Abstract
Background:
In recent decades, because of increasing prevalence of immunocompromised disease, non-tuberculous mycobacteria that have been known already as environmental bacteria presented as an important pathogens. In this study, the prevalence of NTM in Isfahan city water samples was determined bydifferent methods including culture methods with biochemical tests.
Materials and Methods:
Eighty-five water samples were collected from different sources in Isfahan, Iran. The mycobacterial isolates were identified by the growth characteristics, pigment production, semi-quantitative catalase test, Tween 80 hydrolysis, arylsulfatase test (3 and 14 days), heat-stable catalase (pH 7, 68°C), pyrazin amidase (4 and 7 days), urease, nitrate reduction test, and colony morphology.
Results:
Chlorine concentrations of the samples ranged from 0 to 1.8 mg/l. Twenty-one culturable species including M. fortuitum, 23.8% (5 cases); M.smegmatis, 14.3% (3 cases); M. chelonae like organisms, M. terrae complex, M. gordonae and M. mucogenicum, 9.5% (2 cases for each species); M. avium complex, M.phlei, M. xenopi, M. fallax, and M. flavescenc 4.8% (1 case for each species) were identified.
Conclusion:
The results of this study showed the incidence of different species of NTM in this geographical region in Iran. Studies show that the prevalence of immunocompromised disease is increasing in the community and it needs different treatment management strategy; therefore, the results of this study can be useful in this strategy.
Keywords: Isfahan water samples, non tuberculous mycobacterium, phenotypic characteristics
INTRODUCTION
Non-tuberculousmycobacteria (NTM) include all of Mycobacteria species except Mycobacterium tuberculosis complex. In recent decades, because of increase in the prevalence of diseases such as acquired immunodeficiency syndrome (AIDS), misuses of immunosuppressive drugs and corticosteroids, the number of reports of NTM related disease are increasing.[1–4] Pulmonary diseases, dermatitis, soft tissue infection, and other types of infections are also caused by NTM. The most common type of infection by NTM is pulmonary diseases and infection in lymphatic glands, wounds or bones infections, and disseminated diseases.
Lack of evidence on the transmission of NTM from person to person may indicate that the environment is an important source of NTM. Therefore, the researcher focused on various potential and route of transmission of NTM. Based on researches carried out in complex water systems in huge buildings such as hospitals, it seems that water is an important source of Mycobacterial infections.[5] Infection has been also reported after contact with water sources such as warm water, natural water, and pools.[6,7] Biofilm formation ability plays an important role on of constancy of such hydrophobic organism,[5,6] therefore, watering pipes is a significant source of humanetic pathogens by NTM and has taken public health attention.[5] Recently, NTM have been isolated from 75% of water cooling towers in hospitals of USA. These bacteria are evaluated as important causes of hospital infections.[7,8]
In comparison to other bacteria, Mycobacteria show higher resistance pattern to common anti-sepsis agents such as chlorine; therefore, they may enter watering pipe.[9] Therefore, before treatment, knowing about the number of these groups of bacteria in environment and knowing predominant species can be important in treatment strategies for infections caused by NTM. Resistance to primary antituberculosis medicines and causing diseases similar to tuberculosis shows the necessity of diagnosis of NTM species and diagnosis of probable infection sources in order to prevent and control diseases caused by these group of bacteria.[9] Therefore, this study was done in order to achieve prevalence of this group of bacteria in drinking and environmental water samples in Isfahan by using specific and complex phenotypic methods.
MATERIALS AND METHODS
This study was a descriptive analysis study which was done from winter 2009 up to winter 2011. A total of 85 water samples were gathered from swimming pools (n=7) dentistry units (n=9), hemodialysis water (n=7) offices water coolers (n=10), drinking tap water (n=12), undrinkable tap water (n=12), different mineral waters for sale (n=7), fountains and city pools (n=10), river ((n=6), and drinking water with temperature near to boiling point (n=5).
Two samples (one for biochemical test and one for microbiological tests) were taken in Erlenmeyer flasks contained sodium thiosulfate using the grab sampling method (samples were taken at specified points in time and place and with known volume). The temperature, pH, and total chlorine content (using DPD method, Amcor kit) were measured and recorded for each sample.[10] 500 mL of the samples were put under the expose at 0.05% cetylpridinium chloride and filtered by 0.45 μm filter.[10,11] Then filters were transferred directly onto 7H10 Middle Brook solid media, included 15% OADC (Oleic acid, Albumin, Dextrose, Catalase). The plates were examined once a week for 8 weeks. When the colonies appeared, they were subjected to acid-fast staining and acid-fast positive colonies were transferred to Lowenstein-Jensen (LJ) slant media and incubated at 37°C for 8 weeks.
The mycobacterial isolates were identified by the growth characteristics, including growth at 25°C, 37°C and 42°C, pigment production, semi-quantitative catalase test, Tween 80 hydrolysis, arylsulfatase test (3 and 14 days), heat-stable catalase (pH 7, 68°C), pyrazin amidase (4 and 7 days), urease, nitrate reduction test, and colony morphology. The phenotypic identification tests were repeated whenever mycobacteria identification was doubtful. Reference strains of M. smegmatis (PTCC 1307) and M. fortuitum (ATCC 6841) were used as a control species in all steps of this study.[12,13]
RESULTS
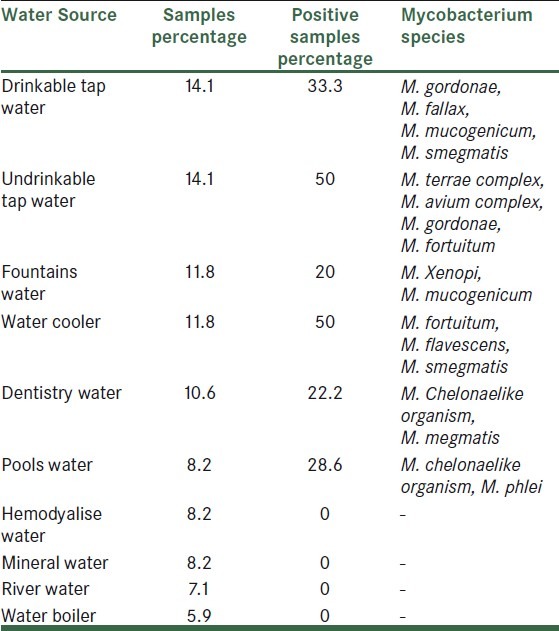
The pH range in an investigated samples was 7-8 and the heat temperature range was 23-24°C. Chlorine concentrations of the samples ranged from 0 to 1.8 mg/L. Sources of samples, percentage of positive samples, and NTM species isolated that are found in water samples are summarized in Table 1.
Table 1.
Parameters related to investigated water samples and identified non-tuberculous mycobacteria species
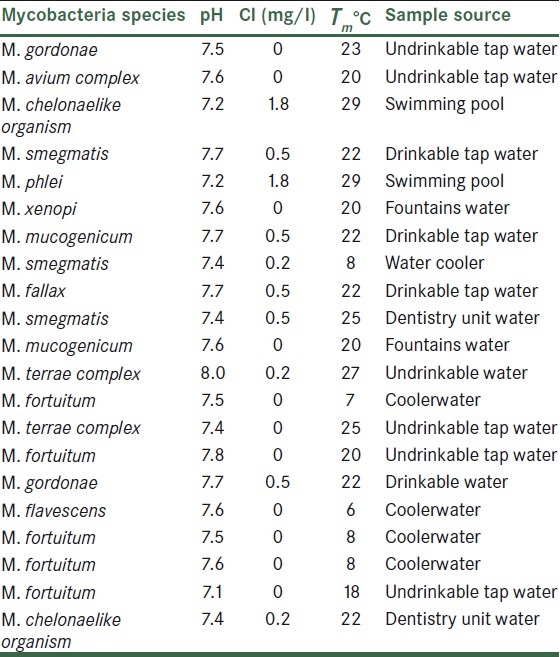
Chart 1.
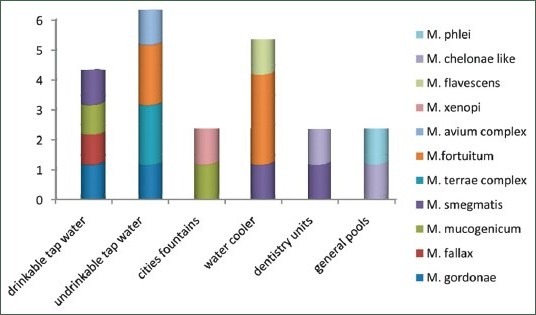
Prevalence of Non-tuberculousmycobacteria species in water samples
21 mycobacteria were isolated and identified by growth characteristics and conventional biochemical tests [Table 2]. Mycobacterium fortuitum (5/23.8%), M. smegmatis (3/14.3%), M. chelonae like, M. terrae complex, M. gordonae and M. mucogenicum (2/9.5%), M. avium complex, M. phlei, M.xenopi, M. fallaxand M. flavescenc 4.8% (1 case for each species) were identified. M. fortuitum was isolated from 60% of samples under temperature 10°C. percentage of Non-tuberculous mycobacteria species isolated from isfahan different water sources was shown in Table 3.
Table 2.
Results of biochemical tests of non-tuberculousmycobacteria isolated from different water sources.
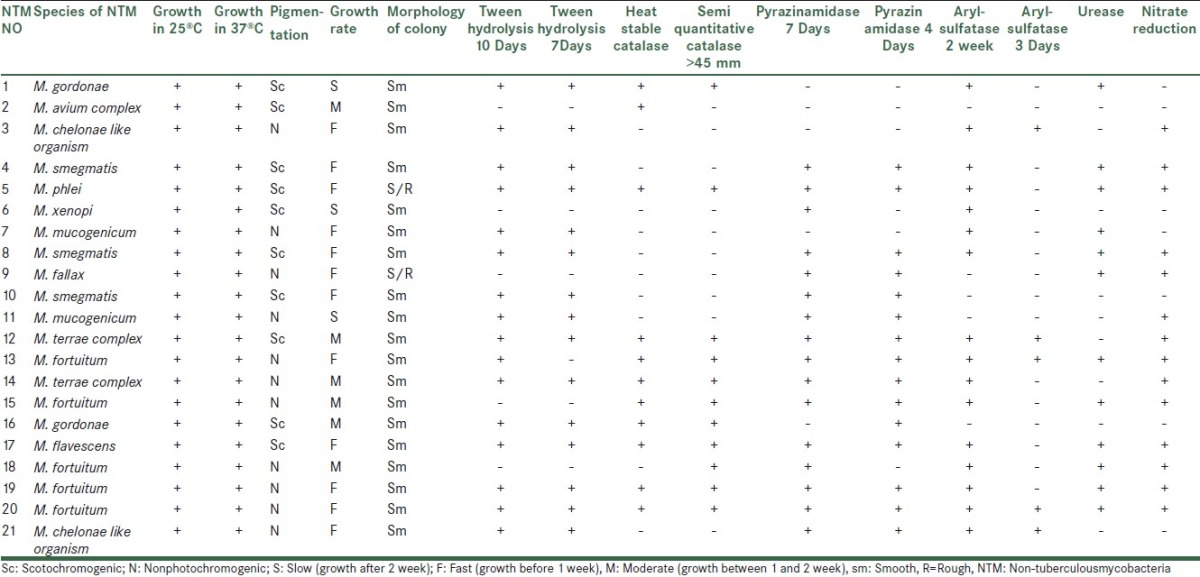
Table 3.
Percentage of non-tuberculous mycobacteria species isolated from isfahan different water sources
DISCUSSION
NTM are widely distributed in the environment, soil, water, and other natural reservoirs. In recent years, NTM have emerged as a major cause of opportunistic infections. Therefore, it is necessary to perform a risk analysis and identify various species existing in the environment. This will help understanding NTM incidence and also making aware clinician. pH, total chlorine, and water temperature of samples showed that mycobacteria can be found in different sources of drinkable and undrinkable water [Table 1]. The vast existence of environmental Mycobacteria in different parts of the city and existence of these bacteria at different Cl concentrations (0-1.8 mg/L) and different heat temperatures[2,9] shows that these factors do not affect the consistency of microorganism.
In this study, it was shown that in swimming pools with high Cl concentrated up to 1.8 mg/L and also in underground and surface water resources in which the Cl concentration is zero, the organisms are still present. Most of the Mycobacteria are isolated from undrinkable water with low Cl concentration. These findings indicate that the free Cl decreases the level of Mycobacteria contamination in drinkable water samples. However, it is not removing NTM completely from these sources.[2,9,14]
Vast temperature changes were seen in samples which contained Mycobacteria (6-29°C) and some species were present in special temperature ranges. For example, M. fortuitum was detected in 60% of samples with temperature under 10°C. The presences of NTM in different ranges of temperatures of were studied in some researches.[13,15,16] Covert and Rodgers also showed that 54% of cool and frozen water and 35% of other drinking water consist of NTM.[17]
Our results are comparable with Chang and Chiao (2002). They isolated acid fast bacilli from 38.7% of hospitals tap water samples in which, NTM species were presented in 20.4% of samples.[18] Chen et al. also studied water samples in different parts of hospital drinking tap water and reported the prevalence of 50% NTM in drinking water samples.[19] Lavania investigated the existence of NTM in 62 drinking water and 31 spring water samples and reported the frequency of NTM as 20.69% and 19.35%, respectively.[20] Special attention should be paid on hemodialyze devices and equipments, because there is contamination in city tap water and disapparition of Mycobacteria from mineral water and hemodialyze water needs high homogenized level of water.
Swimming pools and dentistry units contamination by NTM is important, especially when used by people with a weak immune system. Thus, it is necessary to obey medical rules or present special facilities for this kind of people in medical or other general centers.
In addition to above points, it should be considered although isolated mycobacteria species are predictable by biochemical tests, some phenotypic mycobacteria species have overlapping and cause doubt in diagnosis.[21–24] Although biochemical and phenotypic tests are still the most available facility for identifying species level in most of microbiology laboratories, our experiment show that the results of some of the biochemical tests are variable and using a large number of colonies and changes in amount of bacterial population will affect the biochemical reaction.
ACKNOWLEDGMENT
This study was supported by Grant No. 186056 from Isfahan University of Medical Sciences, Isfahan, Iran. We thank to Mohamad Kazemi for his technical support.
Footnotes
Source of Support: This study was supported by Grant No. 186056 from Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Horsburg CR., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–8. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 2.Peters M, Müller C, Rüsch-Gerdes S, Seidel C, Göbel U, Pohle HD, et al. Isolation of atypical Mycobacteria from tap water in hospitals and homes. J Infect. 1995;31:39–44. doi: 10.1016/s0163-4453(95)91333-5. [DOI] [PubMed] [Google Scholar]
- 3.Covert TC, Rodgers MR, Reyes AL, Stelema GN. Occurrence of non tuberculous mycobacteria in environmental sample. Appl Environ Microbiol. 1999;68:3159–61. doi: 10.1128/aem.65.6.2492-2496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C, Xue C, Ting ZC, Huan DB, Zhong ZJ. identification of mycobacterium marinum 65KD heat shock protein gene by polymerase chain reaction analyse from lesion of swimming pool granuloma. Chin Med J. 2006;119:43–8. [PubMed] [Google Scholar]
- 5.Torvinen E, Suomalainen S, Lehtola MJ, Miettinen IT, Zacheus O, Paulin L, et al. Mycobacteria in water and loose deposits of drinking water distribution system in Finland. Appl Environ Microbiol. 2004;70(4):1973–81. doi: 10.1128/AEM.70.4.1973-1981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42:1756–63. doi: 10.1086/504381. [DOI] [PubMed] [Google Scholar]
- 7.Black WC, Berks SG. Cooling towers a potential environmental source of slow growing mycobacteria species. AIHAJ (Fair fax. Va) 2003;64:238–42. doi: 10.1080/15428110308984813. [DOI] [PubMed] [Google Scholar]
- 8.Kotoch VM. Infections due to non-tuberculous mycobacteria (NTM) Indian J Med Res. 2004;120:290–304. [PubMed] [Google Scholar]
- 9.Taylor RH, Falkinham JO, 3rd, Norton CD, Lechevallier MW. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of M.avium. Appl Environ Microbiol. 2000;66:1702–5. doi: 10.1128/aem.66.4.1702-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.18th ed. Washington DC: American Public Health Association, American water works association, water environmental federation; 1992. Standard methods for the examination of water and wastewater; pp. 1–21. [Google Scholar]
- 11.5th ed. Tehran: Iranian industrial research and standards Organization Publications; 1997. DPD: National drinking water standards. (standard no.1053) [Google Scholar]
- 12.19th ed. Washington DC: American Water Works Association, Water Environment Federation; 1995. Standard methods for the examination of water and wastewater. [Google Scholar]
- 13.Miyamoto M, Yamaguchi Y. Disinfectant effects of hot water, ultraviolet light, silver ions and chlorine on strains of Legionella and nontuberculous mycobacteria. Microbios. 2000;101:7–13. [PubMed] [Google Scholar]
- 14.Naserpour Farivar T, Sharifi B. Prevalence of non-tuberculousis mycobacteria in southeast of Iran. 16th European Congress of Clinical Microbiology and Infectious Diseases. 2006 (ECCMI) [Google Scholar]
- 15.Le Dantec C, Duguet JP, Montiel A, Dumoutier N, Dubrou S, Vincent V. Chlorine disinfection of atypical mycobacteria isolatedfrom a water distribution system. Environ Microbiol. 2000;68:1025–32. doi: 10.1128/AEM.68.3.1025-1032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebakova H, Kozisek F. Incidence of nontuberculous mycobacteria in four hot watersystems using various types of disinfection. Can J Microbiol. 2008;54:891–8. doi: 10.1139/w08-080. [DOI] [PubMed] [Google Scholar]
- 17.Covert TC, Rodgers MR, Reyese AI, Stelma G., Jr Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol. 1999;65:2492–6. doi: 10.1128/aem.65.6.2492-2496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.e Chang CT, Wang LY, Liao CY, Huang SP. Identification of nontuberculous mycobacteria existing in tap water by PCR-restriction fragment length polymorphism. Appl Environ Microbiol. 2002;68:3159–61. doi: 10.1128/AEM.68.6.3159-3161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JH, Lee EJ, Lee HR, Ryu SM, Kim HR, Chang CL, et al. Prevalence of non-tuberculous mycobacteria in hospital environment. J Hosp Infect. 2007;65:143–8. doi: 10.1016/j.jhin.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Lavania M, Katoch K, Parashar D, Sharma P, Das R, Chauhan DS, et al. Predominance of Mycobacterium fortuitum-chelonae complex in Ghatampur Field Area, Endemic for Leprosy. Indian J Lepr. 2008;80:323–30. [PubMed] [Google Scholar]
- 21.Koning B, Amer Ina, Sollich V, Koning W. Intra and interpatient variability of the hsp65 and 16S-23S intergnic gene region in Mycobacterium abscessus strains from patients wilh cystic fibrosis. J Clin Microbiol. 2005;43:3500–3. doi: 10.1128/JCM.43.7.3500-3503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze-Robbecke R, Weber A, Fischeder R. Comparison of decontamination methods for the isolation of Mycobacteria from drinking water samples. J Microbiol Methods. 1991;14:177–83. [Google Scholar]
- 23.Bailey, Scott, Baron . Diagnostic Microbiology. 8th ed. 1990. Fine Gold. (Table 41.6) [Google Scholar]
- 24.Masster RN. U.S.A: 1991. Mycobacteriology. (Table 3.11.4) [Google Scholar]


