2011. [Google Scholar]
- 18.Centers for Disease Control and Prevention; National Center for Health Statistics. Health, United States, 2010 [Internet] Atlanta: Centers for Disease Control and Prevention, National Center for Health Statistics; 2010. Available from: http://www.cdc.gov/nchs/hus.htm. [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.van Klaveren RJ, Hoet PH, Pype JL, Demedts M, Nemery B. Increase in gamma-glutamyltransferase by glutathione depletion in rat type II pneumocytes. Free Radic Biol Med. 1997;22:525–534. doi: 10.1016/s0891-5849(96)00375-9. [DOI] [PubMed] [Google Scholar]
- 23.Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Işimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin Biochem. 2001;34:65–70. doi: 10.1016/s0009-9120(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 24.Tagesson C, Källberg M, Wingren G. Urinary malondialdehyde and 8-hydroxydeoxyguanosine as potential markers of oxidative stress in industrial art glass workers. Int Arch Occup Environ Health. 1996;69:5–13. doi: 10.1007/BF02630732. [DOI] [PubMed] [Google Scholar]
- 25.The Korean Nutrition Society. Dietary Reference Intake for Koreans. 1st revision. Seoul: The Korean Nutrition Society; Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 26.Hjerpsted J, Leedo E, Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr. 2011;94:1479–1484. doi: 10.3945/ajcn.111.022426. [DOI] [PubMed] [Google Scholar]
- 27.McCarron DA. Role of adequate dietary calcium intake in the prevention and management of salt-sensitive hypertension. Am J Clin Nutr. 1997;65:712S–716S. doi: 10.1093/ajcn/65.2.712S. [DOI] [PubMed] [Google Scholar]
- 28.Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. J Am Coll Nutr. 2001;20:428S–435S. doi: 10.1080/07315724.2001.10719180. discussion 440S-442S. [DOI] [PubMed] [Google Scholar]
- 29.Zemel MB. Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem. 1998;188:129–136. [PubMed] [Google Scholar]
- 30.Bohr DF. Vascular smooth muscle: dual effect of calcium. Science. 1963;139:597–599. doi: 10.1126/science.139.3555.597. [DOI] [PubMed] [Google Scholar]
- 31.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 32.Resnick LM, Laragh JH, Sealey JE, Alderman MH. Divalent cations in essential hypertension. Relations between serum ionized calcium, magnesium, and plasma renin activity. N Engl J Med. 1983;309:888–891. doi: 10.1056/NEJM198310133091504. [DOI] [PubMed] [Google Scholar]
- 33.Touyz RM, Panz V, Milne FJ. Relations between magnesium, calcium, and plasma renin activity in black and white hypertensive patients. Miner Electrolyte Metab. 1995;21:417–422. [PubMed] [Google Scholar]
- 34.Maillard MP, Tedjani A, Perregaux C, Burnier M. Calcium-sensing receptors modulate renin release in vivo and in vitro in the rat. J Hypertens. 2009;27:1980–1987. doi: 10.1097/HJH.0b013e32832f0d22. [DOI] [PubMed] [Google Scholar]
- 35.McGrane MM, Essery E, Obbagy J, Lyon J, Macneil P, Spahn J, Van Horn L. Dairy consumption, blood pressure, and risk of hypertension: an evidence-based review of recent literature. Curr Cardiovasc Risk Rep. 2011;5:287–298. doi: 10.1007/s12170-011-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]
- 37.Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011;24:322–332.e6. doi: 10.1016/j.echo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Vaskonen T, Mervaala E, Sumuvuori V, Seppänen-Laakso T, Karppanen H. Effects of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. Br J Nutr. 2002;87:239–245. doi: 10.1079/BJNBJN2001508. [DOI] [PubMed] [Google Scholar]
- 39.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–1138. [PubMed] [Google Scholar]
- 40.de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, Jansen J, Müller M, van der Meer R. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Wit NJ, Bosch-Vermeulen H, Oosterink E, Müller M, van der Meer R. Supplementary dietary calcium stimulates faecal fat and bile acid excretion, but does not protect against obesity and insulin resistance in C57BL/6J mice. Br J Nutr. 2011;105:1005–1011. doi: 10.1017/S0007114510004654. [DOI] [PubMed] [Google Scholar]
- 42.van Meijl LE, Vrolix R, Mensink RP. Dairy product consumption and the metabolic syndrome. Nutr Res Rev. 2008;21:148–157. doi: 10.1017/S0954422408116997. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzen JK, Nielsen S, Holst JJ, Tetens I, Rehfeld JF, Astrup A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am J Clin Nutr. 2007;85:678–687. doi: 10.1093/ajcn/85.3.678. [DOI] [PubMed] [Google Scholar]
- 44.Yu CH, Kim HS, Park MY. Some factors affecting serum lipid levels of Korean rural women. Korean J Nutr. 1999;32:927–934. [Google Scholar]
- 45.Li GY, Fan B, Zheng YC. Calcium overload is a critical step in programmed necrosis of ARPE-19 cells induced by high-concentration H2O2. Biomed Environ Sci. 2010;23:371–377. doi: 10.1016/S0895-3988(10)60078-5. [DOI] [PubMed] [Google Scholar]
- 46.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]