Abstract
Treatment of the xyloglucan isolated from the seeds of Hymenaea courbaril with Humicola insolens endo-1,4-β-d-glucanase I produced xyloglucan oligosaccharides, which were then isolated and characterized. The two most abundant compounds were the heptasaccharide (XXXG) and the octasaccharide (XXLG), which were examined by reference to the biological activity of other structurally related xyloglucan compounds. The reduced oligomer (XXLGol) was shown to promote growth of wheat (Triticum aestivum) coleoptiles independently of the presence of 2,4-dichlorophenoxyacetic acid (2,4-D). In the presence of 2,4-D, XXLGol at nanomolar concentrations increased the auxin-induced response. It was found that XXLGol is a signaling molecule, since it has the ability to induce, at nanomolar concentrations, a rapid increase in an α-l-fucosidase response in suspended cells or protoplasts of Rubus fruticosus L. and to modulate 2,4-D or gibberellic acid-induced α-l-fucosidase.
Xyloglucan is a major hemicellulosic polysaccharide in the primary cell walls of dicotyledons and nongraminaceus monocotyledons and is also present as a storage polysaccharide in the seeds of many dicotyledons (McNeil et al., 1984; Reid, 1985; Lima et al., 1993; York et al., 1993; Braccini et al., 1995). The heterogeneity of xyloglucans results from differences in their molecular mass, distribution, and levels of substituted xylosyl units with galactosyl and fucosyl residues. Thus, the fucosyl residue has been found to be (1→2)-linked to the β-galactosyl residue in suspended cells of Rosa sp. (McDougall and Fry, 1988) and sycamore (Stephen, 1983) or when xyloglucans were isolated from the stems and the roots of etiolated pea (Pisum sativum L.) plants (Guillén et al., 1995). This residue is unusual in seed xyloglucans (Siddiqui and Wood, 1977; Guidley et al., 1991).
The metabolism of xyloglucans in cellulose microfibril networks is important for cell wall expansion. Support for this idea comes from alterations to xyloglucan that contribute to wall extensibility. Endo-1,4-β-d-glucanases, xyloglucanase, α-l-fucosidase, and/or endo-type transferases such as xyloglucan endotransglycosylase are involved in the auxin- or acid-promoted breakdown of xyloglucans (Hayashi et al., 1984; Hetherington and Fry, 1993). Furthermore, xyloglucan-derived oligomers exhibited signaling effects. The Fuc-rich xyloglucan from suspension cultures of Rosa sp. digested by Trichoderma viride cellulase resulted in the formation of a nonasaccharide-containing terminal fucosyl residue (XXFG; Fry et al., 1993a). This oligosaccharide acts as an anti-auxin 2,4-D growth promotor in etiolated pea stems (McDougall and Fry, 1988) and was also able to inhibit GA3 induction of pea segments (Yang et al., 1996), stimulate glycan-synthase activities, and increase the viability of protoplasts (Emmerling and Seitz, 1990). Substitution of the XXXG core with one or two Gal residues to give XXLG and XLLG resulted in growth promotion and in the in vitro stimulation of cellulase (McDougall and Fry, 1990).
Hymenaea courbaril (Leguminosae) is a species that occurs abundantly throughout Brazilian forests from the northeast to the south, and the seeds contain 40 to 45% xyloglucan, the structure of which consists of a cellulosic-type (1→4)-linked β-d-glucan main chain and side chains containing α-d-xylopyranosyl and β-d-galactopyranosyl-(1→2)-α-d-xylopyranosyl residues, each (1→6)-linked to the main chain (Lima et al., 1993, 1995). Here, the ability of endoglucanase I of Humicola insolens to cleave the (1→4)-linked β-d-glucosyl residues of xyloglucan from H. courbaril seeds was carried out. The released oligomers (such as XXLG and XXXG) were then examined under the reduced form in terms of their biological activity with other structurally related xyloglucan compounds. The auxin activity of XXLGol in vivo was investigated using wheat coleoptiles. Parallel to this, a system of Rubus fruticosus L. protoplasts was used to study the signaling effect of the induction of α-l-fucosidase activity.
MATERIALS AND METHODS
Chemicals
The Humicola insolens endoglucanase I (121 endocellulase units mg−1) was a gift of Dr. Schülein (Novo Nordisk, Bagsvaerd, Denmark) and was obtained as described by Armand et al. (1997). Caylase 345 (cellulase) and Caylase M3 (pectinase) were purchased from Cayla (Toulouse, France). α-l-Fuc-(1→2)-β-d-Gal-(1→4)-β-d-Glc (2′-fucosyl-lactose) was from Oxford GlycoSystems (Coger, Paris, France). XXFGol, prepared from Rubus fruticosus L. suspended cells as described by Joseleau et al. (1992), was a gift of Dr. Gérard Chambat (Centre de Recherches sur les Macromolécules Végétales-Centre National de la Recherche Scientifique, Grenoble, France). The trisaccharide methyl α-l-Fuc-(1→2)-β-d-Gal-(1→2)-β-d-Xyl side chain of the xyloglucan molecule was prepared as described by Lopez et al. (1994) and was a gift of Dr. Fernandez-Mayoralas (Instituto de Quimica Organica General, Consejo Superior de Investigaciones Científicas, Madrid, Spain). The oligogalacturonides of DP ranging from 12 to 15 and maltopentaose were a gift of Dr. Alain Heyraud (Centre de Recherches sur les Macromolécules Végétales-Centre National de la Recherche Scientifique). Potassium benzyl penicillin, cycloheximide, actinomycin D, and pnitrophenyl α-l-fucopyranoside were from Sigma. BioGelP2 was from Bio-Rad.
Materials
Suspensions of R. fruticosus L. cultures, originally derived from cambial explants from twigs, were grown as described by Hustache et al. (1975). Hymenaea courbaril seeds were collected in July 1995, at the campus of the University of São Paulo, Ribeirão Preto, Brazil. Seeds of wheat (Triticum aestivum var Festival) were purchased from EARL Benoist (Airvault, France).
Preparation of XXXGol and XXLGol
Preparation of XXXGol and XXLGol was as follows: Purified xyloglucan from H. courbaril seeds was obtained according to the method of Lima et al. (1995). A sample (500 mg) was incubated (96 h, 37°C) in water (100 mL) containing endo-1,4-β-d-glucanase type I (5 mg) from H. insolens (EC 3.2.1.4; 121 endocellulase units/mg). Aliquots (0.25 mL) were removed from the incubated sample at 0, 24, 48, 72, and 96 h, and the reducing sugars were determined. The enzymatic reaction was stopped by heating at 100°C for 5 min, insoluble material was removed by centrifugation, and the supernatant was concentrated and lyophilized. Aliquots of the oligosaccharide mixture (50 mg, 1 mL) were filtered on a cellulose nitrate membrane (0.45 μm) and applied to a column (1.5 × 210 cm; 60°C) of BioGel-P2 (400 mesh). Elution with water was at a flow rate of 0.5 mL min−1, controlled by a peristaltic pump (Milton-Roy, Rochester, NY). Eluted oligosaccharides were monitored with a differential refractometer (model R403, Waters).
Oligosaccharides were reduced as described by York et al. (1993): they were first dissolved (5 mg mL−1) in water containing NaBH4 (5 mg mL−1; 3 h). Excess of borohydride was decomposed with glacial acetic acid, and residual borate was removed by coevaporation with methanol. The resulting oligoglycosyl alditols (5 mg per injection) were filtered on a cellulose nitrate membrane (0.3 μm), desalted, and separated by reverse-phase chromatography on a Nucleosil C-18 semipreparative column (25 × 0.46 cm) eluted with 7% aqueous methanol as the mobile phase at a flow rate of 2 mL min−1; eluted oligosaccharides were detected by monitoring the refractive index.
NMR Spectroscopy
NMR spectra were recorded with a spectrometer (model AC300, Bruker, Billerica, MA). The alditol samples were dissolved in D2O (2 mg mL−1 [1H] and 10 mg mL−1 [13C]). Chemical shifts are reported as δ relative to internal acetone as δ 2.04 (1H) and 29.8 (13C) with respect to the signals for tetramethylsilane at 333 K (1H) and 303 K (13C).
FAB-MS Spectrometry
Low-mass resolution measurements were performed on a quadripole mass spectrometer (model R.10.10C, Nermag, Rveil-Malmaison, France) using a glycerol matrix and FAB(+) mode.
Wheat Coleoptile Growth Biossays
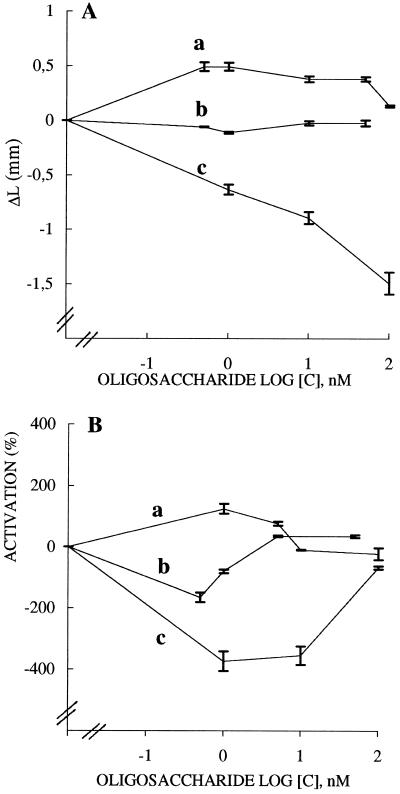
Seeds were grown in the dark and 3-d-old wheat seedings with the first internode measuring 15 mm were selected. These samples were incubated in sterile, plastic Petri dishes (15–20 seeds per dish) in two independent replicate sets containing 15 mL of fresh medium (1% [w/v] Suc, 5 mm KH2PO4 [pH 6.1] and 0.02% potassium benzyl penicillin) with or without effector. The bioassay was started by the addition of 2,4-D (1 μm) and/or XXLGol (0.5, 1, 10, 50, and 100 nm), XXFGol (0.5, 1, 10, and 50 nm), 2′-fucosyl-lactose (1, 10, and 100 nm), and the coleoptile length was measured at intervals up to 62 h. Controls were concomitantly run in the described medium without the addition of 2,4-D or oligosaccharide. The variability in elongation of the 15 to 20 stem segments in a single dish was measured with a Student's t test, and data with P < 0.05 were analyzed. Data points of kinetic curves are each means ± se of 30 to 40 determinations (Fig. 1).
Figure 1.
Effect of the xyloglucan oligomers XXLGol (a) and XXFGol (b) and of 2′-fucosyl-lactose (c) on the straight growth of wheat coleoptiles (A) and on the elongation promoted by 2,4-D (B). Two independent experiments, each conducted from 15 to 20 coleoptile segments treated or not with 2,4-D (1 μm) for 62 h were monitored and the mean increments of length (ΔL) were plotted. In A each value of a curve is the average of 30 to 40 experiments, and vertical bars represent ses. Data are presented as ΔL versus log sugar concentration; ΔL is the additional increase in length between 10 and 20 h. The absolute increase in length of controls (sets in the absence of 2,4-D or oligosaccharides) exhibited the mean value of 3.87 mm. This value was the reference (ΔL = 0). In B data are presented as mean percentages of inhibition or activation of 2,4-D-stimulated growth, calculated as percentages of activation or inhibition = L(2,4-D + oligosaccharide) − (L[2,4-D])/(L[2,4-D]) − L(control) × 100%, where L(2,4-D) is the mean final length of segments treated with 2,4-D, L(control) is the mean final length of segments incubated without 2,4-D, and L(2,4-D + oligosaccharide) is the mean final length of segments treated with 2,4-D plus the oligosaccharide. Plotted data represent the means ± se.
Preparation of Cells and Protoplasts from R. fruticosus L.
Cells in the exponential growth phase (15–18 d after subculturing) were collected by centrifugation at 4000g for 5 min, washed with Heller's medium, and resuspended in 50 mm sodium-citrate buffer, pH 5.9, containing 2% Suc, 4 mm EGTA, 3 mm MgCl2, and 0.06 mm CaCl2. For protoplast isolation, 18- to 20-d-old cells (40 g fresh weight) were incubated overnight at room temperature in 300 mL of the growth medium, pH 5.9, supplemented with 0.56 m mannitol and 0.25% (w/v) cellulase and 0.01% (w/v) pectinase. The released protoplasts were filtered through a 100-μm nylon mesh washed twice with the incubation medium with no cell wall-degrading enzymes before being pelleted at 500g for 5 min. They were resuspended in 25 mm Bis-Tris-HCl buffer, pH 4.8, containing 0.56 m mannitol, 0.06 m Suc, 1 mm KCl, 1 mm CaCl2, and 6% (w/v) Ficoll 400, and were then centrifuged at 500g for 5 min, and finally washed with Bis-Tris-HCl buffer without Ficoll. Protoplast yields ranged from 70 to 85% of the initial number of treated cells.
Bioassays
Protoplasts (2 × 106; or cells) of R. fruticosus were suspended under stirring in 25 mL of buffer (25 mm Bis-Tris-HCl, pH 4.8, containing 0.56 m mannitol, 0.06 m Suc, 1 mm KCl, and 1 mm CaCl2) in the presence or absence of: oligo-, polysaccharide inducer up to 100 nm; 2,4-D or GA3 (10 nm); cycloheximide (1 μm); or actinomycin D (1 μg mL−1). The concentration of oligomers is expressed as molarity, and the molar concentration of the polymer is reported by reference to XXLG repeating units. Protoplasts (or cells) were harvested at various intervals by centrifugation at 300g for 8 min at 4°C, before being subjected to enzyme extraction. The viability of protoplasts was controlled using Evan's blue indicator.
α-l-Fucosidase Assays
Enzymes were extracted in 50 mm Tris-HCl, pH 7.2, containing 1 m NaCl by homogenizing the protoplasts on ice with a polytron at full speed, 15 times for 45 s. The extracts were dialyzed and concentrated using ultrafiltration units equipped with a molecular mass cutoff value of a 10-kD membrane (Ultrafree, Millipore, Bedford, MA). α-l-Fucosidase (EC 3.2.1.51) was assayed as follows: p-nitrophenyl α-l-fucopyranoside (2 mg mL−1) was incubated at 40°C for 0 to 90 min with crude enzyme extract (1 μg of protein based on colorimetric determination; Bradford, 1976) in 200 μL of 0.1 m sodium acetate buffer, pH 5.0. The enzyme reaction was quenched with 100 μL of 0.1 m Na2CO3, and α-l-fucosidase activity was determined by measuring the A410 of the p-nitrophenolate ion according to the method of Lee and Zeikus (1993).
For each oligosaccharide tested, enzyme assays were performed from three to four replications from three independent elicitation sets, and kinetic curves were drawn and fitted with the second-order polynomial regressions. The velocity of enzyme activities was calculated from regression equations using Excel software, and enzyme activation is expressed as R, which is the ratio of the slopes of the fitted curves obtained from treated protoplasts versus controls. The treated protoplasts were elicited by one oligosaccharide and/or one hormone. The controls were the protoplasts suspended in buffer without any effector. Blanks (without enzyme or without substrate) were carried out for each sample.
RESULTS
Preparation and Characterization of Oligosaccharides Derived from Xyloglucan of H. courbaril Seeds
The enzymatic hydrolysis of the xyloglucan isolated from H. courbaril seeds was carried out using endo-1,4-β-d-glucanase I from H. insolens (Armand et al., 1997). The water-soluble oligosaccharides (450 mg) were partially fractionated according to their molecular weights by BioGel-P2 chromatography. Higher-molecular-weight oligosaccharide components represented 50% of eluted material and were not analyzed further. Fractions corresponding to oligomers of DP 5 to 8 were pooled and converted to their corresponding alditol derivatives. The mixture was further fractionated by reverse-phase chromatography. Only the two major oligosaccharides (4 and 42 mg), with retention times of 35 and 41.26 min, respectively, were isolated and characterized.
The oligosaccharides were identified by their 1H-NMR spectra as XXXGol and XXLGol. The shape and chemical shift of their 1H-NMR signals were in agreement with those already published (York et al., 1993), considering the differences in experimental conditions. Our NMR spectra were recorded on a spectrometer (model AC300, Bruker, Wissembourg, France) at 333 K at a concentration of 10 to 20 mg mL−1; York et al. (1993) used a Bruker 500 at 298 K and a concentration of 1 to 10 mg mL−1. In the 1H-NMR spectrum of XXLGol, H-1 signals of α-Xylp units were a doublet (δ 5.03, J 4.0 Hz) and a triplet of two superimposed signals centered at δ 4.81, each with J 4.0 Hz. The H-1 signal of the β-Galp unit was detected as a doublet (δ 4.50, J 10 Hz). The H-1 signal of the β-Glcp gave a broad doublet (δ 4.40, J 8 Hz, 3 units).
The 13C-NMR spectrum of the octasaccharide XXLGol was consistent with the structure (York et al., 1993; Guillén et al., 1995), with C-1 signals at δ 105.5 (β-Galp); 103.8, 103.6, and 103.3 (each β-Glcp); 99.9 (2 × α-Xylp) and 99.3 (α-Xylp); and β-Glcp 4-O-substituted resonances at δ 70.5, 80.3, 80.7, and 80.9. Its positive-ion FAB-MS indicated a molecular weight at 1226 (i.e. GalXyl3Glc3Gol; [M + H]+ at m/z 1227; [M + Na]+ at m/z 1249). Because of the rupture of glycosidic linkages, the ions at m/z 1085, 1065, 983, 791, and 645 were also assigned.
XXXGol was also characterized on the basis of its 1H-NMR and FAB-MS (Guillén et al., 1995). The H-1 region has neither the signal at δ.5.0, which corresponds to Xyl substituted at O-2 by Gal, nor that of β-Galp. Its positive-ion FAB-MS indicated a molecular weight at 1064 (i.e. Xyl3Glc3Gol; [M + Na]+ at m/z 1087).
Growth Induction by Xyloglucan Oligosaccharides and 2′-Fucosyl-Lactose
The coleoptiles incubated in Petri dishes showed no significant variability in elongation. Therefore, all of the samples subjected to a particular treatment on a given day were viewed as a single population, despite the fact that they were distributed between distinct dishes. The ability of the xyloglucan oligosaccharides XXLGol and XXFGol and of 2′-fucosyl-lactose to interact with coleoptile growth, induced or not with 2,4-D, were bioassayed during 62 h. The oligosaccharides were used here at the narrow nanomolar concentration range up to 100 nm, which can promote enzyme-activation responses as detailed below. The trisaccharide was chosen to test the importance of Xyl in the activity by reference to the 2′-fucosyl-lactose. The growth rates between 10 and 20 h of incubation were first investigated as a function of experimental time (not shown) and then expressed as ΔL versus log sugar concentration (Fig. 1A) or as a percentage of growth promotion (percentage of activation) or growth inhibition (percentage of inhibition) by 2,4-D (Fig. 1B).
The results without 2,4-D (Fig. 1A) clearly indicate that XXLGol at a concentration ranging from 0.5 to 100 nm exhibited growth stimulation (curve a), that XXFGol was not active (curve b), and that 2′-fucosyl-lactose showed an inhibiting effect (curve c). In the presence of 2,4-D used at 1 μm (Fig. 1B), XXLGol increased the auxin-induced response (curve a), whereas XXFGol (curve b) and 2′-fucosyl-lactose (curve c) showed anti-auxin activity. It was observed that XXFGol, which was less active than 2′-fucosyl-lactose, had anti-auxin activity at low concentrations but exhibited growth-restoring activity at high concentrations.
Xyloglucosyl Oligomers as Inducers of α-l-Fucosidase Activity
R. fruticosus protoplasts were incubated for 15 min in the presence of sugar inducers at concentrations up to 100 nm. The inducers were the xyloglucan polymer from H. courbaril seeds (Lima et al., 1995), the derived oligosaccharides XXLGol, XXXGol, and XXFGol obtained from R. fruticosus xyloglucan, and the trisaccharide methyl α-l-Fuc(1→2)-β-d-Gal(1→2)-β-d-Xyl. It was verified that protoplast viability was not affected by the treatment and remained identical to the control protoplasts (90%) throughout the experiments.
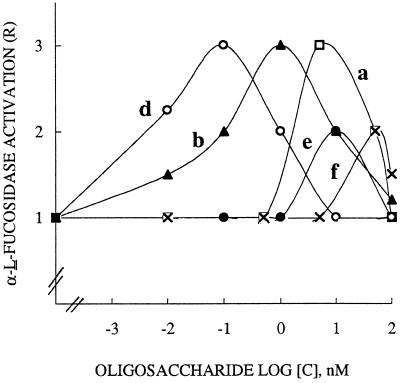
Dose-response curves for α-l-fucosidase activation are shown in Figure 2. With all of the inducers used up to 100 nm, e.g. the oligomers XXLGol, XXFGol, and XXXGol (curves a, b, and d, respectively), the trisaccharide (curve e), and the xyloglucan polymer (curve f), bell-shaped curves were obtained. The highest activities, with maximum R values of approximately 3, were observed for XXXGol, XXFGol, and XXLGol at 0.1, 1, and 5 nm, respectively. The trisaccharide and the polymer with maximal R values of 2 only exhibited higher optimal concentrations (10 and 50 nm, respectively).
Figure 2.
Dose-response curves for α-l-fucosidase response induced by the xyloglucan oligomers XXLGoL (a), XXFGol (b), and XXXGol (d), the trisaccharide methyl α-l-Fuc-(1→2)-β-d-Gal(1→2)-β-d-Xyl (e), and the polymer (f). R. fruticosus protoplasts (2 × 106) in 25 mL of buffer were incubated for 15 min in the presence of inducer up to 100 nm. Each curve was obtained by least-squares regression of data from three to four replications carried out from three independent inducer sets. R is reported as the rate of α-l-fucosidase activity of treated over control protoplasts.
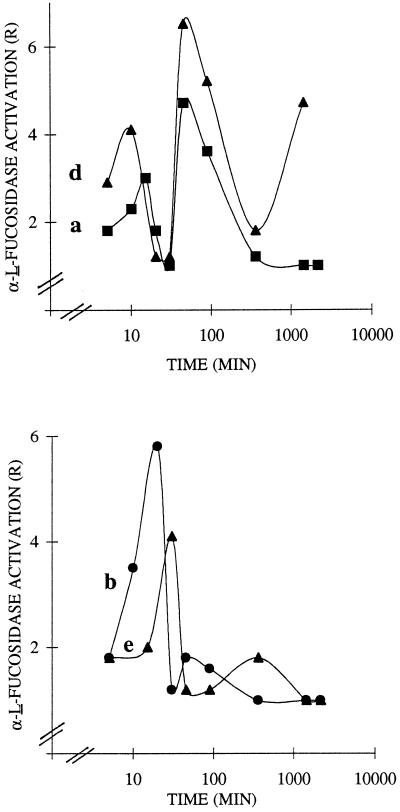
Kinetic measurements of α-l-fucosidase activation in R. fruticosus protoplasts and/or cells in the presence of the inducer used at its optimal concentration and shown in Figure 3 were carried out. We verified that protoplast (or cell) viability was not affected by the treatment and remained as high as in control protoplasts (90%) or control cells (95%) throughout the experiments. When the protoplasts were challenged with XXLGol, XXFGol, XXXGol, and trisaccharide (Fig. 3, curves a, b, d, and e, respectively), the detected responses were biphasic and triphasic with respect to their kinetics. The oscillation of the early responses of protoplasts peaked after 10 to 20 min and 45 min for the oligomers; the response to the synthetic trisaccharide was delayed, since it peaked at 30 min and 6 h. When the inducers were compared at their respective maximal R values, the order of their effectiveness between 10 and 30 min was XXFGol > XXXGol approximately trisaccharide > XXLGol, with respective R values of 5.8, 4.1, and 3. After 45 min, the order was XXXGol > XXLGol > XXFGol, with R values of 6.5, 4.7, and 1.8, respectively.
Figure 3.
Time course for α-l-fucosidase activation in R. fruticosus protoplasts challenged with the sugar inducers XXLGol (a), XXFGol (b), and XXXGol (d) and the trisaccharide methyl α-1-Fuc-(1→2)-β-d-Gal-(1→2)-β-d-Xyl (e). Protoplasts (2 × 106) in 25 mL of buffer were challenged with inducer used at the optimal concentration (0.1 nm XXXGol, 1 nm XXFGol, 5 nm XXLGol, and 10 nm trisaccharide). Each curve was obtained by least-squares regression of data from three to four replications carried out from three independent inducer sets. R is reported as the rate of α-l-fucosidase activity of treated over control protoplasts. Control sets were run without addition of 2,4-D or oligosaccharides.
It is significant that only 10 min was required for XXXGol to trigger a response, as opposed to 15 and 20 min for XXLGol and XXFGol, respectively, and 30 min for the trisaccharide. Incubation for a longer duration of up to 96 h resulted in a large response increase; R values of 6 and 8 were found from XXXGol and XXLGol, respectively (not shown). In the presence of inhibitors of transcription (1 μm cycloheximide) or of translation (1 μg mL−1 actinomycin D), the short responses of up to 45 min were maintained, whereas the long-term treatment resulted in a markedly attenuated response (40 or 50% inhibition at 96 h being detected from the XXLGol inducer). The cell-suspension cultures monitored instead of protoplasts gave rise to plateau responses, and the enzyme activation was largely attenuated, since the highest detected maximal R values were only 1.2 and 3.5 (not shown). Oligosaccharides structurally unrelated to xyloglucan, such as oligogalacturonides of 10 to 15 DP and maltopentose, were also used as potential inducers but failed to promote any response in protoplasts or in cells.
Effects of Xyloglucosyl Oligomers on 2,4-D-Induced α-l-Fucosidase Response
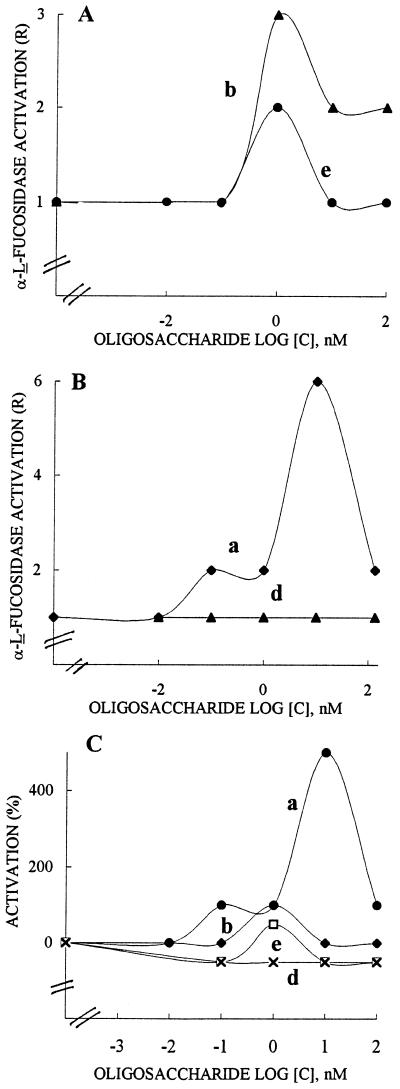
Induction of α-1-fucosidase activity (R value of 1.5) occurred when 2,4-D was used with R. fruticosus protoplasts for 15 min at 10 nm. The oligomers XXFGol, XXLGol, XXXGol, and the trisaccharide methyl α-l-Fuc-(1→2)-β-d-Gal-(1→2)-β-d-Xyl up to 100 nm were assayed for their effect on the auxin-stimulated α-l-fucosidase. One of our aims was to determine which glycosyl residues were required for modifying the α-l-fucosidase response. Dose-response curves in the presence of 10 nm 2,4-D indicated that XXFGol and trisaccharide inducers exhibited a similar behavior (Fig. 4A, curves b and e, respectively). The absence of a fucosyl residue in XXLGol resulted in the promotion of enzyme activation, with an R value of about 6 (Fig. 4B, curve a), whereas XXXGol, which lacks the 2′-fucosyl Gal side chain, was not active (Fig. 4B, curve d). The data shown in Figure 4C are the percentages of modulation of 2,4-D-induced α-l-fucosidase. They clearly show that the terminal galactosyl residue of XXLGol is the structural feature required to promote an α-l-fucosidase response (curve a) and that the lack of the galactosyl residue led to a compound with inhibitory activity only (curve d). The presence of the fucosyl residue attached to the 2-position of the galactosyl unit common to XXFGol (curve b) and the trisaccharide methyl α-l-Fuc-(1→2)-β-d-Gal-(1→2)-β-d-Xyl (curve e) caused either inhibition or activation depending on the sugar concentration, the trisaccharide being the more potent inhibitor of these two sugars.
Figure 4.
Effects of XXLGol (a), XXFGol (b), and XXXGol (d) and the trisaccharide methyl α-l-Fuc(1→2)-β-d-Gal(1→2)-β-d-Xyl (e) on 2,4-D-stimulated α-l-fucosidase in R. fruticosus protoplasts. The results are expressed as the R value in A and B and as the mean percentage of activation (inhibition) of 2,4-D-stimulated response in C. Protoplasts (2 × 106) in 25 mL of buffer were incubated for 15 min with sugar inducer up to 100 nm in the presence of 10 nm 2,4-D. Each curve was obtained by least-squares regression of data from three to four replications carried out from three independent inducer sets. The induced response given as R is reported as the rate of α-l-fucosidase activity of treated protoplasts over controls. The mean percentage of activation (inhibition) is calculated as R(2,4-D + oligosaccharide) − (R[2,4-D])/(R[2,4-D]) − R(control) × 100, where R(2,4-D + oligosaccharide) and R(2,4-D) are R values in protoplasts incubated with sugar inducer and 2,4-D and with 2,4-D, respectively, and R(control) is the R value in protoplasts suspended in buffer without the addition of 2,4-D or oligosaccharides.
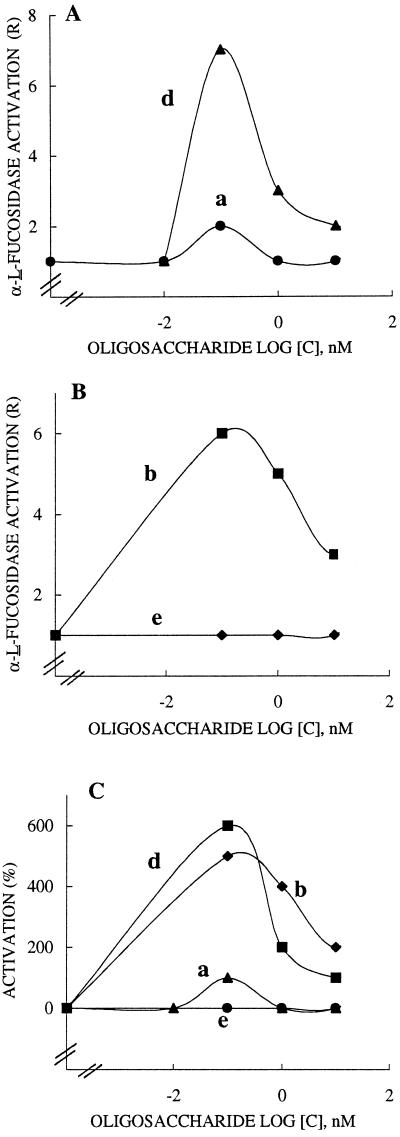
Effects of Xyloglucosyl Oligomers on GA3 α-l-Fucosidase Activation
GA3 acting for 15 min at 10 nm was also found to promote α-l-fucosidase activation in R. fruticosus protoplasts (R value of about 1.5). The dose-response curves resulting from the presence of GA3 (Fig. 5, A and B) and the oligomers showed that XXXGol (curve d) and XXFGol (curve b) were highly effective in the enhancement of enzyme activation, with R values of 7 and 6, respectively. XXLGol (curve a) was less active (R value of 2 only) and the xyloglucosyl side chain of methyl α-l-Fuc-(1→2)-β-d-Gal-(1→2)-β-d-Xyl did not promote an increase in the α-l-fucosidase response (curve e). The data shown in Figure 5C, presented as percentages of activation of GA3-induced-α-l-fucosidase, clearly reveal that the presence of two xylosyl residues attached at final and penultimate Glc units are needed for the biological activity (curves d and b), but the galactosyl residue at position 2 antagonized the inducing effect of xylosyl residue, since XXLGol was poorly effective (curve a). It is significant that the presence of a terminal fucosyl residue in XXFGol (curve b) could only partially restore the activity, but this restoration required a feature mimicking the XXXGol backbone, since the trisaccharide was not active (curve e).
Figure 5.
Effects of XXLGol (a), XXFGol (b), XXXGol (d) and the trisaccharide methyl α-l-Fuc-(1→2)-β-d-Gal-(1→2)-β-d-Xyl (e) on GA3-stimulated α-l-fucosidase in R. fruticosus protoplasts. The results are expressed as the R value in A and B and as the mean percentage of activation of GA3-stimulated response in C. Protoplasts (2 × 106) in 25 mL of buffer were incubated for 15 min with sugar up to 100 nm in the presence of 10 nm GA3. Each curve was obtained by least-squares regression of data from three to four replications carried out from three independent inducer sets. R is reported as the rate of α-1-fucosidase activity of treated protoplasts over controls. The mean percentage of activation is calculated as = R(GA3 + oligosaccharide) − (R[GA3])/(R[GA3]) − R(control) × 100, where R(GA3 + oligosaccharide) and R(GA3) are R values in protoplasts incubated with sugar and GA3 and with GA3, respectively, and R(control) is the R value in protoplasts suspended in buffer without the addition of 2,4-D or oligosaccharides.
DISCUSSION
Sugar-signaling molecules of fungal or plant origin, which are produced by acidic or enzymatic hydrolysis of cell wall polysaccharides or glycoproteins, induce plant-defense responses and/or exhibit effects on growth and development (Aldington and Fry, 1993). In relation to the xyloglucan oligomers, nanomolar or micromolar concentrations with specific structural elements initiate characteristic physiological or biochemical responses. Thus, the structural features required for XXFG to exhibit an anti-auxin effect have been extensively studied (York et al., 1984; McDougall and Fry, 1988, 1989a, 1989b, 1990; Augur et al., 1992). Optimal anti-auxin activity is observed in a nanomolar range of XXFG (but not of XXLG or XXXG). XXFG is as effective as the reduced form XXFGol according to Augur et al. (1992). This effect is mimicked only by the related α-l-Fuc unit containing xyloglucosyl oligomers and by the 2′-fucosyl-lactose. It has also been postulated that the activity of XXFG may be the result of two opposing effects: at lower concentrations, the Fuc-dependent-anti-auxin effect predominates, whereas at higher concentrations the Fuc-independent, growth-promoting effect is expressed.
These results suggest the presence of specific recognition systems for xyloglucan oligomers in plants. It is worth noting that when XLLG, XXXG, and XXFG promoted the elongation of pea stem segments in the absence of 2,4-D, the detected effect differed in several important respects from the growth-inhibiting effect reported above. Indeed, the optimal concentration for growth promotion is approximately micromolar, the fucosyl residue is not required, and some of the Xyl3 Glc4 backbone is required. This prompted Fry et al. (1993a) to suggest that the oligosaccharides exert their effect by acting as substrates of xyloglucan endo-transglycosylase.
We have used the xyloglucan from the seeds of H. courbaril as a source of signaling molecules. The monosaccharide composition of this xyloglucan is Glc, Xyl, and Gal in a ratio of 50:35:13, and its structure was determined by methylation, periodate oxidation, and 13C-NMR spectroscopy (Lima et al., 1993, 1995) to be similar to that of the well-characterized xyloglucan from the seeds of Tamarindus indica (Guidley et al., 1991). The polysaccharide was treated with H. insolens endo-1,4-β-d-glucanase I. Because it belongs to the glycosyl hydrolase family 7, the enzyme is a retaining endoglucanase that needs as a substrate an unsubstituted glucosyl unit in its subsite-1 (Armand et al., 1997), as do most of the endoglucanases used for xyloglucan degradation (Vincken et al., 1995). The enzyme preparation was free of galactosidase and xylosidase, since no monosaccharide was detected on elution with BioGel-P2. The octasaccharide, representing the basic structure of the xyloglucan, was readily obtained, as well as a minor heptasaccharide component. The resulting hepta- and octasaccharide were isolated by size-exclusion chromatography and then by HPLC; the corresponding alditols were characterized by their NMR and FAB-MS spectra. The data are in agreement with those previously published (York et al., 1993; Guillén et al., 1995).
XXLGol and XXXGol were used as signaling molecules. Purified XXLGol was shown to promote, at a nanomolar concentration range, the growth of wheat coleoptiles independently of the presence of 2,4-D, as described previously for micromolar xyloglucan in pea stem segments (McDougall and Fry, 1990). In the presence of 2,4-D, nanomolar concentrations of XXLGol increased the auxin-induced response, but 2′-fucosyl-lactose and XXFGol, to a lesser extent, can exhibit the anti-auxin effect, depending on the concentration used. Therefore, the data obtained for wheat coleoptiles confirmed that the anti-auxin activity depends on the presence of the α-l-fucopyranosyl α-(1→2)-d-galactopyranosyl β-(1→) side chain of the xyloglucan.
The data reported for suspensions of R. fruticosus protoplasts (or cells) revealed that xyloglucan oligomers exhibited signaling molecules by their ability to increase α-l-fucosidase activity and to modulate 2,4-D- or GA3-induced α-l-fucosidase. Here the auxin and/or the anti-auxin activity of xyloglucosyl oligomers in biological systems is totally different from that previously reported, and the GA3-induced response confirmed the results obtained by Warneck and Seitz (1993). The biological responses mainly depended on parameters such as the structural features of the xyloglucosyl oligomers and side chain, the chemical structure of the hormone, and the sugar concentration.
It was interesting that in the presence of 2,4-D the terminal β-d-galactosyl residue was the determinant for biological activity: XXLGol contains the residue promoting an auxin α-l-fucosidase response, and the lack of the galactosyl residue in XXXG led to inhibitory activity. The galactosyl residue can be antagonized by a Fuc molecule attached to the 2 position of the galactosyl unit, as is also the case with XXFGol and synthetic trisaccharide. In the presence of GA3, the xylosyl residue(s) attached in the final or/and penultimate position is required for biological activity. They could be antagonized by the galactosyl residue at position 2, since XXLGol is not active. It was worth noting that the terminal fucosyl of the XXFGol side chain (but not the Fuc unit in a trimeric structure) revealed antagonistic effects toward galactosyl residues and the same order of effectiveness of XXFGol and XXXGol.
The protoplast responses had a very rapid and transient nature, suggesting an early signal transduction cascade. This was especially true in the case α-l-fucosidase induction, which started within a few minutes after the addition of xyloglucan signals. The elicitor treatment up to 180 min did not change the total amount of extractable protein but affected the specific enzyme activity. The induction of such responses did not require the presence of cell walls, and it was fully maintained in the presence of inhibitors of transcription or translation. These results, which strongly suggest the evidence of receptor molecules for xyloglucan at plasma membranes, contribute to a more general application of the receptor hypothesis. Extensive research on signaling sugars has revealed a sequence of biochemical events, including transcription and translation of specific genes, resulting in induction de novo synthesis of enzymes, but the initial process of signal perception and transduction has still not been elucidated (Côté and Hahn, 1994). The presence of high-affinity binding sites, putative receptors for sugars (hepta β-glucoside [Cosio et al., 1992], N-acetylchitooligosaccharide [Shibuya et al., 1993], and the trisaccharide determinant of H-type 1 human determinant [Liénart et al., 1992]), have been reported in plants, and a binding protein for a β-glucan elicitor of Phytophthora megasperma origin has been cloned (Umemoto et al., 1997). However, the initial process of perception and transduction of signaling oligosaccharides remains to be elucidated.
The present study confirmed that xyloglucan oligomers can have hormone-dependent effects in a system other than pea-stem bioassays. This had been previously reported from data on the anti-auxin activity in carrot protoplasts (Emmerling and Seitz, 1990) or on the ability of FG to control the morphogenesis in cultured wheat embryos (Pavlova et al., 1992). In addition, the effect of elongation of XXXG and XXFG was recently correlated with the viscoelastic properties of pea shoots (Cutillas-Iturralde and Lorences, 1997). McDougall and Fry (1990) and Augur et al. (1995) speculated that cellulase and α-l-fucosidase activities participate in the regulation of plant growth by controlling both the hydrolysis of xyloglucan and the concentration of fucosylated oligomers.
This is consistent with our experiments showing that the treatment of R. fruticosus protoplasts by xyloglucosyl oligomers in the presence or absence of hormone greatly modulated the α-l-fucosidase activity within a few minutes of application. When α-l-fucosidase was assayed in plants (Farkas et al., 1991; Augur et al., 1995; Hoson et al., 1995), the enzyme was able to hydrolyze terminal fucosyl residues from XXFG but failed to cleave p-nitrophenyl α-l-fucopyranoside. In contrast, we observed that the enzyme in R. fruticosus hydrolyzes the artificial substrate, as do the mammalian α-l-fucosidases (Johnson and Alhadeff, 1991). Research is now in progress to explain these observations.
Abbreviations:
- DP
degree of polymerization
- FAB
fast-atom bombardment
- XXXG
XXLG, and XXFG, the xyloglucan-derived hepta-, octa-, and nonasaccharide, respectively (see Fry et al., 1993b)
- XXXGol
XXLGol, and XXFGol, the reduced oligomers of XXXG, XXLG, and XXFG, respectively
Footnotes
Financial support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil).
LITERATURE CITED
- Aldington S, Fry SC. Oligosaccharins. Adv Bot Res. 1993;19:1–107. [Google Scholar]
- Armand S, Drouillard S, Schülein M, Henrissat B, Driguez H. A bifunctionalized fluorogenic tetrasaccharide as a substrate to study cellulases. J Biol. 1997;272:2709–2713. doi: 10.1074/jbc.272.5.2709. [DOI] [PubMed] [Google Scholar]
- Augur C, Stiefeld V, Darvill AG, Albersheim P, Puigdomenech P. Molecular cloning and pattern of expression of an α-l-fucosidase gene from pea seedlings. J Biol Chem. 1995;270:24839–24843. doi: 10.1074/jbc.270.42.24839. [DOI] [PubMed] [Google Scholar]
- Augur C, Yu I, Sakai K, Ogawa T, Sinay P, Darvill AG, Albersheim P. Further studies of the ability of xyloglucan oligosaccharides to inhibition auxin-stimulated growth. Plant Physiol. 1992;99:180–185. doi: 10.1104/pp.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braccini I, Hervé du Penhoat C, Michon V, Goldberg R, Clochard M, Jarvis MC, Huang Z-H, Gage DA. Structural analysis of cyclamen seed xyloglucan oligosaccharides using cellulase digestion and spectroscopic methods. Carbohydr Res. 1995;276:167–181. doi: 10.1016/0008-6215(95)00156-n. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cosio EG, Frey T, Ebel J. Identification of a high-affinity binding protein for a hepta β-glucoside phytoalexin elicitor in soybean. Eur J Biochem. 1992;204:1115–1123. doi: 10.1111/j.1432-1033.1992.tb16736.x. [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Cutillas-Iturralde A, Lorences EP. Effect of xyloglucan oligosaccharides on growth, viscoelastic properties, and long-term extension of pea shoots. Plant Physiol. 1997;113:103–109. doi: 10.1104/pp.113.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling M, Seitz HU. Influence of a specific xyloglucan-nonasaccharide derived from cell walls of suspension-cultured cells of Daucus carota L. on regenerating carrot protoplasts. Planta. 1990;182:174–180. doi: 10.1007/BF00197107. [DOI] [PubMed] [Google Scholar]
- Farkas V, Hanna R, Maclachlan G. Xyloglucan oligosaccharide α-l-fucosidase activity from growing pea stems and germinating nasturtium seeds. Phytochemistry. 1991;30:3203–3207. doi: 10.1016/0031-9422(91)83176-l. [DOI] [PubMed] [Google Scholar]
- Fry SC, Aldington S, Hetherington PR, Aitken J. Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol. 1993a;103:1–5. doi: 10.1104/pp.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, York WS, Albersheim P, Darvill AG, Hayashi T, Joseleau JP, Kato Y, Pérez Lorences E, Maclachlan G, McNeil MC and others. An unambigous nomenclature for xyloglucan-derived oligosaccharides. Physiol Plant. 1993b;89:1–3. [Google Scholar]
- Guidley MJ, Lillford PJ, Rowlands DW, Lang P, Dentini M, Crescenzi V, Edwards M, Fanutti C, Reid JSG. Structure and solution properties of tamarind seed polysaccharide. Carbohydr Res. 1991;214:299–314. doi: 10.1016/0008-6215(91)80037-n. [DOI] [PubMed] [Google Scholar]
- Guillén R, York WS, Pauly M, An J, Impallomeni G, Albersheim P, Darvill AG. Metabolism of xyloglucan xylose-deficient oligosaccharide subunits of this polysaccharide in etiolated pea. Carbohydr Res. 1995;277:291–311. doi: 10.1016/0008-6215(95)00220-n. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Wong Y, Maclachlan G. Pea xyloglucan and cellulose. II. Partial hydrolysis by pea endo-1,4 β-glucanases. Plant Physiol. 1984;75:605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington PR, Fry S. Xyloglucan endotransglycosylase activity in carrot cell suspensions during cell elongation and somatic embryogenesis. Plant Physiol. 1993;103:987–992. doi: 10.1104/pp.103.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoson T, Tabuchi A, Masuda Y. Mechanism of xyloglucan breakdown in cell walls of Azuki bean epicotyls. J Plant Physiol. 1995;147:219–224. [Google Scholar]
- Hustache G, Mollard A, Barnoud F. Culture illimitée d'une souche anergiée de Rosa glauca par la technique des suspensions cellulaires. CR Acad Sci Paris. 1975;281:1381–1384. [Google Scholar]
- Johnson SW, Alhadeff JA. Mammalian alpha-l-fucosidases. Comp Biochem Physiol. 1991;99:479–488. doi: 10.1016/0305-0491(91)90327-a. [DOI] [PubMed] [Google Scholar]
- Joseleau JP, Cartier N, Chambat G, Faik A, Ruel K. Structural features and biological activity of xyloglucans from suspension-cultured plant cells. Biochimie. 1992;74:81–88. doi: 10.1016/0300-9084(92)90187-j. [DOI] [PubMed] [Google Scholar]
- Lee YE, Zeikus JG. Genetic organization, sequence and biochemical characterization of recombinant β-xylosidase from Thermoanerobacterium saccharolyticum strain B6A-RI. J Gen Microbiol. 1993;139:1235–1243. doi: 10.1099/00221287-139-6-1235. [DOI] [PubMed] [Google Scholar]
- Liénart Y, Dubois-Dauphin R, Gautier C, Khitri M, Driguez H. Membrane binding sites for the human blood group H-type 2 determinant, an inducer of laminarinase activity in protoplasts of Rubus fruticosus L. Planta. 1992;188:506–512. doi: 10.1007/BF00197042. [DOI] [PubMed] [Google Scholar]
- Lima NN, Rechia CGV, Ganter JLMS, Reicher F, Sierakowski MR. Oligosaccharides derived from the xyloglucan isolated from the seeds of Hymenaea courbaril var. Stilbocarpa. Int J Biol Macromol. 1995;17:413–415. doi: 10.1016/0141-8130(96)81855-1. [DOI] [PubMed] [Google Scholar]
- Lima NN, Reicher F, Correa JB, Ganter JLMS, Sierakowski MR. Partial structure of a xyloglucan from the seeds of Hymenaea courbaril var. Stilbocarpa (jatobá) Cienc Cult. 1993;45:22–26. [Google Scholar]
- Lopez R, Montero E, Sanchez F, Canada J, Fernandez-Mayoralas A. Regioselective acetylation of alkyl β-d-xylopyranosides by use of lipase PS in organic solvents and application to the chemoenzymatic synthesis of oligosaccharides. J Org Chem. 1994;59:7027–7032. [Google Scholar]
- McDougall GJ, Fry SC. Inhibition of auxin-stimulated growth of pea stem segments by a specific nonasaccharide of xyloglucan. Planta. 1988;175:412–416. doi: 10.1007/BF00396348. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Fry SC. Anti-auxin activity of xyloglucan oligosaccharides: the role of groups other than the terminal α-l-fucose residue. J Exp Bot. 1989a;40:233–238. [Google Scholar]
- McDougall GJ, Fry SC. Structure-activity relationships for xyloglucan oligosaccharides with antiauxin activity. Plant Physiol. 1989b;89:883–887. doi: 10.1104/pp.89.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Fry SC. Xyloglucan oligosaccharides promote growth and activate cellulase. Evidence for a role of cellulose in cell expansion. Plant Physiol. 1990;93:1042–1048. doi: 10.1104/pp.93.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M, Darvill AG, Fry SC, Albersheim P. Structure and function of the primary cell wall of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Pavlova ZN, Ash AO, Vnuchkova VA, Babakov AV, Torgov VI, Nechaev OA, Usov AI, Shibaev VN. Biological activity of a synthetic pentasaccharide fragment of xyloglucan. Plant Sci. 1992;85:131–134. [Google Scholar]
- Reid JSG. Cell wall storage carbohydrates in seeds. Biochemistry of the seed “gums” and “hemicelluloses”. Adv Bot Res. 1985;11:125–155. [Google Scholar]
- Shibuya N, Kaku H, Kuchitsu K, Maliarik MJ. Identification of a novel binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction from suspension-cultured rice cells. FEBS Lett. 1993;329:75–78. doi: 10.1016/0014-5793(93)80197-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui R, Wood PJ. Structural investigation of sodium hydroxyde-soluble rapeseed (Brassica campestris) polysaccharides. Carbohydr Res. 1977;53:85–94. doi: 10.1016/s0008-6215(00)81990-4. [DOI] [PubMed] [Google Scholar]
- Stephen AM (1983) Other plant polysaccharides. In GO Aspinall, ed, The Polysaccharides, Vol 2. Academic Press, New York, pp 105–181
- Umemoto N, Karitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I. The structure and function of a soybean β-glucan-elicitor-binding protein. Proc Natl Acad Sci USA. 1997;94:1029–1034. doi: 10.1073/pnas.94.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincken JP, de Keizer A, Beldman G, Voragen AGJ. Fractionation of xyloglucan fragments and their interaction with cellulose. Plant Physiol. 1995;108:1579–1585. doi: 10.1104/pp.108.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneck H, Seitz HU. Inhibition of gibberellic acid-induced elongation growth of pea epicotyls by xyloglucan oligosaccharides. J Exp Bot. 1993;44:1105–1109. [Google Scholar]
- Yang T, Davies PJ, Reid JB. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996;110:1029–1034. doi: 10.1104/pp.110.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, Darvill AG, Albersheim P. Inhibition of 2,4-dichlorophenoxyacetic acid-stimulated elongation of pea stem segments by a xyloglucan oligosaccharide. Plant Physiol. 1984;75:295–297. doi: 10.1104/pp.75.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, Harvey LK, Guillén R, Albersheim P, Darvill AG. Structural analysis of tamarind seed xyloglucan oligosaccharides using β-galactosidase digestion and spectroscopic methods. Carbohydr Res. 1993;248:285–301. doi: 10.1016/0008-6215(93)84135-s. [DOI] [PubMed] [Google Scholar]