Abstract
Aims
Chronic kidney disease (CKD) is a risk factor for left ventricular hypertrophy (LVH) and heart failure. We evaluated the effect of CKD on left ventricular (LV) remodelling among patients with mild heart failure.
Methods and results
REVERSE was a randomized, controlled trial evaluating cardiac resynchronization therapy (CRT) in patients with New York Heart Association (NYHA) class I/II heart failure. CKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. We compared changes in LV function and size over the course of 12 months by CKD status using linear mixed models adjusted for demographics, co-morbidities, medications, cardiomyopathy aetiology, and CRT status. Finally, we evaluated the effect of CKD on cardiac remodelling among patients randomized to CRT on or off. CKD was associated with worsening LV function and dilation compared with the non-CKD group {adjusted, 12-month β coefficients for the CKD group compared with the non-CKD referent group: LV ejection fraction (%) [–1.80, 95% confidence interval (CI) –3.36 to –0.24], LV end-systolic volume (mL) (14.16, 95% CI 3.96–24.36), LV end-diastolic volume (mL) (14.88, 95% CI 2.88–26.76), LV end-systolic diameter (cm) (0.36, 95% CI 0.12–0.48), LV end-diastolic diameter (cm) (0.24, 95% CI 0.012–0.36), mitral regurgitation (%) (3.12, 95% CI 0.48–5.76), and LV shape (0.036, 95% CI 0.012–0.060)}. In participants assigned to CRT, those without CKD had significantly greater improvements in LV structural parameters compared with the CKD group.
Conclusions
In comparison with participants with normal kidney function, CKD is an independent risk factor for ventricular dysfunction and dilation. CRT improves LV function and structure to a lesser extent in patients with CKD than in those with normal kidney function.
Keywords: Chronic kidney disease, Heart failure, Cardiac resynchronization therapy
Introduction
Kidney disease is a frequent complication of congestive heart failure (CHF) and may contribute to the progression of ventricular dysfunction. Advanced stages of CHF may be associated with the cardiorenal anaemia syndrome in which heart failure, kidney disease, and anaemia reflect an extreme form of disease progression.1,2 Regardless of the degree of heart failure, chronic kidney disease (CKD) increases the risk of death and cardiac decompensation.3,4 A host of biological pathways related to neurohormonal dysregulation, vascular calcification, oxidative stress, and renin–angiotensin–aldosterone activation have been implicated as potential mechanisms underlying this increased risk.5–7 Left ventricular hypertrophy (LVH) is a known parameter of cardiac remodelling and has a higher prevalence and incidence among people with impaired kidney function.8–10 LVH is an early subclinical marker of cardiovascular disease and heart failure risk,11–14 and is probably an intermediary step in the pathway leading from kidney dysfunction to heart failure and its complications. The effects of CKD on other left ventricular and myocardial parameters, however, have been less characterized.
We assessed systematically the effect of CKD on left ventricular remodelling among participants enrolled in the Resynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) trial. REVERSE was a clinical trial of cardiac resynchronization therapy (CRT) in patients with asymptomatic and mildly symptomatic heart failure and a prolonged QRS interval. The primary findings from REVERSE contributed to the recent European Society of Cardiology guidelines recommending CRT for patients with New York Heart Association (NYHA) class I or II heart failure.15 Subsequent survey data have reported reductions in death and heart failure hospitalizations after incorporating these guidelines in clinical practice.16 Although clinical trials have highlighted subgroup findings including the effect of CRT by gender, QRS duration, ischaemic vs. non-ischaemic aetiology, presence of atrial fibrillation, and NYHA functional class, limited data exist on the effects of kidney function on cardiac remodelling. We hypothesized that CKD would be an independent risk factor for adverse left ventricular remodelling regardless of randomization to CRT status. In addition, we hypothesized that the reverse remodelling effects known to result from CRT17,18 would be attenuated with the presence of CKD. Finally, we evaluated the effect of CKD on adverse outcomes among this group of participants with systolic dysfunction and mildly symptomatic heart failure.
Methods
Design
The REVERSE study was a prospective, randomized, double-blinded controlled trial of CRT in patients with NYHA class I or II heart failure for at least 3 months before enrolment. All patients were in sinus rhythm with a QRS duration ≥120 ms, left ventricular ejection fraction (LVEF) ≤40%, and left ventricular end-diastolic dimension ≥55 mm. Patient inclusion and exclusion criteria have been published previously.19 All patients were receiving optimal medical heart failure therapy including an angiotensin-converting enzyme (ACE) inhibitor and/or an angiotensin II receptor blocker (ARB), and a beta-blocker.20,21 All participants provided written informed consent before study entry. The ethics committee at each investigator site approved the protocol. The study complies with the Declaration of Helsinki.
Study population
A total of 610 patients were randomized in a 2:1 fashion to the active therapy defined as the cardiac resynchronization device (CRT) to be programmed on (419 patients) or the control group (191 patients) with CRT programmed off for 12 months. Patients were evaluated at 1, 3, 6, and 12 months in a double-blind fashion. In addition two-dimensional, M-mode, and Doppler echocardiograms were recorded along with an electrocardiogram (ECG) at baseline, 6 months, and 12 months.
Kidney function
Creatinine measures were obtained only at the time of study enrolment. REVERSE assessed kidney function using the Modification of Diet in Renal Disease equation.22 Since the design of the original trial, however, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation has been established to provide a more accurate estimate of the glomerular filtration rate (eGFR).23,24 As a result, we calculated the eGFR using the CKD-EPI equation for these analyses. Patients were categorized into the CKD group (eGFR <60 mL/min/1.73 m2) or the non-CKD referent group (eGFR ≥60 mL/min/1.73 m2). Of the 610 patients enrolled in REVERSE, 49 had the race variable missing and were subsequently excluded from the eGFR calculation and this analysis.
Other baseline risk factors for chronic kidney disease and/or ventricular remodelling
Baseline variables, which were assessed as potential confounders, included age, gender, race, systolic blood pressure, diastolic blood pressure, diabetes, smoking, medications (including beta-blockers, ACE inhibitors, ARBs, diuretics), and the aetiology of the cardiomyopathy. Ischaemic cardiomyopathy is defined as a history of myocardial infarction or coronary revascularization and/or evidence of two- or three-vessel disease by coronary angiography,25 and non-ischaemic cardiomyopathy as the absence of these criteria.
Outcome variables
In the present study, the primary outcome variables included echocardiographic measures of left ventricular size and function and the heart failure clinical composite response.26 All echocardiographic measures were performed at one of the core labs in the USA or Europe. Echocardiographers at these labs were blinded to CRT assignment and unaware of kidney function. Echocardiographic parameters evaluated at baseline, 6 months, and 12 months included LVEF, left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), LV mass, mitral regurgitation (MR), LV cavity shape in diastole (LV shape), interventricular mechanical delay (IVMD), and myocardial performance index (MPI). Left ventricular dimensions were recorded with 2D-directed M-mode echocardiography at the tips of the mitral valve leaflets. The images were digitized to obtain left ventricular volumes. LVEF was calculated using Simpson's method of discs.27 LV mass was calculated at end-diastole. The severity of MR was assessed as the average area of the Doppler colour-encoded mitral regurgitant jet within the left atrium. LV shape was computed at end-diastole as the ratio of left ventricular volume to the volume of a sphere with a diameter equal to left ventricular cavity length in the apical four-chamber view.28 IVMD was used as an indicator of interventricular dyssynchrony, defined as the time interval between the onset of antegrade blood flow in the right ventricular outflow tract and in the left ventricular outflow tract. Finally, the MPI was calculated as the sum of isovolumic contraction time and isovolumic relaxation time divided by left ventricular ejection time.29
We also evaluated a worsening in clinical status with the heart failure clinical composite response, which was assessed at the 12-month follow-up visit.19,26 Patients were judged to have a worsening status if they died, were hospitalized due to worsening heart failure, crossed over or discontinued treatment because of worsening symptoms, demonstrated worsening in NYHA functional class, or reported moderately or markedly worse heart failure symptoms following CRT implant.
Statistical analysis
Baseline characteristics of participants were compared by CKD status using χ2 or t-tests where appropriate. Cross-sectional associations between CKD and echocardiographic indices were assessed using linear regression modelling. The estimates were adjusted for potential confounders that are known to affect either kidney function or cardiac remodelling parameters, and included age, gender, race, systolic blood pressure, diastolic blood pressure, diabetes, smoking, cardioprotective medications (beta-blocker, ACE inhibitor, ARB, and diuretic use), cardiomyopathy aetiology, and CRT assignment on or off. A linear mixed effects model with random intercepts and slopes was used to compare linear trends over the baseline, 6-month, and 12-month periods to evaluate the association between CKD and longitudinal measures of echocardiographic indices. This approach takes into account the correlation of observations by subject and adjusted for the same confounders described earlier. Finally, the changes in left ventricular parameters over the 12-month follow-up stratified by CKD and CRT status were assessed.
The association between CKD and a worsening clinical composite response was determined with multivariate Cox proportional hazards regression models and adjusted for the above variables. The proportional hazards assumption was not violated in any of these analyses.
All statistical analyses were performed at academic medical centres. SPSS statistical software (release 19.0.0, SPSS Inc., and IBM Company) and Stata (release 11.2, StataCorp LP, College Station, TX, USA) were used for the analyses. A probability value <0.05 was considered statistically significant.
Results
Study population
The study consisted of 561 patients of whom 29% had CKD (Table 1). Participants with CKD were more likely to be older, diabetic, hypertensive, and have an ischaemic cardiomyopathy compared with the non-CKD group. In addition, participants with CKD were less likely to be on an ACE inhibitor and more likely to use diuretics. With respect to baseline left ventricular measures, CKD patients had a higher LVEF and lower LVESV and LVEDV. The CKD group also had less pronounced IVMD compared with the non-CKD group. Of note, there were no differences in gender, NYHA class, or CRT assignment between the CKD and the non-CKD groups.
Table 1.
Baseline clinical characteristics across kidney function groups
| eGFR ≥60 mL/min/1.73 m2 (n = 401) | eGFR <60 mL/min/1.73 m2 (n = 160) | P-value | |
|---|---|---|---|
| Age, years | 60 ± 11 | 69 ± 9 | <0.01 |
| Men (%) | 310 (77) | 130 (81) | 0.31 |
| White, n (%) | 360 (90) | 150 (94) | 0.14 |
| NYHA class II, n (%) | 327 (82) | 130 (81) | 0.94 |
| Ischaemic CM, n (%) | 201 (50) | 120 (75) | <0.01 |
| Diabetes, n (%) | 79 (20) | 52 (33) | <0.01 |
| Hypertension, n (%) | 203 (51) | 98 (61) | 0.02 |
| ACE-I, n (%) | 323 (81) | 113 (71) | 0.01 |
| ARBs, n (%) | 80 (20) | 41 (26) | 0.14 |
| Beta-blockers, n (%) | 384 (96) | 148 (93) | 0.12 |
| Diuretics, n (%) | 309 (77) | 140 (88) | <0.01 |
| Intrinsic QRS duration, mean ± SD, ms | 153 ± 22 | 153 ± 22 | 0.97 |
| Left ventricular | |||
| Ejection fraction, % | 26 ± 7 | 28 ± 7 | 0.02 |
| End-diastolic diameter, cm | 6.9 ± 1.0 | 6.8 ± 0.8 | 0.37 |
| End-systolic diameter, cm | 5.8 ± 1.1 | 5.6 ± 0.9 | 0.22 |
| End-systolic volume, mL | 205 ± 85 | 188 ± 64 | 0.02 |
| End-diastolic volume, mL | 276 ± 99 | 258 ± 77 | 0.05 |
| Mass, g | 273 ± 79 | 273 ± 74 | 0.92 |
| IVMD, ms | 36 ± 41 | 26 ± 35 | 0.01 |
| Heart rate, mean ± SD, b.p.m. | 68 ± 11 | 66 ± 10 | 0.05 |
| Supine blood pressure, mean ± SD, mmHg | |||
| Systolic | 125 ± 18 | 125 ± 20 | 0.90 |
| Diastolic | 73 ± 11 | 70 ± 11 | <0.01 |
| Weight, kg | 88 (19) | 84 (17) | 0.05 |
| K+, mean ± SD, mmol/dL | 4.31 ± 0.43 | 4.52 ± 0.50 | <0.01 |
| CRT status, n (%) | 0.66 | ||
| Off | 128 (32) | 48 (30) | |
| On | 273 (68) | 112 (70) | |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CM, cardiomyopathy; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; IVMD, interventricular mechanical delay; NYHA, New York Heart Association.
Chronic kidney disease and left ventricular remodelling
After the 12-month follow-up period, in comparison with the non-CKD group, participants with CKD had worse left ventricular function and more dilation after adjustment for all confounding variables including CRT status (Table 2). Specifically, ventricular function decreased and left ventricular volumes and diameters in systole and diastole increased relative to persons without CKD. In addition, compared with the non-CKD group, the LV shape also increased and MR worsened over time among CKD participants.
Table 2.
Effect of chronic kidney disease on left ventricular parameters after 12 months in adjusted linear mixed model analysesa
| Model 1b β (95% CI) | Model 2c β (95% CI) | Model 3d β (95% CI) | |
|---|---|---|---|
| CKD (GFR <60 mL/min/1.73 m2); all β coefficients for CKD | |||
| LVEF (%) | −1.8 (−3.48, −0.24) | −1.80 (−3.36, −0.12) | −1.80 (−3.36, −0.24) |
| LVESV (mL) | 14.04 (3.84, 24.24) | 14.04 (3.84, 24.24) | 14.16 (3.96, 24.36) |
| LVEDV (mL) | 14.76 (2.76, 26.64) | 14.76 (2.76, 26.64) | 14.88 (2.88, 26.76) |
| LVESD (cm) | 0.24 (0.12, 0.48) | 0.36 (0.12, 0.48) | 0.36 (0.12, 0.48) |
| LVEDD (cm) | 0.12 (−0.012, 0.36) | 0.24 (0.012, 0.36) | 0.24 (0.012, 0.36) |
| LV mass (g) | 9.12 (−4.56, 22.68) | 9.12 (−4.44, 22.68) | 8.88 (−4.68, 22.44) |
| Mitral regurgitation (%) | 3.12 (0.48, 5.76) | 3.12 (0.48, 5.76) | 3.12 (0.48, 5.76) |
| LV cavity shape−diastole | 0.036 (0.012, 0.060) | 0.036 (0.012, 0.060) | 0.036 (0.012, 0.060) |
| IVMD (ms) | −2.52 (−11.16, 6.12) | −2.52 (−11.16, 6.00) | −2.52 (−11.16, 6.00) |
| MPI | 25.92 (−4.68, 56.52) | 26.16 (−4.32, 56.64) | 26.04 (−4.44, 56.52) |
CKD, chronic kidney disease; GFR, glomerular filtration rate; IVMD, interventricular mechanical delay; LV cavity shape−diastole, left ventricular cavity shape at end-diastole; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LV mass, left ventricular mass; MPI, myocardial performance index.
aIn all models, CKD is compared with the non-CKD referent group.
bModel 1: unadjusted.
cModel 2: adjusted for age, gender, race, systolic blood pressure, diastolic blood pressure, diabetes mellitus, smoking, medications (beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics), and cardiomyopathy aetiology.
dModel 3: adjusted further for cardiac resynchronization therapy status.
Chronic kidney disease and cardiac resynchronization therapy
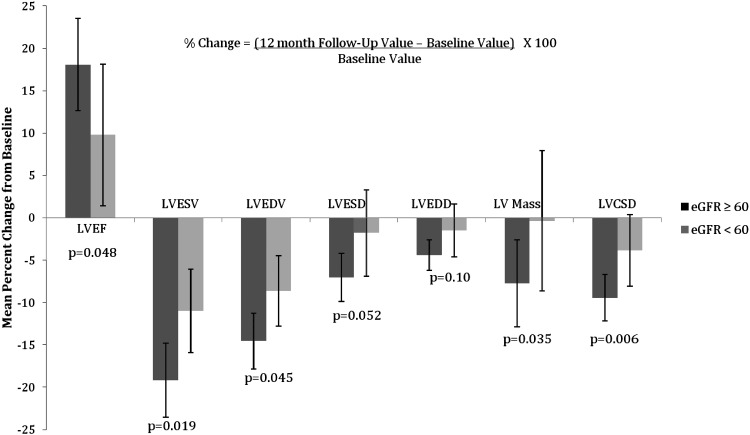
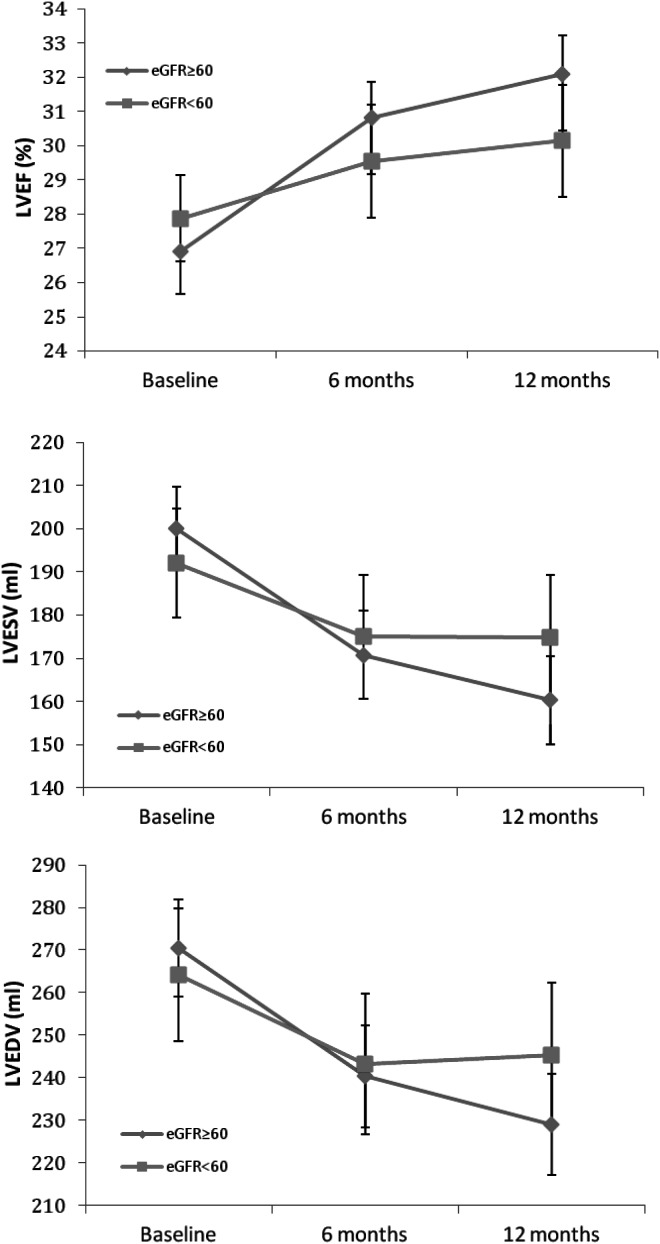
Among participants receiving CRT, the non-CKD group had a significant improvement in LVEF, LVESV, LVEDV, LVESD, LVEDD, LV mass, LV shape, and IVMD after 12 months of resynchronization therapy (Table 3). No significant changes, however, were noted in the degree of MR and the MPI over this time course. Among the CKD group, CRT resulted in an improvement in LVEF, LVESV, and LVEDV, whereas the other parameters demonstrated minimal improvement over the 12-month period and were not significantly different from baseline. The magnitude of the remodelling benefit was consistently better in the non-CKD group compared with participants with CKD (Figure 1). Specifically, non-CKD participants experienced a greater change from baseline in LVEF, LVESV, LVEDV, LVESD, LV mass, MR, and LV shape than the CKD group (Figure 1). These differences between the two kidney function groups appeared as early as 6 months of follow-up and continued for the entire 12-month period (Figure 2). CRT did not appear to have an effect on MPI in either kidney function group. Finally, there was a 34% reduction from baseline in the IVMD in the non-CKD group compared with a 42% reduction in the CKD group. The difference in IVMD changes between the two kidney function groups was not statistically different (P = 0.66).
Table 3.
Changes in left ventricular parameters with cardiac resynchronization therapy on by kidney function groupa
| Baseline | 12 months | Paired difference |
P-value |
||
|---|---|---|---|---|---|
| P (between baseline and 12 months) | P (between the paired differences) | ||||
| LVEF (%) | |||||
| No CKD | 27.1 (0.4) | 32.0 (0.6) | –5.0 (0.6) | <0.001 | 0.048 |
| CKD | 27.6 (0.7) | 30.3 (0.9) | –2.7 (0.9) | 0.002 | |
| LVESV (mL) | |||||
| No CKD | 197.7 (5.1) | 159.8 (5.5) | –37.8 (4.0) | <0.001 | 0.019 |
| CKD | 194.0 (6.9) | 172.7 (7.6) | –21.3 (5.1) | <0.001 | |
| LVEDV (mL) | |||||
| No CKD | 267.2 (6.0) | 228.3 (6.3) | –38.9 (4.4) | <0.001 | 0.045 |
| CKD | 265.8 (8.4) | 242.9 (9.0) | –22.9 (6.2) | <0.001 | |
| LVESD (cm) | |||||
| No CKD | 5.7 (0.1) | 5.3 (0.1) | –0.4 (0.1) | <0.001 | 0.052 |
| CKD | 5.6 (0.1) | 5.5 (0.1) | –0.1 (0.1) | 0.46 | |
| LVEDD (cm) | |||||
| No CKD | 6.8 (0.1) | 6.5 (0.1) | –0.3 (0.1) | <0.001 | 0.10 |
| CKD | 6.8 (0.1) | 6.7 (0.1) | –0.1 (0.1) | 0.23 | |
| LV mass (g) | |||||
| No CKD | 270.4 (6.7) | 249.5 (6.2) | –20.9 (4.6) | <0.001 | 0.035 |
| CKD | 274.3 (11.8) | 273.3 (10.7) | –1.0 (8.4) | 0.91 | |
| Mitral regurgitation (%) | |||||
| No CKD | 14.5 (0.9) | 12.9 (0.9) | –1.6 (0.9) | 0.073 | 0.026 |
| CKD | 15.4 (1.3) | 17.4 (1.7) | 2.0 (1.4) | 0.16 | |
| LV cavity shape–diastole | |||||
| No CKD | 0.53 (0.008) | 0.48 (0.008) | –0.057 (0.008) | <0.001 | 0.0059 |
| CKD | 0.52 (0.01) | 0.50 (0.01) | –0.016 (0.011) | 0.14 | |
| IVMD (ms) | |||||
| No CKD | 37.5 (3.1) | 24.7 (2.7) | –12.8 (3.2) | <0.001 | 0.66 |
| CKD | 24.1 (4.6) | 14.0 (4.4) | –10.1 (5.2) | 0.057 | |
| MPI | |||||
| No CKD | 971 (9.1) | 961 (9.5) | –10.6 (9.3) | 0.26 | 0.23 |
| CKD | 953 (16) | 964 (18) | 11 (16) | 0.50 | |
CKD, chronic kidney disease; IVMD, interventricular mechanical delay; LV cavity shape–diastole, left ventricular cavity shape at end-diastole; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LV mass, left ventricular mass; MPI, myocardial performance index.
aValues represent the mean (standard error).
Figure 1.
Mean percentage change in left ventricular parameters after 12 months of cardiac resynchronization therapy on by kidney function group. eGFR, estimated glomerular filtration rate; LVCSD, left ventricular cavity shape at end-diastole; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume.
Figure 2.
Changes in mean left ventricular ejection fraction (LVEF), left ventricular end-systolic volume (LVESV), and left ventricular end-diastolic volume (LVEDV) during follow-up according to chronic kidney disease status in participants with chronic resynchronizarion therapy on. eGFR, estimated glomerular filtration rate.
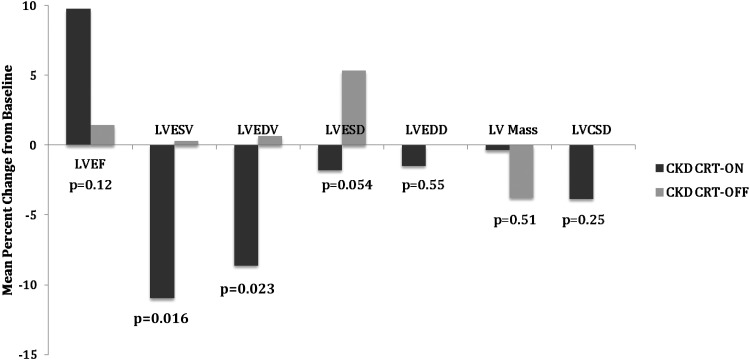
Neither kidney function group demonstrated a significant change in any of the 10 cardiac remodelling parameters over the 12-month period compared with baseline in the CRT off group (Table 4). A direct comparison of the change in LV parameters among CKD participants with and without CRT demonstrated significant differences in the LVESV and LVEDV over the 12-month period with CRT (Figure 3).
Table 4.
Changes in left ventricular parameters with cardiac resynchronization therapy off by kidney function groupa
| Baseline | 12 months | Paired difference |
P-value |
||
|---|---|---|---|---|---|
| P (between baseline and 12 months) | P (between the paired differences) | ||||
| LVEF (%) | |||||
| No CKD | 25.7 (0.7) | 26.3 (0.7) | –0.7 (0.6) | 0.3 | 0.8 |
| CKD | 27.9 (1.0) | 28.3 (1.1) | –0.4 (1.1) | 0.7 | |
| LVESV (mL) | |||||
| No CKD | 216.4 (9.9) | 210.5 (10.8) | 5.9 (4.9) | 0.2 | 0.5 |
| CKD | 182.6 (9.3) | 183.2 (10.2) | –0.54 (7.0) | 0.9 | |
| LVEDV (mL) | |||||
| No CKD | 287.0 (11.2) | 279.2 (11.9) | 7.7 (5.7) | 0.2 | 0.4 |
| CKD | 250.4 (10.4) | 252.0 (11.6) | –1.6 (8.0) | 0.8 | |
| LVESD (cm) | |||||
| No CKD | 5.7 (0.1) | 5.7 (0.2) | 0.02 (0.09) | 0.9 | 0.07 |
| CKD | 5.6 (0.2) | 5.9 (0.2) | –0.3 (0.2) | 0.06 | |
| LVEDD (cm) | |||||
| No CKD | 7.0 (0.1) | 6.9 (0.1) | 0.08 (0.07) | 0.3 | 0.7 |
| CKD | 6.9 (0.2) | 6.9 (0.2) | 0.03 (0.13) | 0.8 | |
| LV mass (g) | |||||
| No CKD | 298.5 (10.5) | 288.9 (10.6) | 9.6 (6.5) | 0.1 | 0.9 |
| CKD | 255.5 (12.7) | 245.9 (15.9) | 9.6 (7.7) | 0.2 | |
| Mitral regurgitation (%) | |||||
| No CKD | 15.1 (1.6) | 14.5 (1.8) | 0.6 (1.2) | 0.6 | 0.03 |
| CKD | 18.4 (1.3) | 20.1 (1.7) | –1.7 (0.9) | 0.2 | |
| LV cavity shape–diastole | |||||
| No CKD | 0.54 (0.01) | 0.53 (0.01) | 0.02 (0.10) | 0.08 | 0.2 |
| CKD | 0.55 (0.02) | 0.55 (0.02) | –0.006 (0.01) | 0.6 | |
| IVMD (ms) | |||||
| No CKD | 34.4 (4.0) | 36.7 (4.1) | –2.3 (3.3) | 0.5 | 0.4 |
| CKD | 23.5 (5.2) | 19.8 (7.3) | 3.7 (6.4) | 0.6 | |
| MPI | |||||
| No CKD | 929 (14) | 915 (15) | 14 (14) | 0.3 | 0.07 |
| CKD | 950 (22) | 994 (34) | –44 (34) | 0.2 | |
CKD, chronic kidney disease; IVMD, interventricular mechanical delay; LV cavity shape–diastole, left ventricular cavity shape at end-diastole; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LV mass, left ventricular mass; MPI, myocardial performance index.
aValues represent the mean (standard error).
Figure 3.
Mean percentage change in left ventricular parameters after 12 months in chronic kidney disease (CKD) participants by cardiac resynchronization therapy (CRT) status. LVCSD, left ventricular cavity shape at end-diastole; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume.
Chronic kidney disease and clinical response
In our analyses, 106 participants had a worsened clinical composite response score at the end of the 12-month follow-up period. Among this group, 68 participants had no CKD (17% of the no CKD group), and 38 participants had CKD (24% of the CKD group) [hazard ratio (HR) 1.46, 95% confidence interval (CI) 0.98–2.18]. After multivariate adjustment, the association was substantially attenuated (HR 1.22, 95% CI 0.78–1.90). In addition, there was no significant interaction between CKD and CRT status on the outcome of clinical events.
Discussion
Our analysis within the REVERSE study demonstrates that regardless of CRT status and other cardiovascular risk factors, CKD participants had a worse LVEF and larger left ventricular size compared to the non-CKD subgroup after the 12-month follow-up period. These findings are partly explained by the attenuation in cardiac remodelling among CKD participants assigned to CRT compared to the non-CKD group. Further, among participants not assigned to CRT, there was no significant difference in any of the functional or structural parameters in either kidney function group over the 1-year follow-up period. Finally, we did not observe a significant association between CKD and worsening heart failure events including death or hospitalization during this relatively short follow-up.
Kidney disease had a strong, independent effect on inhibiting the reverse remodelling effects of CRT. Kidney disease, in part, may be associated with fibrotic changes within the myocardium that subsequently impair the ability of the heart to remodel with CRT. In addition, the lack of deterioration in the cardiac parameters among the REVERSE control group (CRT off) suggests that neither mild heart failure nor kidney disease results in a natural decline in left ventricular function or size over a 1-year time course. These findings suggest that kidney disease is a stronger and more acute inhibitor of reverse remodelling with resynchronization therapy than it is an inducer of intrinsic pathways contributing to progressive cardiac damage. A longer follow-up period would likely be required to appreciate the progressive decline in cardiac structure in the kidney disease population.
Previous findings from the VALIANT study, which enrolled patients with an acute myocardial infarction complicated by heart failure and systolic dysfunction, complement the current analysis. In VALIANT, a reduction in eGFR was associated with an increase in left atrial volumes during the study follow-up, suggesting that diastolic dysfunction may also ensue in patients with kidney disease.30 The effect of CKD on cardiac remodelling may be an intermediate step in the pathway linking kidney disease to progressive heart failure and death. Recent studies also demonstrate that adverse changes in ventricular size and function are associated with heart failure progression and correlate to clinical outcomes.31 The 12-month follow-up period in our study, therefore, was a sufficient duration to appreciate changes in remodelling but probably an inadequate time period to detect differences in a worsening clinical composite response score.
In our study, CRT improved most parameters of left ventricular remodelling in both CKD and non-CKD participants. Subgroup analyses from larger clinical trials have also demonstrated the clinical benefits of CRT across the spectrum of kidney disease in patients with mild to advanced heart failure.32,33 These findings, however, were limited in their assessment of kidney function as they were part of a larger set of analyses.34 Our study provides a systematic evaluation of CKD's effect on CRT in patients with mild heart failure. Observational studies have also demonstrated a decreased benefit in left ventricular remodelling among CKD patients receiving CRT compared with the non-CKD group. These cohorts of patients had more advanced heart failure symptoms35,36 compared with those in the REVERSE trial; as a result, the confounding effect of haemodynamic alterations and volume overload may impact these findings. As stated earlier, CKD may attenuate the positive remodelling observed with CRT due to dysregulation in the neurohormonal axis and renin–angiotensin–aldosterone system—changes that result in interstitial fibrosis.37 Despite this interaction, CRT still appears to benefit CKD participants especially by improving left ventricular dimensions.
Several limitations should be considered when evaluating the results of our study. First, the REVERSE trial excluded participants with a serum creatinine ≥3.0 mg/dL. As a result, the implications regarding CRT therapy in patients with more advanced forms of kidney dysfunction and end-stage renal disease cannot be determined. In addition, we did not find CKD to be an independent risk factor for a worsening clinical composite response score in these analyses. This finding is somewhat concerning given the series of previous publications demonstrating a strong association between kidney disease and adverse heart failure outcomes. As such, these earlier studies probably reflect an adequate follow-up duration. With a longer follow-up period, the increasing differences in cardiac remodelling would most likely lead to greater changes in outcome between study groups. Finally, kidney function was measured at baseline only; as a result, changes in eGFR with CRT in this population cannot be evaluated.
In summary, our findings demonstrate that CKD results in worse left ventricular function and dilation compared with participants with normal kidney function and mild heart failure. In addition, CRT helps with cardiac remodelling among participants with kidney disease; however, this benefit is weaker in magnitude compared with the benefits observed in patients with mild heart failure and no CKD. Future studies should evaluate whether CKD should impact decisions related to CRT implantation.
Conflict of interest: M.S.J.S., M.R.G., and C.L. served as consultants to and received research grants from Medtronic. S.D. received research support from Medtronic and honoraria from Biosense and St. Jude Medical. E.P.G. received research grants from Medtronic and St. Jude Medical. S.G. received honoraria from or consulted for Medtronic. M.R.G. and C.L. served as consultants to and received research grants from St. Jude Medical. M.S.J.S. has also received research support from Paracore. M.R.G. served as a consultant and received research grants from Boston Scientific. The remaining authors report no conflicts of interest.
Funding
Medtronic, Inc., Minneapolis, Minnesota, USA; the National Institutes of Health [grant number K23DK089118 to R.D.].
References
- 1.Scrutinio D, Passantino A, Santoro D, Catanzaro R. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–67. doi: 10.1093/eurjhf/hfq167. [DOI] [PubMed] [Google Scholar]
- 2.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 5.Fujii H, Nakai K, Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther Apher Dial. 2011;15:125–128. doi: 10.1111/j.1744-9987.2010.00883.x. [DOI] [PubMed] [Google Scholar]
- 6.Lonn E, Shaikholeslami R, Yi Q, Bosch J, Sullivan B, Tanser P, Magi A, Yusuf S. Effects of ramipril on left ventricular mass and function in cardiovascular patients with controlled blood pressure and with preserved left ventricular ejection fraction: a substudy of the Heart Outcomes Prevention Evaluation (HOPE) Trial. J Am Coll Cardiol. 2004;43:2200–2206. doi: 10.1016/j.jacc.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 7.Russo D, Corrao S, Battaglia Y, Andreucci M, Caiazza A, Carlomagno A, Lamberti M, Pezone N, Pota A, Russo L, Sacco M, Scognamiglio B. Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int. 2011;80:112–118. doi: 10.1038/ki.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerasola G, Nardi E, Mule G, Palermo A, Cusimano P, Guarneri M, Arsena R, Giammarresi G, Carola Foraci A, Cottone S. Left ventricular mass in hypertensive patients with mild-to-moderate reduction of renal function. Nephrology (Carlton) 2010;15:203–210. doi: 10.1111/j.1440-1797.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 9.Cioffi G, Tarantini L, Frizzi R, Stefenelli C, Russo TE, Selmi A, Toller C, Furlanello F, de Simone G. Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens. 2011;29:565–573. doi: 10.1097/HJH.0b013e3283424188. [DOI] [PubMed] [Google Scholar]
- 10.Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, Lima JA, Siscovick D, Bertoni AG, Shlipak MG. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the multi-ethnic study of atherosclerosis (MESA) Am J Kidney Dis. 2008;52:839–848. doi: 10.1053/j.ajkd.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 12.Liao Y, Cooper RS, Durazo-Arvizu R, Mensah GA, Ghali JK. Prediction of mortality risk by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997;29:641–647. doi: 10.1016/s0735-1097(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 13.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 14.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–991. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 15.Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ. 2010 focused update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur J Heart Fail. 2010;12:1143–1153. doi: 10.1093/eurjhf/hfq192. [DOI] [PubMed] [Google Scholar]
- 16.Bogale N, Priori S, Cleland JG, Brugada J, Linde C, Auricchio A, van Veldhuisen DJ, Limbourg T, Gitt A, Gras D, Stellbrink C, Gasparini M, Metra M, Derumeaux G, Gadler F, Buga L, Dickstein K. The European CRT Survey: 1 year (9–15 months) follow-up results. Eur J Heart Fail. 2012;14:61–73. doi: 10.1093/eurjhf/hfr158. [DOI] [PubMed] [Google Scholar]
- 17.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 19.Linde C, Gold M, Abraham WT, Daubert JC. Rationale and design of a randomized controlled trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with asymptomatic left ventricular dysfunction with previous symptoms or mild heart failure–the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Am Heart J. 2006;151:288–294. doi: 10.1016/j.ahj.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW American College of Cardiology Foundation; American Heart Association. Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;54:2464. doi: 10.1016/j.jacc.2008.11.013. 2009;53:e1–e90. Erratum in: J Am Coll Cardiol. 2009. [DOI] [PubMed] [Google Scholar]
- 21.Authors/Task Force Members; McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines (CPG) Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S; Document Reviewers, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162:548–554. doi: 10.1016/j.ahj.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 26.Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001;7:176–182. doi: 10.1054/jcaf.2001.25652. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Lamas GA, Vaughan DE, Parisi AF, Pfeffer MA. Effects of left ventricular shape and captopril therapy on exercise capacity after anterior wall acute myocardial infarction. Am J Cardiol. 1989;63:1167–1173. doi: 10.1016/0002-9149(89)90173-2. [DOI] [PubMed] [Google Scholar]
- 29.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 30.Verma A, Anavekar NS, Meris A, Thune JJ, Arnold JM, Ghali JK, Velazquez EJ, McMurray JJ, Pfeffer MA, Solomon SD. The relationship between renal function and cardiac structure, function, and prognosis after myocardial infarction: the VALIANT Echo Study. J Am Coll Cardiol. 2007;50:1238–1245. doi: 10.1016/j.jacc.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122:985–992. doi: 10.1161/CIRCULATIONAHA.110.955039. [DOI] [PubMed] [Google Scholar]
- 32.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 33.Richardson M, Freemantle N, Calvert MJ, Cleland JG, Tavazzi L. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007;28:1827–1834. doi: 10.1093/eurheartj/ehm192. [DOI] [PubMed] [Google Scholar]
- 34.Cannizzaro LA, Piccini JP, Patel UD, Hernandez AF. Device therapy in heart failure patients with chronic kidney disease. J Am Coll Cardiol. 2011;58:889–896. doi: 10.1016/j.jacc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Van Bommel RJ, Mollema SA, Borleffs CJ, Bertini M, Ypenburg C, Marsan NA, Delgado V, Van Der Wall EE, Schalij MJ, Bax JJ. Impaired renal function is associated with echocardiographic nonresponse and poor prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2011;57:549–555. doi: 10.1016/j.jacc.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Adelstein EC, Shalaby A, Saba S. Response to cardiac resynchronization therapy in patients with heart failure and renal insufficiency. Pacing Clin Electrophysiol. 2010;33:850–859. doi: 10.1111/j.1540-8159.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 37.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]