Abstract
Neuropathological studies have revealed the presence of a broad variety of inflammation-related proteins (complement factors, acute-phase proteins, pro-inflammatory cytokines) in Alzheimer's disease (AD) brains. These constituents of innate immunity are involved in several crucial pathogenic events of the underlying pathological cascade in AD, and recent studies have shown that innate immunity is involved in the etiology of late-onset AD. Genome-wide association studies have demonstrated gene loci that are linked to the complement system. Neuropathological and experimental studies indicate that fibrillar amyloid-β (Aβ) can activate the innate immunity-related CD14 and Toll-like receptor signaling pathways of glial cells for pro-inflammatory cytokine production. The production capacity of this pathway is under genetic control and offspring with a parental history of late-onset AD have a higher production capacity for pro-inflammatory cytokines. The activation of microglia by fibrillar Aβ deposits in the early preclinical stages of AD can make the brain susceptible later on for a second immune challenge leading to enhanced production of pro-inflammatory cytokines. An example of a second immune challenge could be systemic inflammation in patients with preclinical AD. Prospective epidemiological studies show that elevated serum levels of acute phase reactants can be considered as a risk factor for AD. Clinical studies suggest that peripheral inflammation increases the risk of dementia, especially in patients with preexistent cognitive impairment, and accelerates further deterioration in demented patients. The view that peripheral inflammation can increase the risk of dementia in older people provides scope for prevention.
Introduction
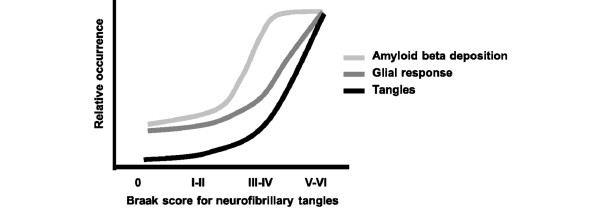
A role for inflammation in the pathogenesis of Alzheimer's disease (AD) had been discussed even in the earliest days of AD research. A hundred years ago Oskar Fischer wrote that cerebral senile plaque formation could be considered as the result of an extracellular deposition of abnormal substance in the cortex that induces a local inflammatory reaction, followed by an aberrant regenerative response of the surrounding nerve fibers. However, he was un-successful in his attempts to show the morphological characteristics of an inflammatory response around plaques and to detect complement proteins by performing complement-binding studies. Seventy years later, with the advent of monoclonal antibodies for immunohistochemistry, complement factors and clustering of activated microglia could be demonstrated within plaques [1]. After the discovery of amyloid-β (Aβ) as the main constituent of senile plaques, the concept was formed that the Aβ peptide itself can induce a local inflammatory response, which was supported by in vitro findings showing that fibrillar Aβ can bind complement factor C1 and activate the classical complement pathway without involvement of antibodies [2]. The inflammatory process in AD brains is not restricted to just a single step of the pathological process; inflammation-related proteins are involved in several crucial pathogenic events of the underlying pathological cascade, such as Aβ generation and clearance, gliosis and increased phosphorylation of tau with accelerated tangle formation [3,4]. It is important to keep in mind that inflammation itself has both beneficial effects, such as the phagocytosis of the toxic Aβ fibrils, and detrimental effects on neighboring cells by prolonged elevation of pro-inflammatory mediators. Clinicopathological studies show that the presence of activated microglia and inflammation-related mediators in the cerebral neocortex of patients with a low Braak stage for AD pathology precedes extensive tau-related neurofibrillary pathology [5] (Figure 1). Clinical research using positron emission tomography with the peripheral benzodiazepine receptor ligand PK-11195 as a marker for activated microglia indicates that activation of microglia precedes cerebral atrophy in AD patients [6]. A positron emission tomography study using the Pittsburg com-pound B for visualization of fibrillar amyloid and the PK-11195 ligand for microglia activation showed that amyloid deposition with microglia activation can be detected in vivo in around 50% of patients with mild cognitive impairment [7]. Thus, neuropathological and neuroradiological studies indicate that inflammatory changes in AD brains are a relatively early pathogenic event that precedes the process of neuropil destruction. The primary focus of the present paper is to review human studies for genetic, epidemiological and clinical evidence for whether, when and how inflammation could increase the risk of developing AD.
Figure 1.
Pathological cascade in Alzheimer's disease brains. The occurrence of amyloid-β deposits, glial response and tau-neurofibrillary pathology in the mid-temporal cortex compared to the neuropathological staging of Alzheimer's disease (modified after [5]).
Genetic evidence
In this section we evaluate the relationship between genetic risk factors for AD and two major components of amyloid plaques in AD brains, namely the presence of complement proteins and clusters of activated microglia, which are a source for the production of pro-inflammatory cytokines.
Aβ-associated proteins
Complement proteins were the first molecules detected in senile plaques in AD brains [8], two years before the identification of Aβ as the core protein of the senile plaques in 1984. In the following years a growing list of other proteins, mostly acute phase proteins, were demonstrated to be associated with Aβ deposits. These so-called Aβ-associated proteins include, next to the complement proteins, α1-antichymotrypsin (ACT), apolipoprotein E (ApoE), clusterin, intercellular adhesion molecule-1, α2-macroglobulin, serum amyloid P component (SAP) and heparan sulfate proteoglycans [9-15]. These proteins play a role in the transport, fibrillogenesis and deposition of Aβ and they are also important for the sequestration of neurotoxic Aβ species in plaques [16]. The presence of certain Aβ-associated proteins within plaques depends on the plaque type (see below) [17,18], and the accumulation of most depends on a certain degree of Aβ fibrillization; for example, SAP is found especially in plaques with fibrillar Aβ deposits but not in diffuse plaques [18]. In vitro studies also indicate that a certain degree of fibril formation is necessary for SAP to bind to Aβ, as SAP was found to bind to mature fibrils but not to protofibrils of Aβ1-42 [19].
Neuropathological studies show that diffuse Aβ deposits, characterized by the presence of non-fibrillar (non-congophilic) Aβ and without neuritic changes or reactive glia, are the predominant plaque types in non-demented controls, and that the amount of fibrillar (congophilic) Aβ deposits increases with progression of the disease [20]. In contrast to the classic plaques, characterized by highly fibrillar Aβ deposits, the list of Aβ-associated proteins present in diffuse plaques is much shorter. Immunohistochemical studies have demonstrated that ApoE, clusterin, complement proteins and ACT are present in diffuse plaques [17]. Studies in transgenic mice expressing the causal AD mutations crossed with transgenic mice overexpressing or genetically depleted for amyloid-associated proteins such as complement factors, ApoE, clusterin and ACT have shown that these proteins have an important role in the dynamic balance between Aβ deposition and removal [21-25]. Four recent large genome-wide association studies in late- onset AD have documented, in addition to the ApoE ε4 allele genotype, nine novel loci as risk factors for developing AD, including genes encoding proteins implicated in inflammatory processes (clusterin (CLU, chromosome 8, rs11136000) and complement receptor-1 (CR1, chromosome 1, rs6656401)) [26,27]. Clusterin is a multifunctional protein involved in both complement attack inhibition and cholesterol metabolism, and complement receptor-1 is the receptor for the cleavage products of complement proteins 3 and 4, which are present in plaques. All nine new genes map onto three pathways leading to late-onset AD: immune system function, cholesterol metabolism, and synaptic dysfunction [28]. Diffuse plaques, the initial pathological lesion in AD, contain a limited number of proteins, which are now all linked with a genetic risk for AD, with ACT as the exception. Abnormalities in the generation of Aβ are considered as the causal factor for the familial forms of early-onset AD, whereas poly-morphisms of the genes encoding for ApoE, clusterin and complement proteins are the genetic risk factors for late-onset AD. Genome-wide association studies for late-onset AD have not revealed genetic factors related to Aβ generation but instead to the Aβ-associated proteins already present in the initial neuropathological lesion. Taken together, the data on the protein composition of diffuse plaques and the recent genetic findings strongly suggest an interaction between Aβ, cholesterol metabolism and complement activation in the initial steps of the pathological cascade in AD.
Glia and pro-inflammatory cytokines
Fibrillar Aβ plaques are associated with clustering of activated microglia and astrocytes. These glial cells play an important role in innate immunity and express a family of receptors, the Toll-like receptors (TLRs), as a first line of defense responsible for recognizing specific pathogen-associated molecular patterns like fibrillar Aβ. TLRs and CD14, both innate immunity receptors, mediate activation of transcription factors such as NF-κB and subsequently the production of inflammatory cyto-kines [29]. Accumulating evidence indicates that CD14 and TLR4 are essential in the interaction of glial cells with Aβ for the production of pro-inflammatory cytokines [30-32]. In vitro studies have shown that Aβ fibrils interact with the TLR2/4 accessory protein CD14 and both TLR2 and TLR4 mediate Aβ-induced production of TNF-α in human monocytes. Mouse microglia show in vitro increased ingestion of Aβ after activation of TLR2, TLR4 or TLR9. Stimulation of the innate immune system via TLR9 is reported to be highly effective at reducing the parenchymal and vascular amyloid burden without apparent toxicity in a transgenic AD mouse model [33]. Both Aβ and the oxidized low-density lipoprotein can trigger inflammatory signaling through a TLR4 and -6 heterodimer. This assemblage is regulated by signals from the scavenger receptor CD36 [34]. These data show that CD36-TLR4-TLR6 activation is a common molecular mechanism by which atherogenic lipids and Aβ stimulate an inflammatory response in arteriosclerosis and AD, respectively. Arteriosclerosis is a major risk factor for late-onset AD and the discussed findings suggest that both disorders share a common pathogenic mechanism rooted in the innate immune system. Introducing a functionally destructive mutant of TLR4 into a transgenic mouse model (APPswe/PS1) results in increased levels of Aβ deposits, as well as reduced microglia activity [35]. Microglia from CD14 null mice failed to affect Aβl-42 damaged neurons and neuronal survival was accompanied by a significant reduction in the production of IL-6, indicative of reduced microglial activation [36]. All these data suggest that activation of TLR4 and CD14 signaling is involved in both the detrimental production of pro-inflammatory cytokines as well as the beneficial removal of Aβ in AD, illustrating the two-edged sword aspect of the neuroinflammatory response in AD brains [37]. Experimental animal studies indicate that microglia 'acivation' is not simply one phenotypic manifestation but includes heterogeneous, functional phenotypes that range from a pro-inflammatory, classic activation state to an alternative activation state involved in repair and extracellular matrix remodeling [38,39].
Lipopolysaccharide (LPS), a bacterial coat component, is widely used as a potent stimulator of the innate immune system and it is recognized by a receptor complex containing fully functional TLR4 and CD14. Chronic neuroinflammation induced by LPS in rats reproduced components of the neurobiology of AD, such as increased activation of microglia and astrogliosis, increased tissue levels of IL-1 and TNF-α, elevated expression of the amyloid precursor protein, and a working memory deficit [40]. Innate immunity responsiveness can be investigated by the incubation of whole blood samples with LPS, followed by the determination of levels of various inflammatory cytokines. Twin studies demonstrate that heritability for serum levels of circulating inflammatory mediators is modest (about 20%). In contrast to circulating inflammatory mediators, however, cytokine production capacity is under strong genetic control. In the non-diseased population, estimates for the heritability of the production of the various cytokines ranges from 53 to 86% [41]. We have studied cytokine production capacity in ex vivo stimulated full blood samples from middle aged off spring with and without a parental history of late-onset AD [42]. We found that the production capacity of the pro-inflammatory cytokines IL-1β, IL-6, TNFα and interferon-γ was significantly higher in off spring with a parental history of AD upon stimulation of whole blood with LPS. Similar results were found for the IL -1β to IL-1 receptor antagonist (IL-1ra) ratio, which was calculated because IL-1ra is the natural antagonist of the pro-inflammatory cytokine IL-1β. This higher ratio reflects a pro-inflammatory genotype. While the ApoE ε4 allele genotype was more frequent among the off spring with compared to those without a parental history of AD, these findings were independent of ApoE4 genotype. In contrast to the stimulated whole blood samples, the evaluation of the unstimulated blood samples does not show significant differences between the off spring with versus without a parental history of late-onset AD. The aim of this study was not to identify the genetic variability of a particular receptor or cytokine but to investigate the genetic contribution of the whole pathway that mediates the production of pro-inflammatory cytokines after activation by LPS of the innate immune receptors CD14 and TLRs by LPS. Similar to LPS, the Aβ-induced cytokine production capacity is also under genetic control, as shown by results from twin studies in which whole blood samples were ex vivo stimulated with Aβ [43].
In conclusion, neuropathological and experimental studies indicate that fibrillar Aβ can activate the innate immunity-related CD14 and TLR signaling pathways for pro-inflammatory cytokine production. The production capacity of this pathway is under genetic control and o spring with a parental history of late-onset AD have a higher production capacity for pro-inflammatory cytokines.
Epidemiological evidence
Prospective case cohort studies have shown that high serum levels of the acute-phase proteins ACT, C-reactive protein and IL-6 could predict cognitive decline or dementia [44-46]. Yaffe and colleagues [47] reported that elderly subjects with a metabolic syndrome and high serum level of IL-6 and C-reactive protein were more likely to experience cognitive decline in the next four years, compared with those with a metabolic syndrome and low levels of these inflammatory proteins. In another population study the metabolic syndrome was also negatively associated with cognition, especially in subjects with high levels of inflammation [48]. In the Framingham study a higher spontaneous production of IL-1β or TNF-α by peripheral blood mononuclear cells was associated with future risk of AD in older individuals [49]. The epidemiological findings from several case cohort studies indicate that non-demented subjects with increased serum levels of acute-phase reactants, indicating a low-grade peripheral systemic inflammation, are at risk for developing a sporadic late-onset form of AD.
The acute phase response is initiated and orchestrated by cytokines, most notably IL-1. AD brains are characterized by overexpression of IL-l and there are strong arguments for an important role for IL-1 in amyloid plaque formation [50]. Aβ deposition and neuronal injury may trigger a self-propagating cytokine cycle, which initiates a vicious feedback of continuing IL-1 elevation when chronically induced. In this way further neuronal dysfunction and Aβ plaque accumulation could be promoted. Taken together, the epidemiological studies suggest that elevated serum levels of acute phase reactants can be considered as a risk factor for AD and neuropathological data demonstrate the presence of acute phase reactants already in human brains with preclinical stages of AD pathology (low Braak score). Studies suggesting that immune blood markers can be used as a clinical test to identify those patients with mild cognitive impairment who progress to clinical AD are consistent with the view that peripheral immune and inflammatory mechanisms contribute to the pathogenesis of AD [51,52].
Clinical evidence
The question of whether systemic inflammation or peripheral chronic inflammation could contribute to AD pathology was a neglected research topic until recently. In particular, the dogmatic belief that the blood-brain barrier excludes cross-talk between both systems hampered studies in this field for a long time. This view has changed dramatically, however, as it became clear that morphological 'delegates' of the immune system, the microglial cells, are present in the brain and that the peripheral lymphoid organs are innervated. A further finding was that cytokines and neurotransmitters, as well as their receptors, are endogenous to both the brain and the immune system. These findings have led to the view that the immune system and brain share a common biochemical language and that their functions are intertwined [53]. Pro-inflammatory cytokines such as IL-1β and TNF-α, which are generated in the periphery, communicate with the brain. Several mechanisms exist by which an initial, exclusively peripheral cytokine signal can be transmitted to the brain, including direct neural pathways (via primary autonomic afferents) that trans-port it across the blood-brain barrier, or entry via the cirvumventricular region, where the blood-brain barrier is non-existent or discontinuous [54].
Several clinical studies suggest that systemic inflammation can be involved in the pathogenesis of AD. In a twin study it was found that AD cases with a history of severe systemic infection tended to have earlier onset than their corresponding twin [55]. A case-control case study reported a positive association between episodes of infection during the four years preceding the diagnosis and an increased likelihood of a diagnosis of AD in older individuals [56]. In a prospective cohort study of community-dwelling subjects with mild to severe AD it was found that acute episodes of systemic inflammation with increased serum levels of TNF-α were associated with a two-fold increase in the rate of cognitive decline over a 6-month period. High baseline levels of TNF-α were associated with a four-fold increase in the rate of cognitive decline while subjects who had low levels of TNF-α throughout the study showed no cognitive decline over the 6-month period [57]. Recent studies indicate that chronic periodontitis has been associated with AD. Periodontitis is a prevalent, persisting peripheral infection associated with Gram-negative, anaerobic bacteria that are capable of exhibiting localized and systemic infection in the host. Emerging evidence suggests that tooth loss and periodontal disease predict cognitive decline in community-dwelling older adults [58]. In addition, patients with AD had higher levels of plasma TNF-α and antibodies against periodontal bacteria com-pared with cognitively normal subjects [59]. Although several case-control and prospective studies indicate periodontitis as a risk factor for cognitive decline, it is important to be cautious to draw any conclusion about a causal relationship. Premorbid cognition is important both for oral health and a risk factor for dementia. Furthermore, factors other than inflammatory mediators could be responsible for the positive association, such as changes in life style and dietary factors, such as a poor nutritional status, especially in relationship to B vitamins.
Most interesting with regard to understanding the contribution of systemic inflammation to the pathogenesis of AD seems to be current research into the role of inflammation in delirium. Delirium is defined as an acute disturbance of consciousness with signs of attention, a typically fluctuating course and a change in cognition (that is, disorientation, disturbed memory). Delirium is the most prevalent neuropsychiatric syndrome that can be observed in the general hospital, especially in older patients with preexisting cognitive impairment. It is independently associated with increased mortality, institutionalization and dementia [60]. Many clinical conditions that are accompanied by systemic inflammatory reactions can induce delirium. A case-controlled neuropathological study has shown an association between severe systemic infection (sepsis) and microglia activation [61]. A recent postmortem study found that delirium is associated with higher immunoreactivity for microglial and astroglial activity and IL-6 compared with age-matched controls without delirium [62]. In healthy persons a severe systemic inflammation, such as sepsis, can lead to delirium, but in patients with preexisting brain pathology even just a mild urinary tract infection can cause it [63]. Experimental animal studies have shown that microglia respond differently to a stimulus if other stimuli precede, coexist or follow it. Microglia can become primed by an initial factor, which prepares them for an enhanced pro-inflammatory cytokine response, even if the subsequent challenges are only mild [64]. Significantly increased serum levels of IL-6 were found in acutely admitted older patients with delirium compared to those without delirium after adjusting for infection, age and cognitive decline [65]. This study indicates that acute phase reactants could contribute to the pathogenesis of delirium. A high incidence of delirium is seen in older patients undergoing surgery for hip fracture. Contrary to popular belief, there is little evidence that general anesthesia is associated with delirium after surgery [66]. The most important predisposing factor for delirium in these patients is preexisting cognitive decline or dementia. The precipitating factors could be related to the release of pro-inflammatory cytokines as a consequence of the fracture and tissue destruction resulting from surgery. In a time course study of cytokines during delirium in older patients admitted for surgery after hip fracture, significant differences in serum levels of IL-6 were found between patients with and without delirium [67].
For a long time it has been a common observation in medicine that, when older patients become delirious while suffering from an acute urinary tract or other common infection, treatment of the infection may go well but the patients emerge with dementia, even when they had appeared cognitively intact or only mildly impaired prior to hospitalization. These patients often fail to recover to their initial level of functioning and some never resume independent life at home. Similar clinical observations have been made after postoperative delirium in elderly hip fracture patients free from preexisting dementia [68-70]. There is now increasing evidence that postoperative delirium after hip surgery is an important predictor of incident dementia in elderly patients living independently at home [70]. In a prospective study it was found that, after a follow-up of 2.5 years, the risk of dementia or mild cognitive impairment is almost doubled in elderly hip surgery patients with postoperative delirium compared with at-risk patients without delirium [68]. It has recently been reported that delirious episodes in a cohort of AD patients accelerate cognitive decline [71].
In conclusion, recent studies suggest that delirium and AD share a neuroinflammatory response as a common pathogenic mechanism that could explain the vulnerability of AD patients for further cognitive worsening after an episode of delirium associated with a systemic inflammatory reaction.
Discussion
The etiology of AD may be heterogeneous, but the underlying pathological cascade has distinct common themes. In the autosomal dominant form of familial AD the etiology is related to causal mutations leading to higher production of Aβ1-42. The subsequent deposition of fibrillar Aβ elicits a brain inflammatory response as a secondary event in the pathological process. In contrast to the monocausal etiology of this rare form of AD, the etiology of the highly prevalent sporadic late-onset form is considered to be multifactorial. Genome-wide association studies of late-onset AD strongly suggest a role for lipoproteins and immune-associated proteins in its etiology and pathogenesis. In addition to the role of ApoE and clusterin in the process of Aβ deposition and drainage, these proteins can also attenuate the activity of NF-κB signaling and the production of pro-inflammatory cytokines [72,73]. The fibrillar Aβ-induced inflammatory response is a relatively early event in the pathological cascade and it is already present in the brain during stages of the disease that precede the stages characterized by tau-related neurofibrillary changes, which are most closely related to a clinical dementia syndrome (Figure 1).
Animal and neuropathological studies in humans show that systemic inflammation can induce an inflammatory response within the brain. This response can lead to acute cognitive disturbance and behavior changes (delirium) [74]. In healthy adults only a severe systemic inflammation can induce delirium, but in older people, especially those with mild cognitive impairment or dementia, even just a mild systemic inflammation can lead to it [75]. Inflammation-induced delirious episodes in adults with preexisting normal cognitive functions are frequently followed by a period of cognitive impairment that can last for months, although there is no evidence in these cases for a further progression of the cognitive symptoms to a clinical AD syndrome. However, inflammation-induced delirium in older patients with preexisting mild cognitive impairment can lead to further cognitive deterioration and dementia (Figure 2). These clinical findings suggest that systemic inflammation does not initiate the pathological AD cascade but can accelerate the underlying cascade.
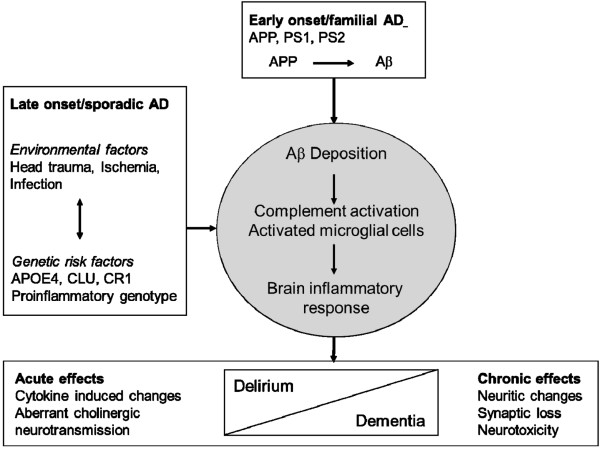
Figure 2.
Relationship between inflammation and the etiology and clinical syndrome of Alzheimer's disease. Schematic diagram showing that interactions between innate immunity-related genetic risk factors and inflammation-inducing events (brain trauma, ischemia and infection) can contribute to the multifactorial etiology of the sporadic late-onset form of AD. The diagram illustrates also that delirium and AD share a neuroinflammatory response as a common pathogenic mechanism that could explain the vulnerability of AD patients to further cognitive worsening after an episode of delirium associated with a systemic inflammatory response. Aβ, amyloid-β peptide; AD, Alzheimer's disease; APOE4, apolipoprotein E4; APP, amyloid precursor protein; CLU, clusterin; CR1, complement receptor-1; PS1, presenilin-1; PS2, presenilin-2.
What makes older patients with peripheral inflammation so vulnerable to dementia? Several mechanisms can play a role. First, neuropathological studies show inflammatory changes in early stages of AD pathology, such as fibrillar Aβ-induced microglia activation. Microglia cells are already 'primed' in preclinical stages of AD for increased production of pro-inflammatory cytokines later on by systemic inflammation as a second challenge. Second, neurotransmitters such as acetylcholine play an active role in controlling glia activation and can inhibit the production of proinflammatory cytokines [76]. AD brains are characterized by cholinergic deficits that can contribute to an uncontrolled neuroinflammatory response when the brain is challenged by peripheral inflammation. Third, arteriosclerosis and other vascular risk factors common in older people lead to blood-brain barrier dysfunction with brain endothelial cell activation resulting in the secretion of multiple neurotoxic and inflammatory factors. These factors could be responsible for the increased susceptibility of the brain to systemic inflammatory mediators [77,78]. This could also explain why the prevalence of delirium in demented patients in late-onset AD and vascular dementia is higher than in those with early onset AD [79].
The pathological, epidemiological and clinical findings reviewed in this paper suggest that several inflammation-related events can contribute to the pathogenesis of AD and accelerate the rate of progression of the clinical course of AD. However, this view does not necessarily indicate that treatment with anti-inflammatory drugs will be effective in AD patients. As discussed earlier, inflammation itself has both beneficial and detrimental effects and every inflammation-based therapeutic strategy influences the delicate balance between both effects. Inhibition of complement activation or blocking of IL-1β have been considered as therapeutic options for treatment of AD patients. However, it appeared that complement C3 deficiency in transgenic AD mice led to accelerated amyloid plaque formation and overexpression of IL-1β to a reduction of amyloid pathology [21,22,80]. These findings indicate potential negative effects of anti-inflammatory drugs in AD patients if these drugs inhibit the primary function of the innate immune system: removal of the pathological agents (fibrillar Aβ) that induce the inflammatory response. Epidemiological and observational studies in humans have found evidence that the use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with a lower risk of developing AD. In contrast, randomized trials have reported no effect of NSAIDs on clinical progression in patients with clinically established AD [81]. An explanation for these apparently divergent conclusions could be the fact that expression levels of cyclooxygenase-1 and -2, as the main targets of NSAIDs, are dependent on the stage of AD pathology [82].
Further directions
The view that peripheral inflammation can increase the risk of dementia in older people offers scope for prevention. In particular, clinical trials are warranted to investigate whether anti-inflammatory drugs can prevent further cognitive decline in patients with mild cognitive impairment during the periods that peripheral inflammation exerts increased inflammatory pressure on the brain. These studies could be performed using available drugs that inhibit microglia activation, such as minocycline, or that restore the cholinergic control of micro-glia activation by cholinomimetics [63]. Minocycline, a tetracycline derivative with anti-inflammatory properties, attenuates the production of pro-inflammatory cytokines by human microglia without affecting their beneficial activities, such as phagocytosis of Aβ fibrils [83].
Conclusion
Genetic, pathological and epidemiological studies show that innate immunity is involved in the early stages of the pathological cascade of AD and can also contribute to the etiology of late-onset AD. Clinical studies suggest that peripheral inflammation increases the risk of dementia, especially in patients with preexisting cognitive impairment, and accelerates further deterioration in demented patients.
Abbreviations
Aβ: amyloid-β; ACT: α1-antichymotrypsin; AD: Alzheimer's disease; ApoE: apolipoprotein E; IL: interleukin; LPS: lipopolysaccharide; NF: nuclear factor; NSAID: non-steroidal anti-inflammatory drug; SAP: serum amyloid P component; TLR: Toll-like receptor; TNF: tumor necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PE, WAG, and JJM were involved in writing the manuscript. AJMR and RV contributed to the reviewed neuropathological findings and EvE to the epidemiological findings. All authors read and approved the final manuscript.
Contributor Information
Piet Eikelenboom, Email: p.eikelenboom@ggzingeest.nl.
Jeroen JM Hoozemans, Email: jjm.hoozemans@vumc.nl.
Rob Veerhuis, Email: r.veerhuis@vumc.nl.
Eric van Exel, Email: e.vanexel@ggzingeest.nl.
Annemieke JM Rozemuller, Email: jm.rozemuller@vumc.nl.
Willem A van Gool, Email: w.a.vangool@amc.uva.nl.
Acknowledgements
Work discussed was supported by an EMGO fellowship of VUMC (EvE), Internationale Stichting Alzheimer Onderzoek (JJMH, ISAO#08513) and ZonMW (WAvG, TOP#40-00812-98-10017).
References
- Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJM, van Gool WA, Hoozemans JJM. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm. 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- Rogers J, Cooper NR, Webster S, Schulz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Lieberburg I. Complement activation by β-amyloid in Alzheimer's disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK, Terwel D, Kummer MP. Neuroinflamatory processes in Alzheimer's disease. J Neural Transm. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Zilka N, Kazmerova Z, Jadhav S, Neradil P, Madari A, Obetkova D, Bugos O, Novak M. Who fans the flames of Alzheimer's disease brains? Misfolded tau on the crossroad of neurodegenerative and inflammatory pathways. J Neuroinflammation. 2012;9:47. doi: 10.1186/1742-2094-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJM, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int J Dev Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, Walker Z, Kennedy A, Fox N, Rossor M, Brooks DJ. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunohistoperoxidase study. Acta Neuropathol. 1982;57:239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor α1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apoliporotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in alzheimer's diseaseand kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Choi-Miura NH, Ihara Y, Fukuchi K, Takeda M, Nakano Y, Tobe T, Tomita M. SP-40,40 is a constituent of Alzheimer's amyloid. Acta Neuropathol. 1992;83:260–264. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- Rozemuller JM, Eikelenboom P, Pals ST, Stam FC. Microglial cells around plaques in Alzheimer's disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci Lett. 1989;101:288–292. doi: 10.1016/0304-3940(89)90547-8. [DOI] [PubMed] [Google Scholar]
- Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Yolk B, Berger M. Interleukin-6 and α2-macroglobulin indicate an acute-phase state in Alzheimer's disease. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- Coria F, Castano E, Prelli F, Larrondo-Lillo M, van Duinen S, Shelanski ML, Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988;58:454–458. [PubMed] [Google Scholar]
- Snow AD, Mar H, Nochlin D, Kimata K, Kato M, Suzuki S, Hassell J, Wight TN. The presence of heparin sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988;133:456–463. [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R, Boshuizen RS, Familian A. Amyloid associated proteins in Alzheimer's and prion disease. Curr Drug Targets CNS Neurol Disord. 2005;4:235–248. doi: 10.2174/1568007054038184. [DOI] [PubMed] [Google Scholar]
- Zhan SS, Veerhuis R, Kamphorst W, Eikelenboom P. Distrubution of β-amyloid associated proteins in plaques in Alzheimer's disease and non-demented elderly. Neurodegeneration. 1994;4:291–297. doi: 10.1016/1055-8330(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, van Breemen, Hoozemans JJM, Morbin M, Ouladhadj J, Tagliavini F, Eikelenboom P. Amyloid-β plaque-associated proteins C1q and SAP enhance the Aβ1-42 peptide-induced cytokine production in adult human microglia in vitro. Acta Neuropathol. 2003;105:135–144. doi: 10.1007/s00401-002-0624-7. [DOI] [PubMed] [Google Scholar]
- Holm Nielsen E, Nybo M, Junker K, Toftedal Hansen P, Rasmussen IM, Svehag SE. Loccalization of human serum amyloid P component and heparin sulphate proteoglycan in vitro-formed Aβ fibrils. Scand J Immunol. 2000;52:110–112. doi: 10.1046/j.1365-3083.2000.00775.x. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in 'normal' aging. Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement inhibited Alzheimer's mice. Proc Natl Acad Sci USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid-β plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du YS, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Ricardo P, Ghetti B, Paul SM. Lack of apoliprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Nilsson LN, Bales KR, DiCarlo G, Gordon MN, Morgan D, Paul SM, Potter H. α1-Antichymotrypsin promotes β-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, O'dell MA, Parsadanian M, Taylor JW, Harmony JA, Bales KR, Paul SM, Aronow BJ, Holtzman DM. Clusterin promotes amyloid plaque formation and it is critical for neuritic toxicity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Morgan K. The three new pathways leading to Alzheimer's disease. Neuropathol Appl Neurobiol. 2011;37:353–357. doi: 10.1111/j.1365-2990.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Guulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke BM Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innateimmunity with Alzheimer's disease. Faseb J. 2004;18:203–2005. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Aβ uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and are required for fibrillar Aβ-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Induction of toll-like receptor 9 signalling as a ethod for ameliorating Alzheimer's disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wikinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulber A, El Khoury J, Golenbock DT, Moore KJ. CD13 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 dimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schaffer W, Kassbender K. Role of Toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Bate C, Veerhuis R, Eikelenboom P, Williams A. Microglia kill amyloid-β1-42 damaged neurons by a CD14-dependent process. NeuroReport. 2004;15:1427–1430. doi: 10.1097/01.wnr.0000132203.76836.16. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease - a double edge sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial "activation" continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- de Craen AJ, Posthuma D, Remarque EJ, van den Biggelaar AH, Westendorp RGJ. Heritability estimates of innate immunity: an extended twin study. Genes Immun. 2005;6:167–170. doi: 10.1038/sj.gene.6364162. [DOI] [PubMed] [Google Scholar]
- van Exel E, Eikelenboom P, Comijs H, Frolich M, Smit JH, Stek ML, Scheltens P, Eefsting JE, Westendorp RGJ. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiat. 2009;66:1263–1270. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Meulenbelt I, de Craen AJM, de Geus EJC, Slagboom PE, Boomsma DI, Westendorp RGJ. Human cytokine response ex vivo amyloid-β is mediated by genetic factors. Twin Res Hum Genet. 2005;8:132–137. doi: 10.1375/1832427053738728. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kilaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MMB. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older patients. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The meatboloc syndrome, inflammation, and the risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Deeg DJ, Kok A, Yaffe K, Pennix BW. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30:2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer's disease: The Framingham study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Sheng JG, Royston MC, Mackenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer's disease; the potential role of a 'cytokine cycle'in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovic GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Avagyan H, Goldenson B, Tse E, Masoumi A, Porter V, Wiedau-Pazos M, Sayre J, Ong R, Mahanian M, Koo P, Bae S, Micic M, Liu PT, Rosenthal MJ, Fiala M. Immune blood biomarkers of Alzheimer disease patients. J Neuroimmunol. 2009;210:67–72. doi: 10.1016/j.jneuroim.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–511. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelly KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee LE, Lippa CF. Alzheimer's disease in 22 twins pairs-13 years follow-up. Hormonal, infectious and traumatic factors. Dement Geriatr Cogn Disord. 1999;10:148–151. doi: 10.1159/000017115. [DOI] [PubMed] [Google Scholar]
- Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer's disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer AR, Morse DE, Holm-Pedersen P, Mortensen EL, Avlund K. Periodontol inflammation in relation to cognitive function in an older Danish population. J Alzheimers Dis. 2012;28:613–624. doi: 10.3233/JAD-2011-102004. [DOI] [PubMed] [Google Scholar]
- Kamer AR, Craig RG, Pirraglia E, Dasanayaka AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlox J, Eurelings LS, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in the elderly and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- Lemstra AW, Groen in't Woud JC, Hoozemans JJ, van Haastert ES, Rozemuller AJ, Eikelenboom P, van Gool WA. Microglia activation in sepsis: a case-control study. J Neuroinflammation. 2007;4:4. doi: 10.1186/1742-2094-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool WA, van de Beek D, Eikelenboom P. Systemic infection; when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Slor CJ, de Jonghe JFM, Groot E, van der Ploeg T, van Gool WA, Eikelenboom P, Snoeck M, Schmand B, Kalisvaart KJ. Anesthesia and postoperative delirium in older adults undergoing hip surgery. J Am Geriatr Soc. 2011;59:1313–1319. doi: 10.1111/j.1532-5415.2011.03452.x. [DOI] [PubMed] [Google Scholar]
- van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, de Rooij SE. Time-course of cytokines during delirium in elderly with hip fractures. J Am Geriatr Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- Kat MG, Vreeswijk R, de Jonghe JF, van der Ploeg T, van Gool WA, Eikelenboom P, Kalisvaart KJ. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half year. Dement Geriatr Cogn Disord. 2008;26:1–8. doi: 10.1159/000140611. [DOI] [PubMed] [Google Scholar]
- Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26:26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- Krogseth M, Wyller TB, Engedal K, Juliebo V. Delirium is an important predictor of incident dementia among elderly hip fracture patient. Dement Geriatr Cogn Disord. 2011;31:63–70. doi: 10.1159/000322591. [DOI] [PubMed] [Google Scholar]
- Fong TG, Jones RN, Shi P, Maracantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Kodvawala A, Hui DY. Apolipoprotein E inhibits Toll-like receptor (TLR)-3 and TLR-4 mediated macrophage activation through distinct mechanisms. Biochem J. 2010;428:47–54. doi: 10.1042/BJ20100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli G, Aronow BJ, Sala A. Essential requirement of apolipoprotein J (clusterin) signaling for IκB expression and regulation of NF-κB activity. J Biol Chem. 2003;278:38214–38219. doi: 10.1074/jbc.C300252200. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, Ely EW. Delirium as predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale D, de Simone R, Minghetti L. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrernegic systems. CNS Neurol Disord Drug Targets. 2007;6:388–397. doi: 10.2174/187152707783399193. [DOI] [PubMed] [Google Scholar]
- Zlokovic BZ. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P. Neurovascular dysfunction, inflammation and endothelial activation. Implications for the pathogenesis of Alzheimer's disease. J Neuroinfl ammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsson B, Blennow K, Gottfries CG, Wallin A. Delirium in dementia. Int J Geriatr Psychiatry. 1998;13:49–56. doi: 10.1002/(sici)1099-1166(199801)13:1<49::aid-gps733>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Miller JN, Jonson RE, O'Banion MK. Sustained hippocampal Il-1β overexpression mediates chronic neuroinfklammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer's disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010;9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJM, Rozemuller JM, van Haastert ES, Veerhuis R, Eikelenboom P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer's disease pathology. Curr Pharm Des. 2008;14:1419–1427. doi: 10.2174/138161208784480171. [DOI] [PubMed] [Google Scholar]
- Familian A, Eikelenboom P, Veerhuis R. Minocycline does not affect amyloid-β phagocytosis. Neurosci Lett. 2007;416:87–91. doi: 10.1016/j.neulet.2007.01.052. [DOI] [PubMed] [Google Scholar]