Abstract
Endoscopic submucosal dissection (ESD) is widely used in Japan as a minimally invasive treatment for early gastric cancer. The application of ESD has expanded to the esophagus and colorectum. The indication criteria for endoscopic resection (ER) are established for each organ in Japan. Additional treatment, including surgery with lymph node dissection, is recommended when pathological examinations of resected specimens do not meet the criteria. Repeat ER for locally recurrent gastrointestinal tumors may be difficult because of submucosal fibrosis, and surgical resection is required in these cases. However, ESD enables complete resection in 82%-100% of locally recurrent tumors. Transanal endoscopic microsurgery (TEM) is a well-developed surgical procedure for the local excision of rectal tumors. ESD may be superior to TEM alone for superficial rectal tumors. Perforation is a major complication of ESD, and it is traditionally treated using salvage laparotomy. However, immediate endoscopic closure followed by adequate intensive treatment may avoid the need for surgical treatment for perforations that occur during ESD. A second primary tumor in the remnant stomach after gastrectomy or a tumor in the reconstructed organ after esophageal resection has traditionally required surgical treatment because of the technical difficulty of ER. However, ESD enables complete resection in 74%-92% of these lesions. Trials of a combination of ESD and laparoscopic surgery for the resection of gastric submucosal tumors or the performance of sentinel lymph node biopsy after ESD have been reported, but the latter procedure requires a careful evaluation of its clinical feasibility.
Keywords: Endoscopic submucosal dissection, Esophageal cancer, Gastric cancer, Colorectal cancer, Laparoscopic surgery, Lymph node metastasis, Perforation, Gastrectomy, Complications
INTRODUCTION
Gastroendoscopic treatments, such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), are standard treatments for early gastrointestinal cancer in Japan. Medical endoscopists often perform these procedures in Japan; however, Masanori Hirao, who first reported endoscopic resection (ER) (a forerunner of ESD) using a local injection of hypertonic saline-epinephrine for the treatment of early gastric cancer[1], was a surgeon with experience in the performance of gastrectomies.
ER was developed to reduce the number of excessive surgeries for early gastric cancer without lymph node metastasis (LNM), but the indications for ER have been expanded to other conditions and organs.
Endoscopic treatment was principally developed for the examination of conditions with no LNM[2-5] and to improve the resectability of tumors[6,7]. ESD has expanded as a new and less invasive treatment that enables complete tumor removal without surgery (Figure 1).
Figure 1.
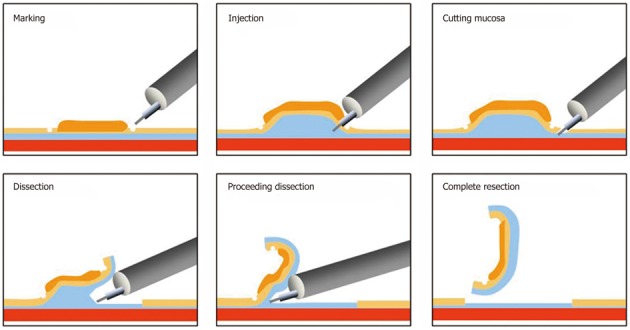
Image of Endoscopic submucosal dissection: Marking is not necessary in a colorectal case because the lesion margins are clear.
ESD has three main advantages over surgery; specifically, it is less invasive, is less expensive, and better preserves physiological function. However, complete resection is impossible in some difficult cases, and the rate of complete resection is not 100% in high-volume institutes. Subsequent surgery may be required for curative treatment in some cases (Figure 2).
Figure 2.
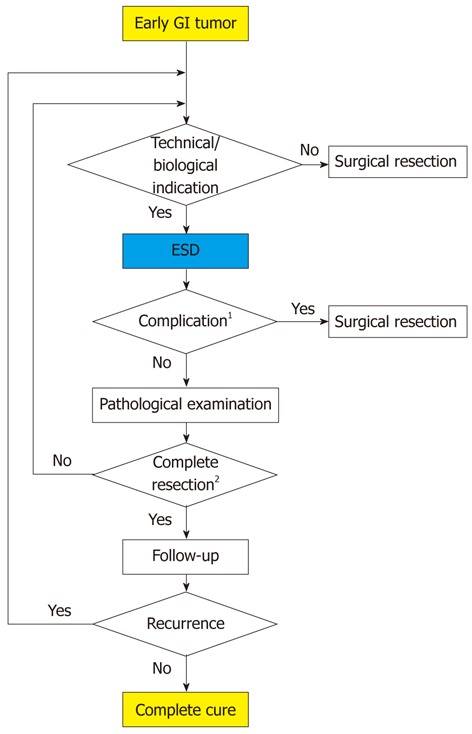
Algorithm for the treatment of early gastrointestinal tumors. 1Perforation or bleeding during endoscopic submucosal dissection which can not be treated endoscopically or delayed perforation; 2See Table 2.
ESD may be applied for secondary lesions after some surgeries, such as esophagectomy and gastrectomy. A new technique in this field is joint laparoscopic surgery and ESD, which has been attempted for the treatment of submucosal tumors, including gastrointestinal stromal tumors (GISTs). The ESD technique is used as a novel, less invasive, natural orifice transluminal endoscopic surgery (NOTES).
This article discusses the relationship between ESD and surgical treatment from several viewpoints.
ADDITIONAL SURGERY AFTER ESD
Additional surgery with lymph node dissection is recommended when ER is histologically non-curative. Non-curative ESD is defined as the presence of cancer cells in the lateral or deep margins, invasion to the deep layer, the presence of lymphatic vessel invasion, the presence of an undifferentiated cell type, or a combination of these conditions. The definitions vary according to the affected organ.
Esophagus
Mortality after esophagectomy is 14%[8], which is much higher than that after ESD. Therefore, the decision to perform an esophagectomy for esophageal cancer should be carefully considered. The 2007 guidelines of the Japanese Esophageal Society for the diagnosis and treatment of esophageal squamous cell carcinoma (SCC) state that intraepithelial tumors (M1) and tumors invading the lamina propria (M2) that spread to less than two-thirds of their circumference are absolute indications for esophageal ER, and tumors that are in contact with or invading the muscularis mucosa (M3) without clinical lymph node involvement are a relative indication for ER.
Intensive pathological investigations of a large number of surgical resection cases have demonstrated that less than 5% of M1 and M2 cases exhibit LNM, and the 5-year survival rate is greater than 90%; most deaths result from other causes. However, M3 and submucosa (SM1) cases (i.e., tumors that invade the most superficial third of the submucosa) exhibit a 10%-32% risk of LNM[3,6,9]. Therefore, additional treatment is not necessary for M1 and M2 cases, but additional treatment, such as esophagectomy or chemoradiation, must be considered for M3 and SM1 cases. The indication for additional surgery is controversial in M3 cases. Eguchi et al[9] investigated 464 consecutive patients who had undergone radical esophagectomy with lymphadenectomy and found that 42% of patients with M3 lesions and lymphatic invasion (LY) exhibited LNM, but only 10% of patients with M3 lesions without LY exhibited LNM. The authors concluded that M3 lesions with LY should be treated similarly to SM lesions, and patients with M3 lesions without LY can be followed up without any additional treatment. Shimizu et al[10] found that 16 patients with esophageal carcinoma that invaded the muscularis mucosa or upper submucosa who underwent EMR followed by chemoradiotherapy but refused esophagectomy exhibited no local recurrence or metastasis. The overall survival rate of these 16 patients at 5 years was 100%, but 39 patients with similarly staged cancer who underwent esophagectomy after EMR exhibited a survival rate of 87.5%[10]. A phase II study to evaluate the efficacy and safety of combined EMR and chemoradiotherapy treatment for clinical stage I esophageal cancer (T1b: SM1-3) is ongoing[11]. The necessity of additional surgical treatment and alternative treatments should be considered based on the pathological findings, age, comorbidities, and the patient’s wishes, and the clinical protocol should be strictly followed.
Stomach
ESD was first applied in the stomach, and most of the clinical and pathological data for this technique can be pooled. The en bloc resection and complete resection rates are 83%-95% and 73%-92%, respectively[12-18], which suggests that complete resection is not obtained in 8%-27% of cases. Additional treatments are required in these cases. The 2004 guidelines for the diagnosis and treatment of stomach carcinoma from the Japanese Gastric Cancer Society[19] recommend ER for the lesion, which meets the following criteria: macroscopic mucosal cancer, less than 2 cm, differentiated type (pap, tub1, tub2), and the absence of ulceration and scarring in the case of the depressed type. Expanded criteria include differentiated mucosal cancer without ulceration irrespective of the tumor size, differentiated mucosal cancer with ulceration if the size is less than 3 cm, and differentiated lesions less than 3 cm in size that invade less than 500 μm into the submucosa and without evidence of lymphovascular invasion on computed tomography or endoscopic ultrasound[4,20].
Patients who undergo treatment according to these expanded criteria exhibit similar long-term survival and outcomes as patients who are treated according to the basic criteria[21]. Patients are considered to have undergone non-curative ER when histological examinations reveal that the resected specimen does not meet the criteria for curative ER (Table 1) according to the Japanese Classification of Gastric Carcinoma[22]. Surgery remains the standard treatment after non-curative ER in patients with a possible risk of LNM[23]. The incidence of residual tumors in surgical specimens of gastric cancer was 24.6% (out of 118 cases) after incomplete ESD, and radical gastrectomy should be performed if pathological examination reveals a positive lateral resection margin after ESD[24]. However, gastrectomy with lymph node dissection should be considered for patients with submucosal invasion regardless of margin status because of possible residual tumor or LNM[25]. A suspicious margin status should be managed similarly to a positive margin status after endoscopic treatment because of the high rate of residual cancer. These authors emphasized that gastrectomy is sometimes unnecessary because recurrence may involve only the mucosa, and more minimally invasive treatment modalities, such as re-ESD and other endoscopic interventions, are recommended in these cases. Incomplete resection by gastric ESD significantly increases the local recurrence risk, and most initial recurrence lesions that are treated with additional argon plasma coagulation recur[26].
Table 1.
Histological criteria for curative endoscopic resection for gastric cancer
| Factors associated with no risk of lymph node metastasis |
| Intestinal-type histology |
| No lymphatic or vascular infiltration |
| Intramucosal cancer, regardless of tumor size, without ulcer findings |
| or intramucosal cancer less than 30 mm in size with ulcer findings |
| or minute submucosal invasive cancer (SM1) less than 30 mm in size |
| Factors associated with the resection margin |
| Tumor-free horizontal margin |
| Tumor-free vertical margin |
Elderly patients should not undergo radical surgery if they have comorbid diseases or a limited life expectancy. However, additional surgery following non-curative ER improves overall and disease-free survival compared with nonsurgical observation in stomach cancer patients > 75 years of age[27], and surgery should be considered following non-curative ER in elderly patients.
Colon and rectum
The 2010 Guidelines for the Treatment of Colorectal Cancer from the Japanese Society for Cancer of the Colon and Rectum recommends colorectal ER for intramucosal cancer (M) and cancers with slight invasion into the submucosal layer of less than 2 cm in diameter, irrespective of the shape, based on a large number of studies on resected specimens[5,28,29]. The guideline-recommended indications for additional surgical treatment are presented in Table 2. The budding grade and histological type exhibit a greater association with LNM than the depth of submucosal invasion[30,31], and well-differentiated adenocarcinoma without budding may be observed after complete ER even if the cancer invades the submucosal layer.
Table 2.
Factors for which additional treatment after endoscopic submucosal dissection should be recommended
| Esophagus (squamous cell carcinoma) |
| Tumors in contact with or invading the muscularis mucosa |
| Tumors invading the submucosal layer |
| Stomach |
| Positive lateral margins |
| Deep submucosal invasion, regardless of positive vertical margins |
| (> 500 μm) |
| Vascular or lymphatic invasion |
| Diffuse-type histology |
| Colon and rectum |
| Positive vertical margins at the site of submucosal invasion |
| Depth of submucosal invasion greater than 1000 μm |
| Vascular or lymphatic invasion |
| Poorly differentiated adenocarcinoma, signet ring cell carcinoma, or |
| mucinous carcinoma |
| High-grade tumor budding1 |
Tumor budding: An isolated single cell or a cluster composed of fewer than five cancer cells in the stroma of the actively invasive region.
ESD FOR RESIDUAL/RECURRENT CANCER
Repeat EMR as a cure for locally recurrent gastrointestinal tumors is difficult to perform because the initial ER or chemoradiotherapy produces submucosal fibrosis. Surgical resection is necessary in these cases. However, ESD allows for a submucosal dissection through the fibrosis to achieve an en bloc resection.
Esophagus
EMR was evaluated as a salvage treatment after chemoradiotherapy for esophageal cancer prior to the introduction of ESD, but the rate of en bloc resection was not high enough to warrant its use. Saito et al[32] reported that 100% of cases of locally recurrent or residual superficial esophageal SCCs after chemoradiotherapy were successfully resected en bloc using ESD, and these results were superior to the en bloc resection rate of 47% for EMR.
Stomach
The rates of en bloc resection, complete resection, and curative resection for EMR for locally recurrent gastric cancer are 0%, 41%, and 33%-41%, respectively[17,33]. Surgical resection after EMR should be considered for non-curative cases. The rates of en bloc resection, complete resection, and curative resection for ESD are 89%, 95%, and 76%-80%, respectively, even in locally recurrent cases[17,18,33]. Furthermore, the rate of perforation is 3%-9%[17,33], which is comparable to the 3%-10%[12-16,18,34] perforation rate for ESD in new cases. Conservative treatment was performed after closure with an endoscopic clip in all of the perforation cases[17,33]. ESD is a useful treatment that can prevent the need for surgical resection for locally recurrent gastric cancer.
Colon and rectum
Kuroki et al[35] used ESD in 34 consecutive cases of residual or locally recurrent colorectal tumors. The perforation rate was 14.7% (five cases), emergency surgery was required in one case, and the rates of en bloc resection and complete resection were 93% and 82%, respectively. These authors concluded that the use of ESD for residual/locally recurrent lesions was curative and efficacious, and this technique reduces surgical resection requirements. The en bloc[36] resection rate of ESD in 30 cases of residual or locally recurrent colorectal tumors was 93%, and the complete resection rate was 83%. However, these favorable results were reported at high-volume institutions, and this procedure is not easily performed because of submucosal fibrosis, spastic bowel movements, and the low controllability of the scope in the right colon[37]. The use of ESD for a recurrent lesion requires a highly advanced technique, and the laparoscopic resection of colonic cancer may be performed within almost the same time frame as ESD at some institutions[38]. Therefore, the treatment modality for a residual/recurrent tumor in the colon should be chosen according to the technical abilities of the physician.
TRANSANAL RESECTION, TRANSANAL ENDOSCOPIC MICROSURGERY, AND ESD
Transanal resection (TAR) (Figure 3A) and TEM (Figure 3B) are well-developed surgical procedures for the local excision of rectal adenomas, intramucosal cancers, and superficial submucosal cancers of the rectum. ESD is associated with a longer procedure time than TAR (131 min vs 63 min), but ESD is more effective than TAR for the treatment of non-invasive rectal tumors, with a lower recurrence rate (0% vs 15.5%) and shorter hospital stays (4.9 d vs 7 d)[39]. TEM provides better visualization than TAR, and the clinical results with TEM are superior. However, the recurrence rate is as high as 4%-8%[40-43]. TEM exhibits a technical advantage because it enables the removal of the full thickness of the rectal wall, but the recurrence rates are 0% for SM1, 17% for SM2, and 30% for SM3. Therefore, TEM alone is not a feasible treatment for massive SM rectal cancers, and its indication is not broader than ESD. TEM suffers from poor visualization of the operative field near the dentate line, relatively high recurrence rates, and high instrumentation costs. Therefore, ESD may be a better technique for adenoma, intramucosal cancer, and slightly invasive submucosal cancer in the rectum. Figure 4 presents an example case of ESD for a rectal tumor larger than 10 cm.
Figure 3.
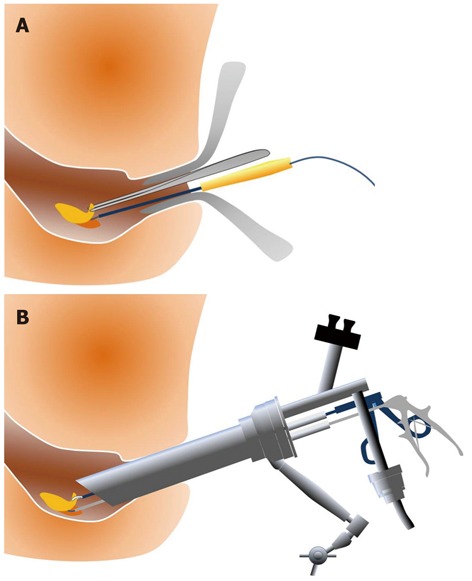
Resection of a rectal tumor. A: Transanal resection; B: Transanal endoscopic microsurgery.
Figure 4.

A case of a rectal tumor resected by endoscopic submucosal dissection in which laparotomy was required. A: A broad-based tumor spreading to over half of the circumference is observed in the rectum; B: Chromoendoscopy with indigo carmine; C: Mucosal incision with the Flush knife; D: Appearance of the mucosa after complete resection by endoscopic submucosal dissection; E: The fixed resected specimen was 115 mm in diameter.
SURGERY FOR ESD COMPLICATIONS
Perforation and bleeding are the major complications during the ESD procedure, and perforation, bleeding, and stenosis may occur after the operation. Most of the bleeding may be treated using an endoscopic approach, but all perforations are traditionally treated using salvage laparotomy with closure of the perforation and intensive intra-abdominal lavage with a large volume of physiological saline. However, perforation during the ESD procedure may be nonsurgically treated using endoscopic closure followed by adequate intensive conservative treatment[44].
Esophagus
The perforation rate in esophageal ESD is 0%-20%[6,45-51]. Perforation may cause severe emphysema or mediastinal inflammation, which may require surgical or interventional mediastinal drainage[52]. However, immediate endoscopic closure of the perforation followed by intensive conservative treatment can avoid the need for surgery in most perforations during the ESD procedure[44]. Pneumomediastinum, which does not cause clinically significant complications, is frequently detected after esophageal ESD by chest computed tomography (31%)[53]. Esophageal ESD is associated with esophageal stenosis, particularly when ESD involves the entire circumference of the esophageal lumen. Various techniques, including intralesional steroid injection[54], oral prednisolone administration[55,56], balloon dilatation[55-58], and the placement of a self-expanding metal stent, are used[59,60], and surgical treatment is not generally necessary. However, a small risk of perforation has been observed during balloon dilatation[61] and steroid injection[54]; therefore, these procedures should be performed carefully.
Stomach
Perforation during ESD for stomach tumors occurs in 2%-10% of cases, and most perforations are treated using endoscopic and conservative treatments without a surgical approach[12-18]. However, delayed perforation after gastric ESD generally requires emergency surgery. Hanaoka et al[62] demonstrated that delayed perforation after gastric ESD occurred in six of 1159 patients (0.45%), and five of these six patients required emergency surgery. Three of these patients were treated using omentoplasty. However, two patients required gastrectomy because the perforation hole was too large to be closed with an omental patch in one patient, and the omentum had been removed in a previous colectomy in the other patient.
Colon and rectum
The perforation rate in colorectal ESD is 4%-10%, and most cases are treated nonsurgically[35,62-69]. However, delayed perforation after colorectal ESD generally requires emergency surgery. Saito et al[64] observed perforations after colorectal ESD in 54 of 1111 cases (4.9%) and delayed perforation in 4 cases (0.4%). Two immediate perforations with ineffective endoscopic clipping and three delayed perforations required emergency surgery. Fujishiro et al[70] reported 11 (5.5%) immediate perforations in 200 cases of colorectal ESD that were successfully managed conservatively, but one delayed perforation (0.5%) required laparotomy.
ESD AFTER GASTROENTEROLOGICAL SURGERY
The remnant stomach after gastrectomy remains at high risk for a second primary gastric cancer. Traditionally, the outcome for patients with gastric cancer in the remnant stomach has been poor, and the standard treatment for remnant gastric cancer was total gastrectomy with extended lymph node dissection[71]. However, Takeda et al[72] observed that none of the 15 patients with early remnant gastric cancer in his consecutive series presented LNM, and the prognosis of these patients was good. The quality of life after total gastrectomy is markedly reduced. Therefore, ER is a highly desirable method for the preservation of the stomach. ER of the lesion in the remnant stomach is technically difficult using the conventional method of EMR because of the narrow inner space and the massive fibrosis around the staples in the suture line. The rates of en bloc resection and complete resection in the ESD of remnant gastric tumors are 97%-100% and 74%-92%, respectively[73-75]. The 0%-13% perforation rate is higher than the perforation rate in the normal stomach, but all perforations have been successfully treated[73-75]. ESD improves the avoidance of surgical resection in early lesions in the remnant stomach after gastrectomy.
ESD is also highly indicated for tumors in the gastric tube after esophagectomy. The en bloc resection rate of ESD for gastric tube tumors is 88%-90%[76,77], which is higher than the rate associated with EMR[76]. One case of successful ESD for a tumor in a colonic interposition after esophagectomy has been reported[78].
A retrospective analysis of 639 cases of esophageal cancer in patients who underwent gastric tube reconstruction and survived more than 10 years revealed that gastric cancer developed at a constant rate, even many years after esophagectomy, with a 10-year cumulative incidence rate of 8.1%[76]. The duration of survival has improved due to progress in the diagnosis and treatment of esophageal cancer. A lesion in a reconstructed organ, such as a gastric tube or colonic interposition, will likely appear more often, and ESD will play a more important role in the treatment of these lesions.
COMBINATION OF ESD AND LAPAROSCOPIC SURGERY
Sentinel lymph node biopsy after ESD
ESD is applied to benign or early malignant lesions without LNM, and the indication criteria for its use in each organ are discussed above. Local excision may be permitted if no LNM is specifically proven in cases that do not meet the criteria, such as intramucosal undifferentiated carcinoma or massively invaded submucosal cancer. The use of laparoscopic sentinel lymph node biopsy (SLNB) for the examination of LNM after ESD for early gastric cancer that does not meet the standard or expanded indication criteria has been reported[79]. Additional curative surgical resection was performed when LNM was observed. Cahill et al[80] applied NOTES after ESD of the stomach and sigmoid colon in a porcine model based on the same criteria. However, a systemic review of 21 articles on SLNB in gastric cancer patients revealed that the overall sensitivity of SLNB was 85.4%[81]. The authors concluded that there was insufficient evidence to introduce SLNB into the treatment protocol for gastric cancer[81]. A meta-analysis of 53 studies on SLNB in colon and rectal cancer revealed a pooled sensitivity of only 76%[82]. The accuracy of SLNB varies according to the biopsy procedure, experience, and method of staining. Therefore, technical improvements in SLNB may lead to the clinical application of the combination therapy of ESD and SLNB using laparoscopic surgery or NOTES.
Gastric submucosal tumors
The combination of ESD and laparoscopic surgery as a novel treatment method for gastric submucosal tumors, such as GISTs, has been reported at some institutions. Laparoscopic excision has been applied to gastric submucosal tumors, but the precise location of the lesion could not be detected in the laparoscopic view. Therefore, excessive resection is inevitable for complete resection. The ESD technique is used for mucosal cutting and full-thickness wall cutting around the lesion prior to laparoscopy to minimize excessive resection. Complete resection is then performed using a laparoscopic approach[79,83-86] (Figure 5).
Figure 5.
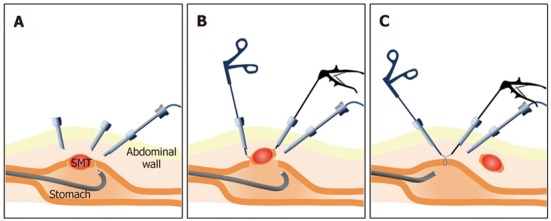
Combination of endoscopic submucosal dissection and laparoscopic surgery. A: Confirmation of tumor location and mucosal cutting around the tumor using endoscopic submucosal dissection; B: The full thickness of the stomach wall was cut using a laparoscopic instrument, such as Ligasure®; C: The gastric wall was closed using a laparoscopic hand-sewn technique or laparoscopic suturing device, such as End-GIA.
Application of the ESD technique to NOTES
NOTES has attracted considerable attention as a mini-mally invasive surgery. Endoscopic transesophageal mediastinal lymph node dissection and epicardial coagulation using mediastinal and thoracic approaches in a porcine model have been reported previously[87] (Figure 6). An element of the ESD technique has been applied to these novel surgeries.
Figure 6.
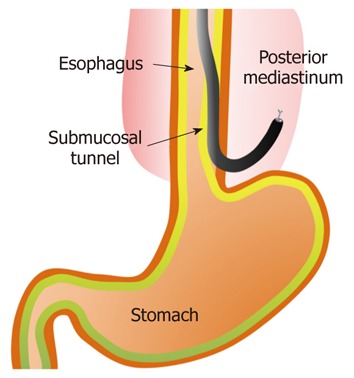
Natural orifice transluminal endoscopic surgery using the endoscopic submucosal dissection technique (in a porcine model).
CONCLUSION
Additional treatments, including surgery with lymph node dissection, should be recommended to patients with non-curative resection following ESD. ESD may be successfully applied for a residual/recurrent gastrointestinal tumor after ER, a second primary tumor in the remnant stomach after gastrectomy, or a tumor in the gastric tube or colon that was reconstructed after gastroenterological surgery. ESD is superior to TAR and TEM for the treatment of an early rectal tumor. Most of the perforations that occur during the ESD procedure can be treated nonsurgically using endoscopic closure, but delayed perforations require emergency surgery. This review summarized novel trials of the combination of ESD and laparoscopic surgery, and the application of an element of the ESD technique to NOTES was reported.
Footnotes
Peer reviewers: Jaekyu Sung, MD, PhD, Assistant Professor, Division of Gastroenterology, Department of Internal Medicine, Chungnam National University Hospital, 33 Munhwa-ro, Jung-gu, Daejeon 301-721, South Korea; Shotaro Enomoto, MD, PhD, Second Department of Internal Medicine, Wakayama Medical University, 811-1, Kimiidera, Wakayama 641-0012, Japan; Taro Osada, MD, PhD, Associate Professor, Department of Gastroenterology, Juntendo University, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan; Satoru Kakizaki, MD, PhD, Assistant Professor, Department of Medicine and Molecular Science, Gunma University, Graduate School of Medicine, 3-39-15 Showa-machi, Maebashi, Gunma 371-8511, Japan
S- Editor Song XX L- Editor A E- Editor Zhang DN
References
- 1.Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 2.Gotoda T, Sasako M, Ono H, Katai H, Sano T, Shimoda T. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg. 2001;88:444–449. doi: 10.1046/j.1365-2168.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 3.Tajima Y, Nakanishi Y, Ochiai A, Tachimori Y, Kato H, Watanabe H, Yamaguchi H, Yoshimura K, Kusano M, Shimoda T. Histopathologic findings predicting lymph node metastasis and prognosis of patients with superficial esophageal carcinoma: analysis of 240 surgically resected tumors. Cancer. 2000;88:1285–1293. [PubMed] [Google Scholar]
- 4.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 5.Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. doi: 10.1007/s00535-004-1339-4. [DOI] [PubMed] [Google Scholar]
- 6.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video) Gastrointest Endosc. 2007;66:966–973. doi: 10.1016/j.gie.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 8.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–1197. doi: 10.1136/bmj.327.7425.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eguchi T, Nakanishi Y, Shimoda T, Iwasaki M, Igaki H, Tachimori Y, Kato H, Yamaguchi H, Saito D, Umemura S. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol. 2006;19:475–480. doi: 10.1038/modpathol.3800557. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Kato M, Yamamoto J, Nakagawa S, Tsukagoshi H, Fujita M, Hosokawa M, Asaka M. EMR combined with chemoradiotherapy: a novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc. 2004;59:199–204. doi: 10.1016/s0016-5107(03)02688-9. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa Y, Muto M, Minashi K, Boku N, Fukuda H. A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan Clinical Oncology Group Study JCOG0508. Jpn J Clin Oncol. 2009;39:686–689. doi: 10.1093/jjco/hyp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P) Dig Liver Dis. 2009;41:201–209. doi: 10.1016/j.dld.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y, et al. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821–826. doi: 10.1111/j.1440-1746.2006.04505.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746–750. doi: 10.1055/s-0029-1215010. [DOI] [PubMed] [Google Scholar]
- 17.Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Clinical advantages of endoscopic submucosal dissection for gastric cancers in remnant stomach surpass conventional endoscopic mucosal resection. Dig Endosc. 2010;22:17–20. doi: 10.1111/j.1443-1661.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 18.Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer Society. The Guidelines for Diagnosis and Treatment of Carcinoma of the Stomach, 2004. Available from: http//www.jgca.jp/PDFfiles/Guidelines2004_eng.pdf.
- 20.Haruta H, Hosoya Y, Sakuma K, Shibusawa H, Satoh K, Yamamoto H, Tanaka A, Niki T, Sugano K, Yasuda Y. Clinicopathological study of lymph-node metastasis in 1,389 patients with early gastric cancer: assessment of indications for endoscopic resection. J Dig Dis. 2008;9:213–218. doi: 10.1111/j.1751-2980.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- 21.Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–871. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 22.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 23.Oda I, Gotoda T, Sasako M, Sano T, Katai H, Fukagawa T, Shimoda T, Emura F, Saito D. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95:1495–1500. doi: 10.1002/bjs.6305. [DOI] [PubMed] [Google Scholar]
- 24.Jung H, Bae JM, Choi MG, Noh JH, Sohn TS, Kim S. Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg. 2011;98:73–78. doi: 10.1002/bjs.7274. [DOI] [PubMed] [Google Scholar]
- 25.Song KY, Hyung WJ, Kim HH, Han SU, Cho GS, Ryu SW, Lee HJ, Kim MC. Is gastrectomy mandatory for all residual or recurrent gastric cancer following endoscopic resection? A large-scale Korean multi-center study. J Surg Oncol. 2008;98:6–10. doi: 10.1002/jso.21074. [DOI] [PubMed] [Google Scholar]
- 26.Park JC, Lee SK, Seo JH, Kim YJ, Chung H, Shin SK, Lee YC. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24:2842–2849. doi: 10.1007/s00464-010-1060-8. [DOI] [PubMed] [Google Scholar]
- 27.Kusano C, Iwasaki M, Kaltenbach T, Conlin A, Oda I, Gotoda T. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol. 2011;106:1064–1069. doi: 10.1038/ajg.2011.49. [DOI] [PubMed] [Google Scholar]
- 28.Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka S, Haruma K, Oh-E H, Nagata S, Hirota Y, Furudoi A, Amioka T, Kitadai Y, Yoshihara M, Shimamoto F. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep. 2000;7:783–788. doi: 10.3892/or.7.4.783. [DOI] [PubMed] [Google Scholar]
- 30.Komori K, Hirai T, Kanemitsu Y, Shimizu Y, Sano T, Ito S, Senda Y, Misawa K, Ito Y, Kato T. Is “depth of submucosal invasion > or = 1,000 microm” an important predictive factor for lymph node metastases in early invasive colorectal cancer (pT1)? Hepatogastroenterology. 2010;57:1123–1127. [PubMed] [Google Scholar]
- 31.Kazama S, Watanabe T, Ajioka Y, Kanazawa T, Nagawa H. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer. 2006;94:293–298. doi: 10.1038/sj.bjc.6602927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito Y, Takisawa H, Suzuki H, Takizawa K, Yokoi C, Nonaka S, Matsuda T, Nakanishi Y, Kato K. Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy. Gastrointest Endosc. 2008;67:355–359. doi: 10.1016/j.gie.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212–218. doi: 10.1016/j.gie.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol. 2009;24:1102–1106. doi: 10.1111/j.1440-1746.2009.05811.x. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki Y, Hoteya S, Mitani T, Yamashita S, Kikuchi D, Fujimoto A, Matsui A, Nakamura M, Nishida N, Iizuka T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010;25:1747–1753. doi: 10.1111/j.1440-1746.2010.06331.x. [DOI] [PubMed] [Google Scholar]
- 36.Hurlstone DP, Shorthouse AJ, Brown SR, Tiffin N, Cross SS. Salvage endoscopic submucosal dissection for residual or local recurrent intraepithelial neoplasia in the colorectum: a prospective analysis. Colorectal Dis. 2008;10:891–897. doi: 10.1111/j.1463-1318.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto T, Saito Y, Matsuda T, Fukunaga S, Nakajima T, Fujii T. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc. 2011;25:255–260. doi: 10.1007/s00464-010-1169-9. [DOI] [PubMed] [Google Scholar]
- 38.Waters JA, Chihara R, Moreno J, Robb BW, Wiebke EA, George VV. Laparoscopic colectomy: does the learning curve extend beyond colorectal surgery fellowship? JSLS. 2010;14:325–331. doi: 10.4293/108680810X12924466006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiriyama S, Saito Y, Matsuda T, Nakajima T, Mashimo Y, Joeng HK, Moriya Y, Kuwano H. Comparing endoscopic submucosal dissection with transanal resection for non-invasive rectal tumor: a retrospective study. J Gastroenterol Hepatol. 2011;26:1028–1033. doi: 10.1111/j.1440-1746.2011.06684.x. [DOI] [PubMed] [Google Scholar]
- 40.Vorobiev GI, Tsarkov PV, Sorokin EV. Gasless transanal endoscopic surgery for rectal adenomas and early carcinomas. Tech Coloproctol. 2006;10:277–281. doi: 10.1007/s10151-006-0305-y. [DOI] [PubMed] [Google Scholar]
- 41.Bretagnol F, Merrie A, George B, Warren BF, Mortensen NJ. Local excision of rectal tumours by transanal endoscopic microsurgery. Br J Surg. 2007;94:627–633. doi: 10.1002/bjs.5678. [DOI] [PubMed] [Google Scholar]
- 42.Guerrieri M, Baldarelli M, de Sanctis A, Campagnacci R, Rimini M, Lezoche E. Treatment of rectal adenomas by transanal endoscopic microsurgery: 15 years’ experience. Surg Endosc. 2010;24:445–449. doi: 10.1007/s00464-009-0585-1. [DOI] [PubMed] [Google Scholar]
- 43.Morino M, Allaix ME, Caldart M, Scozzari G, Arezzo A. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc. 2011;25:3683–3690. doi: 10.1007/s00464-011-1777-z. [DOI] [PubMed] [Google Scholar]
- 44.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, et al. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001–1006. doi: 10.1055/s-2006-944775. [DOI] [PubMed] [Google Scholar]
- 45.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 46.Nonaka K, Arai S, Ishikawa K, Nakao M, Nakai Y, Togawa O, Nagata K, Shimizu M, Sasaki Y, Kita H. Short term results of endoscopic submucosal dissection in superficial esophageal squamous cell neoplasms. World J Gastrointest Endosc. 2010;2:69–74. doi: 10.4253/wjge.v2.i2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coda S, Trentino P, Antonellis F, Porowska B, Gossetti F, Ruberto F, Pugliese F, D’Amati G, Negro P, Gotoda T. A Western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer. 2010;13:258–263. doi: 10.1007/s10120-010-0544-5. [DOI] [PubMed] [Google Scholar]
- 48.Ishii N, Uchida S, Itoh T, Horiki N, Matsuda M, Setoyama T, Suzuki S, Uemura M, Iizuka Y, Fukuda K, et al. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife for superficial esophageal neoplasia. Is it safe for elderly patients? Surg Endosc. 2010;24:2110–2119. doi: 10.1007/s00464-010-0907-3. [DOI] [PubMed] [Google Scholar]
- 49.Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626–631. doi: 10.1111/j.1442-2050.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 50.Neuhaus H, Wirths K, Schenk M, Enderle MD, Schumacher B. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc. 2009;70:112–120. doi: 10.1016/j.gie.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 51.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Miyashita M, Nomura T, Makino H, Hagiwara N, Takahashi K, Sakata Y, Nagata K, Iwakiri K, Tajima H, Tajiri T. Computed tomography-guided mediastinal drainage for iatrogenic perforation of the esophagus. J Nippon Med Sch. 2006;73:337–340. doi: 10.1272/jnms.73.337. [DOI] [PubMed] [Google Scholar]
- 53.Tamiya Y, Nakahara K, Kominato K, Serikawa O, Watanabe Y, Tateishi H, Takedatsu H, Toyonaga A, Sata M. Pneumomediastinum is a frequent but minor complication during esophageal endoscopic submucosal dissection. Endoscopy. 2010;42:8–14. doi: 10.1055/s-0029-1215215. [DOI] [PubMed] [Google Scholar]
- 54.Kochhar R, Poornachandra KS. Intralesional steroid injection therapy in the management of resistant gastrointestinal strictures. World J Gastrointest Endosc. 2010;2:61–68. doi: 10.4253/wjge.v2.i2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isomoto H, Yamaguchi N, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46. doi: 10.1186/1471-230X-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi N, Isomoto H, Shikuwa S, Nakayama T, Hayashi T, Ohnita K, Takeshima F, Kohno S, Nakao K. Effect of oral prednisolone on esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: a case report. Digestion. 2011;83:291–295. doi: 10.1159/000321093. [DOI] [PubMed] [Google Scholar]
- 57.Wong VW, Teoh AY, Fujishiro M, Chiu PW, Ng EK. Preemptive dilatation gives good outcome to early esophageal stricture after circumferential endoscopic submucosal dissection. Surg Laparosc Endosc Percutan Tech. 2010;20:e25–e27. doi: 10.1097/SLE.0b013e3181c922a7. [DOI] [PubMed] [Google Scholar]
- 58.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661–665. doi: 10.1055/s-0029-1214867. [DOI] [PubMed] [Google Scholar]
- 59.Saito Y, Tanaka T, Andoh A, Minematsu H, Hata K, Tsujikawa T, Nitta N, Murata K, Fujiyama Y. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci. 2008;53:330–333. doi: 10.1007/s10620-007-9873-6. [DOI] [PubMed] [Google Scholar]
- 60.Kim SH, Lee SO. Circumferential intramural esophageal dissection successfully treated by endoscopic procedure and metal stent insertion. J Gastroenterol. 2005;40:1065–1069. doi: 10.1007/s00535-005-1692-y. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi H, Arimura Y, Okahara S, Uchida S, Ishigaki S, Tsukagoshi H, Shinomura Y, Hosokawa M. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy. 2011;43:184–189. doi: 10.1055/s-0030-1256109. [DOI] [PubMed] [Google Scholar]
- 62.Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H, et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112–1115. doi: 10.1055/s-0030-1255932. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka S, Tamegai Y, Tsuda S, Saito Y, Yahagi N, Yamano HO. Multicenter questionnaire survey on the current situation of colorectal endoscopic submucosal dissection in Japan. Dig Endosc. 2010;22 Suppl 1:S2–S8. doi: 10.1111/j.1443-1661.2010.00952.x. [DOI] [PubMed] [Google Scholar]
- 64.Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Nishiyama H, Isomoto H, Yamaguchi N, Ishii H, Fukuda E, Machida H, Nakamura T, Ohnita K, Shikuwa S, Kohno S, et al. Endoscopic submucosal dissection for laterally spreading tumours of the colorectum in 200 consecutive cases. Surg Endosc. 2010;24:2881–2887. doi: 10.1007/s00464-010-1071-5. [DOI] [PubMed] [Google Scholar]
- 66.Fujishiro M, Kodashima S, Goto O, Ono S, Niimi K, Yamamichi N, Oka M, Ichinose M, Omata M. Endoscopic submucosal dissection for esophageal squamous cell neoplasms. Dig Endosc. 2009;21:109–115. doi: 10.1111/j.1443-1661.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida N, Naito Y, Kugai M, Inoue K, Wakabayashi N, Yagi N, Yanagisawa A, Yoshikawa T. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol. 2010;16:4180–4186. doi: 10.3748/wjg.v16.i33.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723–729. doi: 10.1055/s-0030-1255675. [DOI] [PubMed] [Google Scholar]
- 69.Toyonaga T, Man-i M, Fujita T, East JE, Nishino E, Ono W, Morita Y, Sanuki T, Yoshida M, Kutsumi H, et al. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy. 2010;42:714–722. doi: 10.1055/s-0030-1255654. [DOI] [PubMed] [Google Scholar]
- 70.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678–683; quiz 645. doi: 10.1016/j.cgh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Sasako M, Maruyama K, Kinoshita T, Okabayashi K. Surgical treatment of carcinoma of the gastric stump. Br J Surg. 1991;78:822–824. doi: 10.1002/bjs.1800780718. [DOI] [PubMed] [Google Scholar]
- 72.Takeda J, Toyonaga A, Koufuji K, Kodama I, Aoyagi K, Yano S, Ohta J, Shirozu K. Early gastric cancer in the remnant stomach. Hepatogastroenterology. 1998;45:1907–1911. [PubMed] [Google Scholar]
- 73.Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Matsumura S, Suzuki S. Treatment of gastric remnant cancer post distal gastrectomy by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol. 2008;14:2550–2555. doi: 10.3748/wjg.14.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JY, Choi IJ, Cho SJ, Kim CG, Kook MC, Lee JH, Ryu KW, Kim YW. Endoscopic submucosal dissection for metachronous tumor in the remnant stomach after distal gastrectomy. Surg Endosc. 2010;24:1360–1366. doi: 10.1007/s00464-009-0779-6. [DOI] [PubMed] [Google Scholar]
- 75.Takenaka R, Kawahara Y, Okada H, Tsuzuki T, Yagi S, Kato J, Ohara N, Yoshino T, Imagawa A, Fujiki S, et al. Endoscopic submucosal dissection for cancers of the remnant stomach after distal gastrectomy. Gastrointest Endosc. 2008;67:359–363. doi: 10.1016/j.gie.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 76.Bamba T, Kosugi S, Takeuchi M, Kobayashi M, Kanda T, Matsuki A, Hatakeyama K. Surveillance and treatment for second primary cancer in the gastric tube after radical esophagectomy. Surg Endosc. 2010;24:1310–1317. doi: 10.1007/s00464-009-0766-y. [DOI] [PubMed] [Google Scholar]
- 77.Osumi W, Fujita Y, Hiramatsu M, Kawai M, Sumiyoshi K, Umegaki E, Tokioka S, Yoda Y, Egashira Y, Abe S, et al. Endoscopic submucosal dissection allows less-invasive curative resection for gastric tube cancer after esophagectomy - a case series. Endoscopy. 2009;41:777–780. doi: 10.1055/s-0029-1215024. [DOI] [PubMed] [Google Scholar]
- 78.Bando H, Ikematsu H, Fu KI, Oono Y, Kojima T, Minashi K, Yano T, Matsuda T, Saito Y, Kaneko K, et al. A laterally-spreading tumor in a colonic interposition treated by endoscopic submucosal dissection. World J Gastroenterol. 2010;16:392–394. doi: 10.3748/wjg.v16.i3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908–1913. doi: 10.1007/s00464-008-0317-y. [DOI] [PubMed] [Google Scholar]
- 80.Cahill RA, Asakuma M, Perretta S, Leroy J, Dallemagne B, Marescaux J, Coumaros D. Supplementation of endoscopic submucosal dissection with sentinel node biopsy performed by natural orifice transluminal endoscopic surgery (NOTES) (with video) Gastrointest Endosc. 2009;69:1152–1160. doi: 10.1016/j.gie.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 81.Lips DJ, Schutte HW, van der Linden RL, Dassen AE, Voogd AC, Bosscha K. Sentinel lymph node biopsy to direct treatment in gastric cancer. A systematic review of the literature. Eur J Surg Oncol. 2011;37:655–661. doi: 10.1016/j.ejso.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 82.van der Pas MH, Meijer S, Hoekstra OS, Riphagen II, de Vet HC, Knol DL, van Grieken NC, Meijerink WJ. Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12:540–550. doi: 10.1016/S1470-2045(11)70075-4. [DOI] [PubMed] [Google Scholar]
- 83.Wong DC, Wong SK, Leung AL, Chung CC, Li MK. Combined endolaparoscopic intragastric excision for gastric neoplasms. J Laparoendosc Adv Surg Tech A. 2009;19:765–770. doi: 10.1089/lap.2009.0067. [DOI] [PubMed] [Google Scholar]
- 84.Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–1735. doi: 10.1007/s00464-007-9696-8. [DOI] [PubMed] [Google Scholar]
- 85.Tanabe K, Urabe Y, Tokumoto N, Suzuki T, Yamamoto H, Oka S, Tanaka S, Ohdan H. A new method for intraluminal gastrointestinal stromal tumor resection using laparoscopic seromuscular dissection technique. Dig Surg. 2010;27:461–465. doi: 10.1159/000320458. [DOI] [PubMed] [Google Scholar]
- 86.Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, Koike K. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183–187. doi: 10.1007/s10120-011-0014-8. [DOI] [PubMed] [Google Scholar]
- 87.Turner BG, Gee DW, Cizginer S, Kim MC, Mino-Kenudson M, Sylla P, Brugge WR, Rattner DW. Endoscopic transesophageal mediastinal lymph node dissection and en bloc resection by using mediastinal and thoracic approaches (with video) Gastrointest Endosc. 2010;72:831–835. doi: 10.1016/j.gie.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


