Abstract
Introduction:
The main obstacle for tissue engineering is to find the most appropriate cell which is able to produce extracellular matrix (ECM) similar or better than natural chondrocytes in vitro. This study compared aggrecan synthesis's potential between differentiated chondrocytes (DCs) from adipose-derived stem cells (ADSCs) and natural articular chondrocytes (NCs) in 3D culture in vitro.
Materials and Methods:
Human ADSCs were isolated from sub-cutaneous adipose tissue and then the surface markers including CD 14, 45 CD105, CD90, CD44 were analyzed by flow cytometry. Also human articular chondrocytes were yielded of non-weight bearing area of Knee cartilage. Both types of the cells were encapsulated in alginate scaffolds and cultured in chondrogenic medium with and without TGFβ3 for 3 weeks. Then the extent of aggercan (AGC) production was evaluated by ELISA on days 14 and 21.
Results:
Our findings indicated that differentiated chondrocytes (DCs) with and without TGFβ3 synthesized more AGC than natural chondrocytes (NCs) on day 14. But DCs without TGFβ3 had higher production than other groups on day 21. Application of TGFβ3 resulted in an increase of amount of AGC in DCs on day 14 but a decrease on day 21 than same group.
Conclusion:
Since, aggrecan is an important chondrogenic marker, it was concluded that ADSCs can be possible reliable alternative cell source for cartilage tissue engineering in future.
Keywords: Adipose-derived stem cell, articular chondrocyte, alginate, scaffold, TGFβ3
INTRODUCTION
Articular cartilage is an avascular and aneural tissue so it cannot self-regenerate once damaged by sports and accidental injuries or articular diseases.[1,2] Different surgical strategies such as: Articular debridement, subchondral drilling, periosteal, or perichondrial resurfacing have relieved pain and improved joint function but the repaired tissues are fibrocartilage rather than hyaline cartilage.[3–5] So the investigators have been employed a new strategy, tissue engineering, for repairing of articular cartilage damages during the past two decades. The basis of tissue engineering involves the use of cells, scaffolds, and signaling factors.[6] Since 1994, Autologous Chondrocyte transplantation (ACT) procedure has been used for articular cartilage repair with successful clinical results.[7,8] But, several disadvantages have been reported such as donor-site morbidity, low number of available cells, and dedifferentiation of chondrocytes in vitro culture.[9] Due to limitations, stem cells seem to be a promising alternative source for repairing cartilage defects. Mesenchymal stem cells (MSC) are obtained easily from different tissues such as bone marrow,[10] adipose tissue,[11] synovial membrane,[12] trabecular bone[13] muscle,[14] and other tissues. Their proliferation capacity and high multilineage differentiation potential make them attractive alternative for chondrocytes in cartilage regeneration.[5,15] These cells could be easily differentiated into chondrocytes in vitro.[16] Bone marrow is the most frequently used source of mesenchymal stem cells applied for cartilage repair but it is not the optimal source, because its sampling procedure is dangerous and painful.[17]
In recent years, human adipose-derived stem cells (ADSCs) are considered as a suitable alternative source for cartilage tissue engineering.[18–20] Adipose tissue can be easily obtained without invasive manner and a large number of stem cells can be harvested from low proportion of fat which is 100 times more, compared to bone marrow.[21] Several researchers have studied and compared chondrogenic potential between ADSCs and BM-MSCs conflicting but they rendered different results. Some of them concluded that differentiation potential of ADSCs is good or similar to BM-MSCs and others reported BM-MSCs were better.[22] It is expected, when MSCs are employed for articular cartilage repair, they could be differentiated into chondrocytes which comparable to native articular chondrocytes.[17,23] Nowadays, the main obstacle for cartilage tissue engineering is to use the most appropriate cell which is able to produce extracellular matrix (ECM) similar or better than natural chondrocytes. However, this challenge has been extended up to now and needs to be studied more.[5,24] Selecting a suitable scaffold is the other important factor in tissue engineering. Alginate---a natural polysaccharide found in brown seaweed---in the presence of calcium or other 2-valent cations transforms into hydrogel. It is a nontoxic and injectable scaffold that stimulates the expression of chondrogenic phenotype.[25–27]
Proteoglycans constitute the important part of extracellular matrix of articular cartilage which can resist against to mechanical loading. Aggrecan is a large proteoglycan (80-90%) consist of a core protein with glycosaminoglycan side chains.[1,28]
In this study, we decided to compare the amount of AGC produced by differentiated chondrocytes (DCs) from ADSCs and natural articular chondrocytes (NCs) in alginate, with and without TGFβ3, on days 14 and 21.
MATERIALS AND METHODS
Isolation and proliferation of adipose derived stem cells
Human ADSCs were extracted from subcutaneous abdominal adipose tissue harvested from four patients (30-50 years). Consent was obtained from the patients previously. Adipose tissue was mechanically minced and washed with PBS (Sigma) and then it was digested with 0.075% type I collagenase (Sigma) solution at 37°C for 30 min. After inactivation of the collagenase with DMEM- LG (Sigma) and 10% fetal bovine serum (FBS) (Invitrogen), the cell solution was centrifuged at 1500 rpm for 10 min. The supernatant was removed and the resultant pellet was resuspended in culture medium contained DMEM-LG supplemented with 10% FBS, 1% penicillin and streptomycin (Gibco) and then cultured at 37°C, 5% CO2 conditions. Medium was replaced every 4 days. When the cells reached 80% confluence, they were passaged with 0.05% trypsin/0.53 mM EDTA (Sigma) solution. For preparation of alginate beads, the cells at passage 3 to 5 were detached by trypsin/EDTA, centrifuged, and counted.
Flow cytometry
The cell markers were quantified by flow cytometry. ADSCs should characteristically show positive for CD105 (a receptor for TGFβ), CD90 (marker of thymic antigen), CD 44 (hyaluronic acid receptor) and negative for CD 14, CD 45(hematopoietic markers). Cells were released with trypsin-EDTA, rinsed, and suspended in PBS. Cell suspension were split into aliquots (100 μl); an unstained group, 5 μl mouse antibody IgG1,2 (negative control), 5 μl mouse anti-human monocolonal CD105(Abcam)(unconjugated) and mouse anti-human monocolonal CD 44(DAKO Cytomation) conjugated with phycoerythrin (PE), 5 μl mouse anti-human monocolonal CD 14,45(IQ Product) and mouse anti-human monocolonal CD 90(IQ Product) conjugated with fluoroisothiocyanate (FITC). Then the samples were incubated for 30 min in the dark at 4°C. The cells were washed with PBS and centrifuged at 1500 rpm for 10 min. The supernatant was removed, the labeled cell pellet resuspended in 200 μl PBS, and subjected to FACS analysis (BD FACS Caliber).
Chondrocyte isolation
In this study, articular cartilage was obtained from knee joints of 5 patients, 20 to 30 years old, by arthroscopy. The cartilage specimens were removed from non-weight bearing areas and transported to laboratory in PBS.Consent was obtained from the patients previously. Then the cartilage slices were minced into 1-2 mm pieces and digested by type II collagenase solution (350 u/ml) (Sigma) for 4 h at 37°C. After digestion, the solution was centrifuged and cells cultured in DMEM/F12 (Gibco) 10% FBS, 1% penicillin-streptomycin. Medium was replaced every 4 days. When the cells reached 80% confluence, they were passaged with trypsin/ EDTA solution. For preparation of alginate beads, the cells at passage 2 to 4 were detached by trypsin/EDTA, centrifuged, and counted.
Encapsulation of ADSC and chondrocytes in Alginate
The isolated ADSCs (P3 to P5) and monolayer chondrocytes (P2 to P4) were separately resuspended in 1.5% alginate (Sigma)[29] at 5 million cells/ml. The alginate/cell suspension was expressed through a 23-gauge needle into a 102 mM CaCl2 solution (Merck). The alginate beads after 15 min were washed twice in 0.9% saline solution and once in DMEM. The new alginate beads cultured in 12-well plate and 2 ml chondrogenic culture media was added to each well and incubated at 37°C, 5% CO2 for 14 and 21 days.
Chondrogenic culture media contained: DMEM-HG (High Glucose)(Gibco), penicillin and streptomycin 1% (Gibco), dexamethasone 10/7 M(Sigma), ascorbat-2-phosphate 50 μg/ml(Sigma), bovine serum albumin 1% (Sigma), linoleic acid 5 μg/ml (Sigma), insulin-transferrin-selenium (ITS) 1% (Sigma), with and without adding transforming growth factor-β3 (TGFβ3) 10 ng/ml (Sigma). Medium was replaced every 4 days and l ml of supernatant medium on days 14 and 21 was frozen at –20°C for enzyme-linked immunosorbent assay (ELISA).
MTT(3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide) assay
The viabilities of NCs and DCs in alginate beads were assessed by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay on days 14 and 21. At first, the medium of each well was removed, rinsed with PBS, and replaced with 800 μl serum free medium and 80 μl MTT solution (5 mg/ml in PBS). Then it incubated at 37°C, 5% CO2 for 4 h, so that purple formazan crystals formed in the alginate beads. Then the medium was discarded and added 800 μl DMSO (Sigma) to each well, and incubated in dark for 2 h. DMSO dissolved the formazan crystals and created a purple color. Then 100 μl of the solution transferred to 96-well plate and absorbance of each well was read at 570 nm with ELISA reader (Hiperion MPR4). The MTT assay was also applied to the scaffolds without cells as controls and the data was subtracted from measured values. The assays were performed in triplicate.
Enzyme-linked immunosorbent assay
Aggrecan was quantified in supernatant culture media on days 14 and 21, according to protocol of Human aggrecan Direct ELSA kit (Invitrogen). Briefly, supernatant media as antigens were added to ELISA plate and AGC molecules bound to coated antibodies. Then secondary enzyme conjugated antibodies were added which linked to antigens and formed sandwich. Finally, enzyme's substrate was added and the absorbance of the mixture was measured at a wavelength of 450 nm by spectrophotometer.
Statistical tests
The Kolmogorov-Smirnov test was used for assessing normal distribution of variables and ANOVA (one-way-analysis of variance) with the LSD post hoc test was used for the comparison of MTT and concentration of ELISA results in different groups.
RESULTS
Flow cytometry
Flow cytometric analysis of undifferentiated human adipose-derived stem cells (ADSCs) was performed. The results showed they were negative for CD 14, 45(0.14%) but expressed CD 44, CD90 (89.69%), and CD105 (94.64%) at high level [Figure 1].
Figure 1.
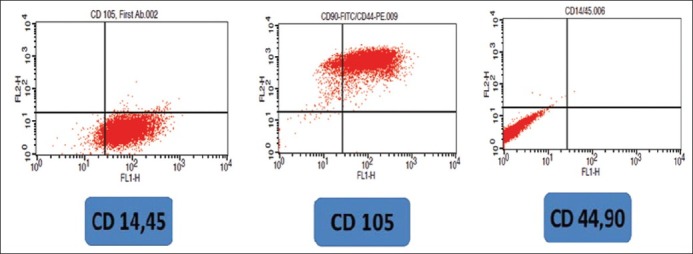
Flow cytometry dot plots of CD 105, CD 90, CD 44, CD 14, 45 in human adipose-derived stem cells
Chondrocyte culture
Some chondrocytes in primary culture adhered to plastic flasks after 7 days and made cell colonies. They lost their round phenotype gradually and transformed to fibroblast-like morphology at passage one [Figure 2]. But, when monolayer chondrocytes at passage 2 to 4 seeded in alginate, they gained their spherical shape [Figure 3].
Figure 2.
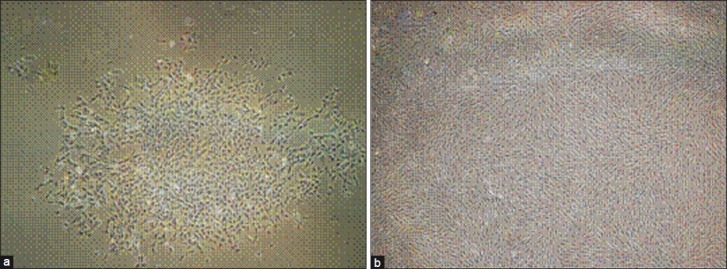
(a) Inverted microscope images of monolayer chondrocytes (a) at passage 0 after 7 days. Note the cells make colonies. (b) at passage one showing fibroblast like morphology (×60)
Figure 3.
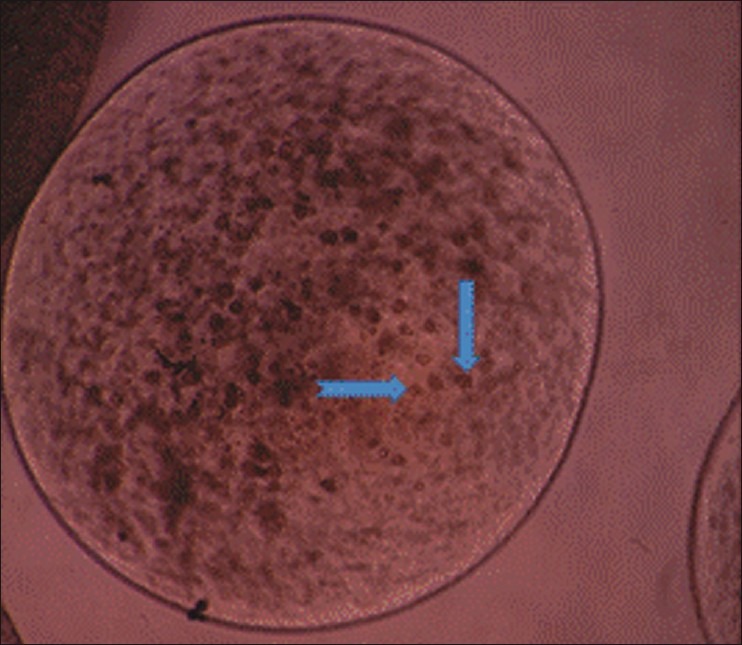
Inverted microscope images of chondrocytes seeded in alginate beads. Note the spherical shape of the cells (Arrows) within the bead (×60)
MTT
MTT assay was applied for eight groups of alginate beads, NCs and DCs in two different chondrogenic media (with and without TGFβ3) on days 14 and 21. Followed by treatment with MTT solution, the dark blue formazan crystals were seen in cells seeded in alginate beads, which indicated their metabolic activity. However, the addition of TGFβ3 improved viability in NCs and declined in DCs at two time points, but the comparison of all results showed [Figure 4] that they have not significant differences (P >0.05).
Figure 4.
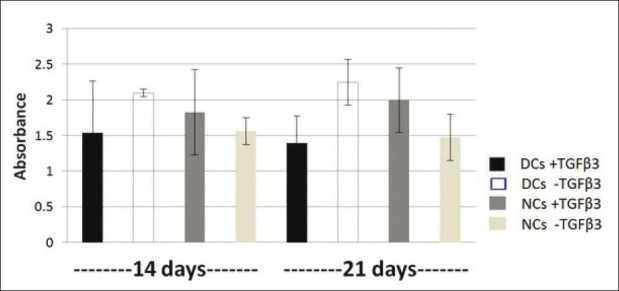
Comparison of MTT assay results between eight groups. They have not significant differences (P > 0.05)
ELISA
The results showed that the content of AGC in tissues produced by DCs with and without TGFβ3 are more than NCs with same conditions on day 14 and there was a significant difference (P <0 .05) [Figure 5]. The addition of TGFβ3 to chondrogenic media results in enhancing the AGC production in DCs than the same group without TGFβ3, and they have significant differences (P <0.05). However, its amount, to some extent was superior in tissues generated by NCs without TGFβ3 and they have not significant differences (P>0.05). The results showed that AGC synthesis in four groups on day 21 significantly decreased than DCs with TGFβ3 on day 14 (P <0 .05). However, its proportion in DCs without TGFβ3 on day 14 is significantly higher than all groups on day 21 (P <0.05) with exception of DCs without TGFβ3, which their difference was not significant (P >0.05). TGFβ3 led to decreasing of AGC production in DCs significantly on day 21 than the other group without it (P <0 .05). Our findings indicated that in the presence of TGFβ3, NCs could produce more AGC than other same group, but there was no significant difference (P <0 .05)
Figure 5.
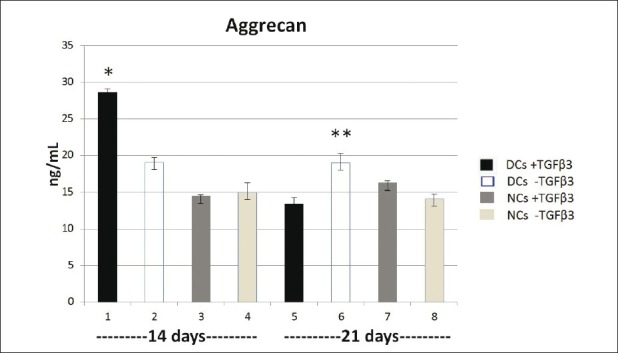
The concentration of aggrecan in eight groups on days 14 and 21. * P < 0.001,** P < 0.05
DISCUSSION
Chondrocytes synthesize the extracellular matrix components, collagens, and proteoglycans (PGs).[30] Aggrecan, the main part of PGs, consists of a core protein and glycosaminoglycan chains are attached to it covalently.[29] A great number of AGC molecules are attached to a central hyaluronic acid by non-covalent link proteins.[31] Side chains of AGC have negative charges and lead to a high osmotic environment for resistance against mechanical loading.[30]
The TGFβ family including five types (TGFβ1-5) and TGFβ1, 2, 3 are able to stimulate chondrocytes to synthesis type II collagen and proteoglycans and they also are used for inducing chondrogenic differentiation of different MSCs.[32] According to previous studies, the day 14 is the most important time point in chondrogenesis in vitro, because the highest proportion of extracellular matrix is produced at that time. They showed the concentration of extracellular matrix components can negatively reduce the metabolic activity of chondrocytes[33,34] which is inconsistent with our findings.
The characterization of ADSCs was analyzed by flow cytometry. Our results revealed the positive expression of CD90, CD44, CD105 and negative of CD14, 45. These results are inconsistent with marker list of International Society for Cellular Therapy.[35,36] MTT results showed the metabolic activity in eight groups of cells in alginate beads but there was no significant difference (P >0.05). Some authors reported that the viability of encapsulated chondrocytes in alginate increased as the time passed.[37–39] However, other experiments found lower viability of chondrocytes in alginate beads over the time.[40–42] The review of literature denoted that TGFβ led to improved proliferation of chondrocytes[43] but some investigators reported its inhibitory function.[44] However, in our study, TGFβ3 resulted in increasing of viability in NCs and decreasing in DCs on days 14 and 21 than the same groups without TGFβ3 but these differences were not statistically significant (P >0 .05).
ELISA results indicated DCs with and without TGF β3 produced more AGC than NCs on day 14 (P <0.05) but on day 21 only DCs without TGF β3 has significantly higher production than other three groups on day 21 (P <0 .05). Interestingly, the proportion of AGC between DCs without TGFβ3 on day14 and 21 was stable continuously. Our results of two DCs groups on day 14 and DCs group without TGF β3 on day21 are in agreement with the recent findings of Tigli et al. They demonstrated that embryonic stem cell-derived MSCs, embryonic stem cells, BM-MSC and ADSCs had superior ability in expression of chondrogenic markers such as, AGC, collagen type II and sox-9 than articular chondrocytes in silk and chitosan scaffolds.[45] Our results about the DCs group with TGFβ3 on day 21 is in agreement with previous findings.[24,46,47] They indicated ADSCs in hyaluronic acid, BM- MSCs in alginate, and agarose have lower capacity to produce cartilage matrix than articular chondrocytes and in comparison BM-MSCs in hyaluronic acid showed equal matrix to articular chondrocytes. Mahmoudifar et al. reported that AGC gene expression of ADSCs seeded in polyglycolic acid (PGA) scaffold was less than fetal chondrocytes.[48]
Also TGFβ3 led to promotion of the AGC production in DCs on day 14 and reduction on day 21 compared to the same group without TGFβ3. This growth factor did not show significant AGC production variations on NCs groups on two time points which may be due to the maturation of these cells. It is suggested that different isoforms of TGF β are able to regulate chondrogenesis. For instance, TGFβ2 lead to synthesis of proteoglycan in chondrocytes seeded in alginate beads.[49,50] Also authors reported that TGFβ2 and TGFβ3 resulted in improved synthesis of AGC in chondrocytes differentiated from BM-MSCs in pellet and micromass cultures.[51,52] A recent report indicated that fetal chondrocytes without TGF-β1 expressed more AGC than the same group with TGF-β1 in polyglycolic acid (PGA) scaffolds (48). Also, other members of TGF-β such as TGF-β1, BMP-2, and BMP-9 resulted to increased expression of AGC gens and type II collagen in MSCs encapsulated in alginate beads.[53,54] According to our observations, NCs have constantly generated equal AGC on days 14 and 21, so they maintained their phenotype after 3 weeks, as reported previously.[55]
The findings of this study indicated that DCs from adipose-derived MSCs have higher potential to synthesis aggrecan than natural articular chondrocytes in vitro and may be a reliable alternative cell source for cartilage tissue engineering in future.
AKNOWLEDGMENT
This study was supported financially by IRAN National Science Found for project No. 9000737
Footnotes
Source of Support: IRAN National Science Found for project No. 9000737
Conflict of Interest: None declared.
REFERENCES
- 1.Bonassar LJ. “Cartilage reconstruction”. USA: Academic Press; 2002. Methods of tissue engineering. Chap. 93; pp. 1027–39. [Google Scholar]
- 2.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–42. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: Basic science and clinical progress.A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.Ochi M, Adachi N, Nobuto H, Yanada S, Ito Y, Agung M. Articular cartilage repair using tissue engineering technique: Novel approach with minimally invasive procedure. Artif Organs. 2004;28:28–32. doi: 10.1111/j.1525-1594.2004.07317.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243–62. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risbud M. Tissue engineering: Implications in the treatment of organ and tissue defects. Biogerontology. 2001;2:117–25. doi: 10.1023/a:1011585117310. [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 8.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: Results at two to ten years. J Bone Joint Surg Am. 2003;85:17–24. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 9.Risbud MV, Sittinger M. Tissue engineering: Advances in vitro cartilage generation. Trends Biotechnol. 2002;20:351–6. doi: 10.1016/s0167-7799(02)02016-4. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 12.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–9. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 14.Nawata M, Wakitani S, Nakaya H, Tanigami A, Seki T, Nakamura Y, et al. Use of bone morphogenetic protein 2 and diffusion chambers to engineer cartilage tissue for the repair of defects in articular cartilage. Arthritis Rheum. 2005;52:155–63. doi: 10.1002/art.20713. [DOI] [PubMed] [Google Scholar]
- 15.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Pelttari K, Steck E, Richter W. The use of mesnchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Hashemi Beni B, Razavi S, Esfandiary E, Karbasi S, Mardani M, Nasresfahani M. Induction of Chondrogenic differentiation of human adipose-derived stem cells with TGF-ß3 in pellet culture system. Iranian Journal of Basic Medical Sciences. 2008;11:10–7. [Google Scholar]
- 19.Hashemi Beni B, Razavi S, Esfandiary E, Karbasi S, Mardani M, Sadeghi F. The effect of BMP-6 growth factor on differentiation of adipose-derived stem cells into chondrocyte in pellet culture. Journal of Isfahan Medical School. 2009;27:613–44. [Google Scholar]
- 20.Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–32. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 21.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, et al. Effect of tissue harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008;332:415–26. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: A crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307–14. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Jakobsen RB, Shahdadfar A, Reinholt FP, Brinchmann JE. Chondrogenesis in a hyaluronic acid scaffold: Comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthrosc. 2010;18:1407–16. doi: 10.1007/s00167-009-1017-4. [DOI] [PubMed] [Google Scholar]
- 25.Mulder L. Eindhoven: Faculty Biomedical Engineering, Eindhoven University of Technology; 2002. Cell adhesion on alginate scaffolds for tissue engineering of aortic valve – a review; pp. 22–34. [Google Scholar]
- 26.Park K, Huang J, Azar F, Jin RL, Min BH, Han DK, et al. Scaffold free, engineered porcine cartilage construct for cartilage defect repair – in vitro and in vivo study. Artif Organs. 2006;30:586–96. doi: 10.1111/j.1525-1594.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 27.Melero-Martin JM, Dowling MA, Smith M, Al-Rubeai M. Expansion of chondroprogenitor cells on macroporous microcarriers as an alternative to conventional monolayer systems. Biomaterials. 2006;27:2970–9. doi: 10.1016/j.biomaterials.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Knudson CB, Knudson W. “Cartilage proteoglycans”. Semin Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 29.Bittencourt CR, Pereira HR, Felisbino SL, Ferreira RR, Guilherme GR, Moroz A, et al. Chondrocyte cultures in tridimentional scaffold: Alginate hydrogel. Acta Ortop Bras. 2009;17:242–6. [Google Scholar]
- 30.Jeffery AK. Articular cartilage and the orthopaedic surgeon. Part I: Structure and function. Curr Orthop. 1994;8:38–44. [Google Scholar]
- 31.Mow VC, Gu WU, Chen FH. “Structure and function of articular cartilage and meniscus”. Philadelphia: Lippincott Williams and Wilkins; 2005. Basic orthopedic biomechanics and mechano-biology. Chap. 5; pp. 181–258. [Google Scholar]
- 32.Grimaud E, Heymann D, Redini F. Recent advances in TGF-b effects on chondrocyte metabolism. Potential therapeutic roles of TGF-b in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–57. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 33.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells in Vitro and in Vivo. Biochem Biophys Res Commun. 2002;290:763–9. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 34.Aguiar DJ, Knudson W, Knudson CB. Internalization of the hyaluronan receptor CD44 by chondrocytes. Exp Cell Res. 1999;52:292–302. doi: 10.1006/excr.1999.4641. [DOI] [PubMed] [Google Scholar]
- 35.Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M. Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: A biochemical, morphological and ultrastructural study. Histochem Cell Biol. 2007;128:507–20. doi: 10.1007/s00418-007-0337-z. [DOI] [PubMed] [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells.The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, De Isla N, Decot V, Marchal L, Cauchois G, Huselstein C, et al. Influences of construct properties on the proliferation and matrix synthesis of dedifferentiated chondrocytes cultured in alginate gel. Biorheology. 2008;45:527–38. [PubMed] [Google Scholar]
- 38.Zhang L, Song H, Zhao X. OptimumCombination of Insulin-Transferrin-Selenium and Fetal Bovine Serumfor Culture of Rabbit Articular Chondrocytes in Three-Dimensional Alginate Scaffolds. Int J Cell Biol. 2009;2009:747016. doi: 10.1155/2009/747016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baghaban Eslaminejad MR, Taghiyar L, Falahi F. Quantitative analysis of the proliferation and differentiation of rat articular chondrocytes in alginate 3D Culture. Iran Biomed J. 2009;13:153–60. [PubMed] [Google Scholar]
- 40.Van Susante JL, Buma P, van Osch GJ, Versleyen D, van der Kraan PM, van der Berg WB, et al. Culture of chondrocytes in alginate and collagen carrier gels. Acta Orthop Scand. 1995;66:549–56. doi: 10.3109/17453679509002314. [DOI] [PubMed] [Google Scholar]
- 41.Chubinskaya S, Hakimiyan AA, Rappoport L, Yanke A, Rueger DC, Cole BJ. Response of human chondrocytes crepared for autologous implantation to growth factors. J Knee Surg. 2008;21:192–9. doi: 10.1055/s-0030-1247818. [DOI] [PubMed] [Google Scholar]
- 42.Yamaoka H, Asato H, Ogasawara P, Nishizawa S, Takahashi T, Nakatsuka T, et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J Biomed Mater Res A. 2006;78:1–11. doi: 10.1002/jbm.a.30655. [DOI] [PubMed] [Google Scholar]
- 43.Venezian R, Shenker BJ, Datar S, Leboy PS. Modulation of chondrocyte proliferation by ascorbic acid and BMP-2. J Cell Physiol. 1998;174:331–41. doi: 10.1002/(SICI)1097-4652(199803)174:3<331::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 44.Blanco FJ, Geng Y, Lotz M. Differentiation-dependent eff ects of IL-1 and TGF-beta on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J Immunol. 1995;154:4018–26. [PubMed] [Google Scholar]
- 45.Tigli RS, Ghosh S, Laha MM, Shevde NK, Daheron L, Gimble J, et al. Comparative chondrogenesis of human cell sources in 3D scaffolds. J Tissue Eng Regen Med. 2009;3:348–60. doi: 10.1002/term.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gleghorn JP, Dines ME, Cox M, Ayala P, Bonassar LJ. Comparison of chondrogenesis by MSCs and chondrocytes in alginate: Evaluation of biochemical composition and mechanical properties (Abstract) Trans Orthop Res Soc. 2005 Poster #1782. [Google Scholar]
- 47.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. OsteoArthritis Cartilage. 2006;14:179–89. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoudifar N, Doran PM. Extent of cell differentiation and capacity for cartilage synthesis in human adult adipose-derived stem cells: Comparison with fetal chondrocytes. Biotechnol Bioeng. 2010;107:393–401. doi: 10.1002/bit.22798. [DOI] [PubMed] [Google Scholar]
- 49.Van Osch GJ, Van Der Veen SW, Buma P, Verwoerd-Verhoef HL. Effect of transforming growth factor β on proteoglycan synthesis by chondrocytes in relation to differentiation stage and the presence of pericellular matrix. Matrix Biol. 1998;17:413–24. doi: 10.1016/s0945-053x(98)90101-9. [DOI] [PubMed] [Google Scholar]
- 50.Van Susante JL, Buma P, Van Beuningen HM, Van Den Berg WB, Veth RP. Responsiveness of bovine chondrocytes to growth factors in medium with different serum concentrations. J Orthop Res. 2000;18:68–77. doi: 10.1002/jor.1100180111. [DOI] [PubMed] [Google Scholar]
- 51.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 52.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 53.Awad HA, Halnorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor β1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived Stromal Cells. Tissue Eng. 2003;9:1301–12. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 54.Majumdar WK, Wang E, Morris EA. BMP-2 and BMP-9 promote chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell Physiol. 2001;189:275–84. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 55.Lin YJ, Yen CN, Hu YC, Wu YC, Liao CJ, Chu IM. Chondrocytes culture in three-dimensional porous alginate scaffolds enhanced cell proliferation, matrix synthesis and gene expression. J Biomed Mater Res A. 2009;88:23–33. doi: 10.1002/jbm.a.31841. [DOI] [PubMed] [Google Scholar]


