Abstract
Nr4a1 and Nr4a2 are transcription factors and immediate early genes belonging to the nuclear receptor Nr4a family. In this study, we examine their role in long-term memory formation for object location and object recognition. Using siRNA to block expression of either Nr4a1 or Nr4a2, we found that Nr4a2 is necessary for both long-term memory for object location and object recognition. In contrast, Nr4a1 appears to be necessary only for object location. Indeed, their roles in these different types of long-term memory may be dependent on their expression in the brain, as NR4A2 was found to be expressed in hippocampal neurons (associated with object location memory) as well as in the insular and perirhinal cortex (associated with object recognition memory), whereas NR4A1 showed minimal neuronal expression in these cortical areas. These results begin to elucidate how NR4A1 and NR4A2 differentially contribute to object location versus object recognition memory.
It is well established that long-term memory (LTM) formation requires transcription (for review, see Alberini 2009). Transcription regulated specifically by cAMP responsive element binding protein (CREB) has been shown to be essential for long-term memory (Bourtchuladze et al. 1994; Yin et al. 1994; Guzowski and McGaugh 1997; Pittenger et al. 2002; Sekeres et al. 2010; but see Balschun et al. 2003). Two CREB-dependent immediate early genes that have been implicated in LTM are Nr4a1 and Nr4a2 (Pena de Ortiz et al. 2000; von Hertzen and Giese 2005a,b; Colon-Cesario et al. 2006). Nr4a1 (Nur77) and Nr4a2 (Nurr1) are members of the nuclear steroid/thyroid hormone receptor superfamily that bind in an apparently ligand-independent manner to Nerve Growth Factor1-B (NGFI-B) response elements (Baker et al. 2003; Wang et al. 2003).
Expression of both Nr4a1 and Nr4a2 has been shown to increase in the hippocampus following learning. Nr4a1 expression increased in the CA1 region of the hippocampus during context shock memory consolidation, and Nr4a2 increased in CA1 and CA3 pyramidal cell layers of the rat hippocampus following a spatial discrimination task (Pena de Ortiz et al. 2000; von Hertzen and Giese 2005a,b; Keeley et al. 2006; Hawk and Abel 2011). An exception to these findings was a study from Malkani and Rosen (2000) who found increased Nr4a1 expression in the cortex and amygdala following context shock memory consolidation, but failed to see a change in area CA1 of the hippocampus. These patterns of expression suggest a role for the Nr4a family in learning and memory.
Transcription of both Nr4a1 and Nr4a2 appear to be regulated by chromatin modification via histone acetylation and deacetylation. Histone deacetylase (HDAC) activity was shown to interfere with formation of the pre-initiation complex at the Nr4a1 promoter, suggesting that acetylation is necessary for its transcription (Fass et al. 2003). In addition, during memory consolidation, the HDAC inhibitor Trichostatin A (TSA) maintained the expression of both Nr4a1 and Nr4a2 during memory consolidation, without maintaining the expression of several other CREB-dependent genes (Vecsey et al. 2007).
We recently showed that HDAC3 may modulate long-term memory formation in an Nr4a2-dependent manner (McQuown et al. 2011). Deletion of HDAC3 in the dorsal hippocampus transformed a subthreshold training event that normally does not result in LTM into an event that led to robust LTM. Deletion of HDAC3 also facilitated Nr4a2 expression following subthreshold training. In contrast, the HDAC3 deletion had no effect on Nr4a2 expression in naive-handled mice, suggesting that the increase in Nr4a2 required an activity-dependent event. Notably, siRNA knockdown of Nr4a2 in animals with HDAC3 deletions prevented the enhanced LTM that occurred following the subthreshold training (McQuown et al. 2011). Together, these studies suggest that Nr4a1 and Nr4a2 are key target genes regulated by acetylation during long-term memory formation.
The studies discussed above have demonstrated an important role for Nr4a1 and Nr4a2 in long-term memory formation. They also indicate that expression of these genes during consolidation is regulated by chromatin modification through histone acetylation and deacetylation. The current study aimed to further understand the role of these genes in long-term memory formation.
To investigate the role of Nr4a1 and Nr4a2 in learning and memory processes, we examined the basal and activity-dependent levels of NR4A1 and NR4A2 proteins in brain regions involved in learning and memory. We chose to examine regions involved in object location memory and object recognition memory, as the training for these tasks is the same, but the testing depends on different brain regions. (Bermudez-Rattoni et al. 2005; Balderas et al. 2008; Roozendaal et al. 2010; Haettig et al. 2011). We performed immunohistochemistry on 20-μm coronal sections of formaldehyde fixed brain tissue from adult male mice to examine expression of NR4A1 and NR4A2 in the hippocampus as well as the insular, perirhinal, and entorhinal cortices. We also used transient siRNA knockdown to specifically examine the roles of Nr4a1 and Nr4a2 in learning and memory as measured by object location and novel object recognition memory tasks. The findings presented here provide new insight into how Nr4a1 and Nr4a2 regulate memory formation in different tasks utilizing different brain regions.
We found that NR4A1 protein was strongly expressed throughout the hippocampus, including the CA1–CA3 regions (Fig. 1B–D). Notably, in addition to hippocampal expression (Fig. 1H–J), NR4A2 showed strong expression in discrete foci within cortical layer VI (Allen Brain Atlas coordinates: −2.25 to −5.25 ventral to bregma) (Fig. 1K,L). Double labeling for NR4A2 and NeuN showed that cortical NR4A2 is expressed in neurons (Fig. 1L). These findings demonstrate that both NR4A1 and NR4A2 were expressed in the hippocampus, whereas NR4A2 also exhibited strong expression in cortical layer VI.
Figure 1.
NR4A1 and NR4A2 are expressed in the hippocampus and NR4A2 is also expressed in cortical layer VI extending through the insular, perirhinal, and entorhinal cortices. Representative images showing (A) Nuclear DAPI staining 4× magnification. (B) NR4A1-immune-positive cells, 4× magnification. (C) NR4A1-immune-positive cells in CA1 of the hippocampus, 20× magnification. (D) NR4A1-immune-positive cells in CA2 and CA3 of the hippocampus, 20× magnification. (E) NR4A1-immunoreactivity in cortical layer VI, showing diffuse staining. (F) Minimal colocalization of NeuN- and NR4A1-immunopositive cells. (G) Nuclear DAPI staining, 4× magnification. (H) NR4A2-immune-positive cells, 4× magnification. (I) NR4A2-immune-positive cells in the CA1 area of the hippocampus, 20× magnification. (J) NR4A2-immune-positive cells in CA2 and CA3 of the hippocampus, 20× magnification. (K) NR4A2-immune-positive cells in cortical layer VI, showing strong focal expression. (L) Colocalization of NeuN- with NR4A2-immunopositive cells. The primary antibodies were directed against NR4A1 (sc-5569, Santa Cruz), NR4A2 (sc-5568, Santa Cruz), and NeuN (MAB377, Millipore Corporation). Images were acquired with an Olympus (BX51) microscope using 4× (A,B,G,H) or 20× (C–F,I–L) objectives and a CCD camera (QImaging) using QCapture Pro 6.0 software (QImaging).
Next we examined changes in expression of NR4A1 and NR4A2 following training for an object memory task that activates these brain regions (Bermudez-Rattoni et al. 2005; Balderas et al. 2008; Roozendaal et al. 2010; Haettig et al. 2011). Adult male C57Bl/6J mice were habituated to the context for 4 d and then on the fifth day were returned to the same context and either exposed to two identical objects for 10 min (trained) or without objects (habituated). Preliminary time-course studies showed that Nr4a2 mRNA levels increased for 60 min following a learning event, and Jo et al. (2009) demonstrated that NR4A2 protein is stable for >2 h. Therefore, 2 h after training animals were perfused and formaldehyde-fixed brains were collected for immunohistochemistry. The NR4A1 and NR4A2 antibodies we used for immunohistochemistry are directed against the variable N-terminal region of the two proteins, and the specificity of the antibodies was verified by Western blot analysis (Supplemental Fig. 1).
We quantified NR4A1 and NR4A2 expression in area CA1 of the hippocampus and cortical layer VI of the insular, perirhinal, and entorhinal cortices. Following training, there were significant 40% increases in both NR4A1- (habituated: 1.03 ± 0.07 N = 23 vs. trained: 1.43 ± 0.07 N = 31, P = 0.0003) (Fig. 2B) and NR4A2-immune-positive cells in area CA1 (habituated: 1.00 ± 0.19 N = 12 vs. trained: 1.39 ± 0.11 N = 16, P = 0.04) (Fig. 2C). There was no significant change in cortical NR4A1-immune-positive cells (habituated: 1.00 ± 0.36 N = 23 vs. trained: 0.72 ± 0.15 N = 26, P = 0.24) (Fig. 2D). In addition, we found a significant twofold increase in cortical NR4A2-immune-positive cells in mice that received a 10-min training compared with habituated animals (habituated: 1.00 ± 0.12 N = 21 vs. trained: 1.96 ± 0.19 N = 27 P = 0.0002) (Fig. 2E). Representative images of hippocampal NR4A1 (Fig. 2F,G) and hippocampal (Fig. 2H,I) and cortical NR4A2 (Fig. 2J,K) are shown.
Figure 2.
Object memory training increased expression of both NR4A1 and NR4A2 proteins in area CA1 of the hippocampus and increased cortical expression of NR4A2, but not NR4A1. (A) Mice received a 10-min training with two identical objects in a previously habituated context. Two hours later tissue was collected for immunohistochemistry. (B) The number of NR4A1-immune-positive cells per area in CA1 of the hippocampus is increased in trained animals as compared with habituated animals (P = 0.0003). (C) The number of NR4A2-immune-positive cells per area in CA1 of the hippocampus is increased in trained animals as compared with habituated animals (P = 0.04). (D) The number of NR4A1-immune-positive cells per area in cortical layer VI is not significantly different in trained animals as compared with habituated animals (P = 0.24). (E) The number of NR4A2-immune-positive cells per area in cortical layer VI is increased in trained animals as compared with habituated animals (P = 0.0002). (F–K) Images were acquired using a BX61 microscope with a 10× objective, XC10 camera, and VSW-ASW FL software (Olympus). A single optimized acquisition exposure time was used for all images acquired from each slide with all treatment groups represented. The number of immune-positive cells per area (B–E) was quantified using ImageJ software (NIH). Representative images of NR4A1 and NR4A2 expression in habituated vs. trained animals in area CA1 of the hippocampus (F–I) and the perirhinal cortex (PRC) (J,K). Images (F–K) were acquired with an Olympus (BX51) microscope using a 20× objective (A–F,G–J) and a CCD camera (QImaging) using QCapture Pro 6.0 software (QImaging).
To further investigate the role of Nr4a1 and Nr4a2 in learning and memory, we used small interfering (si) RNAs to transiently knock down expression of Nr4a1 and Nr4a2. Targeted SMART pool siRNAs (Dharmacon) were prepared with jetSI (Polyplus Transfection) at a final concentration of 4 μM before injection. A nontargeting control siRNA with an RNA-induced silencing complex (RISC)-free modification was used as a control (siRISC). Mice received intrahippocampal infusions of RISC-free control, Nr4a1, or Nr4a2 siRNA directly into the dorsal CA1 area of the hippocampus (AP, −2.0 mm; DV, −1.5 mm; ML, ±1.5 mm). To assay specific knockdown of each transcript, RNA was isolated from 1-mm hippocampal punches and quantitative real-time RT-PCR was performed as previously described (McQuown et al. 2011) using a Roche LightCycler:
Nr4a1 probe 93: 5′-tctggtcc-3′, forward primer: 5′-agcttgggtgttgatgttcc-3′, reverse primer: 5′-aatgcgattctgc agctctt-3′;
Nr4a2 probe 2: 5′-ttctcctg-3′, forwardprimer: 5′-ttgcagaatatgaacatcgaca-3′, reverse primer: 5′-gttccttgagcccgtg tct-3′;
Gapdh probe: 5′-tggccgtattgg-3′, forward primer: 5′-atggtgaaggtcggtgtga-3′, reverse primer: 5′-aatctccactttgc cactgc-3′.
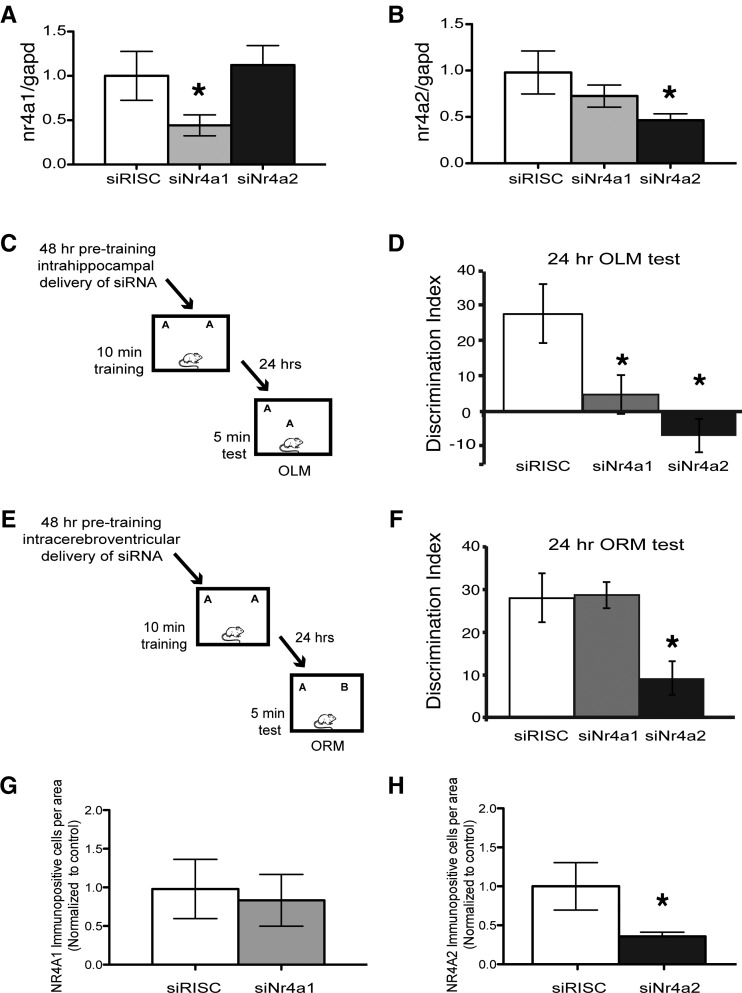
Hippocampal infusion with siRNA against Nr4a1 significantly and specifically decreased expression of Nr4a1 by more than twofold as compared with the RISC-free control (siRISC = 1.58 ± 0.43, siNr4a1 = 0.70 ± 0.18; P = 0.04). There was no significant difference in expression of Nr4a2 following infusion with siNr4a1 (1.99 ± 0.37; P = 0.25) (Fig. 3A). Infusion with siNr4a2 also significantly and specifically knocked down Nr4a2 expression more than twofold when compared with RISC-free control (siRISC = 0.97 ± 0.23, siNr4a2 = 0.46 ± 0.07; P = 0.02). No significant difference was found in expression of Nr4a1 following infusion with siNr4a2 (0.72 ± 0.11; P = 0.17) (Fig. 3B). Each primer–probe set produced a single real-time RT-PCR product. In addition, there were no significant differences in Gapdh mRNA expression following siRNA knockdown (Supplemental Fig. 2).
Figure 3.
Knockdown of Nr4a2, but not Nr4a1, in cortical regions including the insular, perirhinal, and entorhinal cortices reduces preference for the novel object in the object recognition (ORM) task, while hippocampal knockdown of both Nr4a1 and Nr4a2 affects performance in the object location (OLM) task. (A) siRNA directed against Nr4a1 significantly reduced hippocampal expression of Nr4a1 (P = 0.04) without affecting Nr4a2 (P = 0.25). (B) siRNA against Nr4a2 significantly reduced hippocampal expression of Nr4a2 (P = 0.02) without affecting Nr4a1 expression (P = 0.17). (C) Forty-eight hours after infusion, habituated animals received 10 min of training with two identical objects. Another 24 h later they were given a retention test in which one object was moved to a novel location. (D) During a 24-h retention test, mice that received hippocampal infusions of either siNr4a1 or siNr4a2 displayed no preference in the OLM task in contrast to animals that received control RISC-free siRNA (P = 0.002). (E) Habituated mice received intracerebroventricular (ICV) infusions of control RISC-free, Nr4a1, or Nr4a2 siRNA. Forty-eight hours later they received 10 min of training with two identical objects. Twenty-four hours later they were given a retention test where one object was replaced with a novel object. (F) During a 24-h retention test, mice that received ICV infusions of siNr4a2 displayed no preference for the novel object in contrast to animals that received control RISC-free siRNA or siNr4Aa1 (P = 0.03). (G) Intracerebroventricular infusions of Nr4a1 siRNA did not change expression of NR4A1 in the cortex, as measured by immunohistochemistry (P = 0.4). (H) Intracerebroventricular infusions of Nr4a2 siRNA significantly decreased expression of NR4A2 in the cortex, as measured by immunohistochemistry (P = 0.02).
We then used the object location memory (OLM) task to investigate the roles of Nr4a1 and Nr4a2 in the hippocampus. Two days prior to training, mice received hippocampal siRNA infusions and 48 h after infusion were trained with two identical objects for 10 min (Fig. 3C). The percentage of time spent exploring the objects during training did not differ significantly between the groups (statistics calculated using ANOVA). Twenty-four hours after training, the animals were placed in the identical context with one object moved to a novel location (OLM) (Fig. 3C). Animals that spent more time with the object in the novel location are considered to demonstrate memory for the object in the familiar location. Animals that received infusions of the control RISC-free siRNA exhibited memory for the familiar object and spent more time with the object in the novel location (% discrimination index [DI] = 27.7 ± 8.4 N = 8) (Fig. 3D). In contrast, animals infused with siRNA against either Nr4a1 or Nr4a2 had significantly impaired memory for the familiar object (% DI for Nr4a1 = 4.8 ± 5.5 N = 9, % DI for Nr4a2 = −6.9 ± 4.7 N = 10, P = 0.002). These results suggest that both Nr4a1 and Nr4a2 are required for hippocampus-dependent long-term memory for object location.
We next examined the role of Nr4a1 and Nr4a2 in long-term memory in a standard novel object recognition task (ORM) (Fig. 3E). Two days prior to training, mice received intracerebroventricular (ICV) infusions (AP, −0.34 mm; DV, −2.1 mm; ML, ±1.0 mm) of small interfering (si) RNAs to transiently knock down expression of Nr4a1 and Nr4a2. In the ORM task, 48 h after siRNA infusion, animals previously habituated to the context were trained with two identical objects for 10 min (Fig. 3E). There were no significant differences in total exploration times between groups during training or testing (statistics calculated using ANOVA).
Twenty-four hours after training, animals were given a retention test in which one familiar object was replaced with a novel object (Fig. 3E). At testing, animals infused with siRNA targeted against Nr4a2 had a significantly lower discrimination index (% DI) for the novel object (% DI = 10.1 ± 4.5 N = 6; P = 0.03) than mice infused with either control siRISC (% DI = 27.88 ± 5.7 N = 9) or siRNA directed against Nr4a1 (% DI = 29.1 ± 2.6 N = 7). There was no difference between control animals and animals infused with siNr4a1 (Fig. 3F). The siRNAs targeted to Nr4a2 resulted in a significant knockdown of NR4A2 protein in layer VI of the insular, perirhinal, and entorhinal cortices 2 h after training with objects, (siRISC = 1.00 ± 0.30 N = 13, siNr4a2 = 0.35 ± 0.05 N = 15, P = 0.02) (Fig. 3H). As predicted from the lack of neuronal NR4A1 expression in cortical areas, no significant difference in cortical expression was observed following Nr4a1 knockdown (siRISC = 0.97 ± 0.38 N = 13, siNr4a1 = 0.83 ± 0.33 N = 15, P = 0.38) (Fig. 3G). These data show that cortical knockdown of Nr4a2 impaired long-term memory for the object itself.
Both Nr4a1 and Nr4a2 mRNAs are highly expressed in the CA1 and CA3 regions of the hippocampus (Honkaniemi et al. 1995; Xiao et al. 1996; this study), an area critical for context and location-dependent memory formation (O'Keefe and Burgess 1999; Fanselow 2000; Maren and Holt 2000). Through a number of recent studies, it has been found that mRNA expression of Nr4a1 and Nr4a2 increases during memory acquisition and consolidation, suggesting that induction of these genes is integral to the formation of long-term memory (Pena de Ortiz et al. 2000; von Hertzen and Giese 2005a,b; Colon-Cesario et al. 2006). Acquisition and LTM for a spatial discrimination task in rats is disrupted by oligodeoxynucleotide knockdown of Nr4a2 in the CA3 area of the hippocampus (Colon-Cesario et al. 2006). In this study, we showed that NR4A1 and NR4A2 protein expression is increased in the hippocampus following an object memory-training task. siRNA knock down of either of these genes affects learning and memory in tasks relying on the hippocampus. These data demonstrate critical roles for Nr4a1 and Nr4a2 in the hippocampus during long-term memory formation.
Although their expression has been previously examined, little is known about the role of the Nr4a genes in cortex-dependent memory. Several studies have reported that Nr4a1 mRNA is expressed throughout the cerebral cortex (Chan et al. 1993; Honkaniemi et al. 1995; Xiao et al. 1996; Zetterström et al. 1996), while Nr4a2 mRNA is not as widespread in the CNS (Saucedo-Cardenas and Conneely 1996; Xiao et al. 1996). However, expression of Nr4a2 mRNA in regions associated with memory acquisition and consolidation has been proposed to play a role in memory processing (Xing et al. 1997). Our findings support this, showing that following a learning event, expression of NR4A2 protein increased significantly in cortical layer VI. siRNA targeted to Nr4a2 in the cortex led to a significant reduction in NR4A2 and impaired a cortex-dependent memory task. Together these findings suggest a critical role for NR4A2, but not NR4A1 in cortex-dependent memory formation.
While the perirhinal cortex is considered essential for poly-modal object recognition (Albasser et al. 2011), the hippocampus is believed to be necessary for object location. However, a functional interaction between the hippocampus and the perirhinal cortex is important for multisensory processing and memory (Barker and Warburton 2011). The results presented here suggest that NR4A1 is a critical signaling molecule involved in object location memory, while NR4A2 is critical for both object location and recognition memory.
Previously, we showed that Nr4a2 was necessary for memory enhancement resulting from a focal hippocampal deletion of HDAC3 (McQuown et al. 2011). In the present study, we showed that in addition to Nr4a2, Nr4a1 is critical for hippocampal memory formation. Further studies have shown that HDAC activity interferes with transcription of Nr4a1 and an HDAC inhibitor increases expression of both Nr4a1 and Nr4a2 in the hippocampus (Fass et al. 2003; Vecsey et al. 2007). Therefore, further studies will be needed to answer whether HDAC3 is also a critical regulator of Nr4a1 expression during LTM formation. We also found that cortical NR4A2 is specifically required for long-term memory for object recognition. These data suggest that Nr4a1 and Nr4a2 are critical for specific types of memory and represent different mechanisms required for consolidation of long-term memory in different brain regions.
Acknowledgments
This work was supported by grants from NIMH (MH081004) and NIDA (DA025922; DA031989) to M.A.W., a predoctoral NRSA fellowship (F31MH85494) to R.M.B.; a Re-Entry into Biomedical and Behavioral Career supplement (MH081004-02S1) to S.E.M.; a predoctoral NRSA fellowship (F31DA29368) to M.M.; Biomedical Research Support (MBRS) Program, NIH Grant GM-55246 to M.F.D. and N.H. We especially thank Dr. John Guzowski for the use of his Olympus BX61 microscope and XC10 camera.
Footnotes
[Supplemental material is available for this article.]
References
- Albasser MM, Amin E, Iordanova MD, Brown MW, Pearce JM, Aggleton JP 2011. Separate but interacting recognition memory systems for different senses: The role of the rat perirhinal cortex. Learn Mem 18: 435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM 2009. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 89: 121–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Shewchuk LM, Kozlova T, Makishima M, Hassell A, Wisely B, Caravella JA, Lambert MH, Reinking JL, Krause H, et al. 2003. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell 113: 731–742 [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F 2008. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem 15: 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP 2003. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci 23: 6304–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC 2011. When is the hippocampus involved in recognition memory? J Neurosci 31: 10721–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL 2005. Insular cortex is involved in consolidation of object recognition memory. Learn Mem 12: 447–449 [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59–68 [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE 1993. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci 13: 5126–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Cesario WI, Martinez-Montemayor MM, Morales S, Felix J, Cruz J, Adorno M, Pereira L, Colon N, Maldonado-Vlaar CS, Pena de Ortiz S 2006. Knockdown of Nurr1 in the rat hippocampus: Implications to spatial discrimination learning and memory. Learn Mem 13: 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS 2000. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res 110: 73–81 [DOI] [PubMed] [Google Scholar]
- Fass DM, Butler JE, Goodman RH 2003. Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J Biol Chem 278: 43014–43019 [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL 1997. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci 94: 2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA 2011. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem 18: 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk JD, Abel T 2011. The role of NR4A transcription factors in memory formation. Brain Res Bull 85: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkaniemi J, Sagar SM, Pyykonen I, Hicks KJ, Sharp FR 1995. Focal brain injury induces multiple immediate early genes encoding zinc finger transcription factors. Brain Res Mol Brain Res 28: 157–163 [DOI] [PubMed] [Google Scholar]
- Jo AY, Kim MY, Lee HS, Rhee YH, Lee JH, Baek KH, Park CH, Koh HC, Shin I, Lee YS, et al. 2009. Generation of dopamine neurons with improved cell survival and phenotype maintenance using a degradation-resistant nurr1 mutant. Stem Cells 27: 2238–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley MB, Wood MA, Isiegas C, Stein J, Hellman K, Hannenhalli S, Abel T 2006. Differential transcriptional response to nonassociative and associative components of classical fear conditioning in the amygdala and hippocampus. Learn Mem 13: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB 2000. Induction of NGFI-B mRNA following contextual fear conditioning and its blockade by diazepam. Brain Res Mol Brain Res 80: 153–165 [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W 2000. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res 110: 97–108 [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, et al. 2011. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31: 764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N 1999. Theta activity, virtual navigation and the human hippocampus. Trends Cogn Sci 3: 403–406 [DOI] [PubMed] [Google Scholar]
- Pena de Ortiz S, Maldonado-Vlaar CS, Carrasquillo Y 2000. Hippocampal expression of the orphan nuclear receptor gene hzf-3/nurr1 during spatial discrimination learning. Neurobiol Learn Mem 74: 161–178 [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34: 447–462 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA 2010. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30: 5037–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo-Cardenas O, Conneely OM 1996. Comparative distribution of NURR1 and NUR77 nuclear receptors in the mouse central nervous system. J Mol Neurosci 7: 51–63 [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Neve RL, Frankland PW, Josselyn SA 2010. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn Mem 17: 280–283 [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. 2007. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen LS, Giese KP 2005a. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J Neurosci 25: 1935–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen LS, Giese KP 2005b. α-isoform of Ca2+/calmodulin-dependent kinase II autophosphorylation is required for memory consolidation-specific transcription. Neuroreport 16: 1411–1414 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu YZ, Wong PT, Farook JM, Teo AL, Lee LK, Moochhala S 2003. cDNA microarray analysis of gene expression in anxious PVG and SD rats after cat-freezing test. Exp Brain Res 149: 413–421 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Castillo SO, Nikodem VM 1996. Distribution of messenger RNAs for the orphan nuclear receptors Nurr1 and Nur77 (NGFI-B) in adult rat brain using in situ hybridization. Neuroscience 75: 221–230 [DOI] [PubMed] [Google Scholar]
- Xing G, Zhang L, Heynen T, Li XL, Smith MA, Weiss SR, Feldman AN, Detera-Wadleigh S, Chuang DM, Post RM 1997. Rat nurr1 is prominently expressed in perirhinal cortex, and differentially induced in the hippocampal dentate gyrus by electro-convulsive vs. kindled seizures. Brain Res Mol Brain Res 47: 251–261 [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79: 49–58 [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Williams R, Perlmann T, Olson L 1996. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res 41: 111–120 [DOI] [PubMed] [Google Scholar]