Abstract
Background:
It has been recently demonstrated that Royal jelly (RJ) has a beneficial role on neural functions. Alzheimer's disease (AD) is associated with impairments of learning and memory. Therefore, the present study was designed to examine the effect of RJ on spatial learning and memory in rats after intracerebroventricular injection of streptozotocin (icv-STZ).
Materials and Methods:
Rats were infused bilaterally with an icv injection of STZ, while sham rats received vehicle only. The rats were feed with RJ-contained food (3% w/w) (lyophilized RJ mixed with powdered regular food) or regular food for 10 days. Then spatial learning and memory was tested in the rats by Morris water maze test.
Results:
Results showed that in icv-STZ group latency and path length were increased as compared to sham group, also icv-STZ rats less remembered the target quadrant that previously the platform was located; however, these were protected significantly in STZ group that received RJ-containing food.
Conclusions:
Our findings support the potential neuroprotective role of RJ and its helpful effects in AD.
Keywords: Alzheimer's disease, rat, Royal jelly, spatial learning and memory, streptozotocin
INTRODUCTION
Alzheimer's disease (AD) is a disorder with a deadly outcome and unknown etiology in human that afflicted many people worldwide.[1] This disease is characterized by a progressive and irreversible neurodegeneration in various brain regions, especially in the hippocampus, which is an important area for memory and cognition.[2,3] The increased production and accumulation of amyloid-β peptide (Aβ) contribute to progressive neuronal degeneration.[4,5] Therefore, Alzheimer's is associated with deficits in cognitive abilities such as learning and memory in human beings.[5] Currently, there isn’t any conclusive treatment for AD and the common treatments just slow the progression of the disease and manage some of the symptoms.[3]
Royal jelly (RJ) is an essential food for the honey bee young larva and the queen herself, it thought to play important nutritional roles in the queen.[6] It is a viscous substance secreted by the hypopharyngeal and mandibular glands of the worker honey bee of the species, Apis mellifera. It has been reported to have a variety of biological activities toward various types of cells and tissues of animal models.[6]
RJ is composed of proteins, carbohydrates, lipids (including sterols and fatty acids), and traces of mineral salts and vitamins.[7] This material has been determined to exhibit a variety of pharmacological activities including antitumor, antimicrobial, vasodilative, and hypotensive activities, as well as growth stimulating and infection preventing, antihypercholesterolemic and anti-inflammatory activities. Several studies have been shown the antioxidant activity of RJ.[8] For these reasons, for more than 30 years, RJ has been used commercially in medical products, healthy foods, and cosmetics, to a wide extent.[8]
In addition, RJ facilitates the differentiation of all types of brain cells including neurons from cultured neural stem/progenitorcells (NS/NPCs).[6] RJ or its components would facilitate in vivo neurogenesis in the hippocampal dentate gyrus (DG).[9] However, there are no reports so far showing the effects of RJ on cognition behaviorally, especially when there is a background of neurodegenerative disease.
One of the relevant animal models of Alzheimer's disease is intracerebroventricular streptozotocin (icv-STZ) injection.[1,10] Streptozotocin is a diabetogenic drug that used to induce diabetes mellitus,[11] and recent evidence suggests that the intracerebroventricular (icv) injection of streptozotocin (STZ) to rats in a subdiabetogenic dose causes prolonged impairment of memory and brain metabolic process, as a sporadic dementia of the Alzheimer's type (SDAT) that comprises more than 90% of Alzheimer's patients in the world.[1,10] Therefore, the aim of this study was to evaluate the effect of RJ on learning and memory in rats after intracerebroventricular injection of streptozotocin (icv -STZ).
MATERIALS AND METHODS
Male Wistar rats (320 ± 20 g) were housed four per cage and maintained on a 12 h light–dark cycle in an air-conditioned constant temperature (23 ± 1°C) room, with food and water made available ad libitum. The Ethic Committee for Animal Experiments at Isfahan University approved the study and all experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
Animals were divided into four groups (n = 9). The first group was the sham control (sham group), the second group consisted of sham animals receiving RJ-contained food (sham-RJ group), the third group was the ICV-STZ control (lesion group), and the fourth group consisted of ICV-STZ animals receiving RJ-contained food (lesion-RJ). Rats in the control groups received regular food.
Five days after surgical procedure, for recovery, rats received RJ-contained food for 10 days and then spatial learning and memory was tested in the rats by Morris water maze test.
RJ was administrated in the form of a mixed food prepared by adding freeze-dried RJ at 3% (w/w) in regular food powder. The mixture and pure regular food were made into pellets with a small amount of water and desiccated under vacuum overnight.[9,12]
Surgical procedure
The rats were anesthetized with chloral hydrates (400 mg/kg, i.p.) and their heads were fixed in a stereotaxic frame. A heating pad was used to maintain body temperature at 36.5 ± 0.5°C. The skull was exposed and two small holes were drilled and injection canula was lowered into the lateral ventricles (anterior-posterior = -0.8 mm; medial-lateral = ±1.6 mm; and dorsal-ventral = -4.2 mm with reference to bregma).[13] Injection canula was connected to a Hamilton syringe attached to a microinjector unit. The lesion group received a bilateral ICV injection of STZ (1.5 mg/kg in 4 μl per site) as in previous studies.[10] The sham group underwent the same surgical procedures, but the same volume of saline was injected instead of STZ.
Morris water maze test
The circular tank (180 cm in diameter) was filled with water (22 ± 2°C) made opaque and was surrounded by a variety of extra-maze cues. The tank was divided into four quadrants and four start positions were located at the interactions of the quadrants. Data were recorded using custom software (Radiab 1). Twenty-four hours before water maze testing, all rats were habituated to the water and apparatus.
In the spatial acquisition phase, the rats learned to find a submerged platform using extra-maze cues. A transparent Lucite platform (10 × 10 cm) was submerged 2 cm underneath the water in north-east quadrant of the tank, where it remained for all spatial trials [Figure 1a]. Each rat participated in 16 trials, which were organized into daily block of four trials (1 trial/start position within a block) for 4 consecutive days. For each trial, the rat was given a maximum time of 60 s to locate the platform, after which it remained there for 30 s. If the rat did not locate the platform within 60 s, it was guided to it by the experimenter. The next trial started immediately after removal from the platform. Escape latencies (s), swim distance (cm), and swim speed (cm/s) were recorded.
Figure 1.
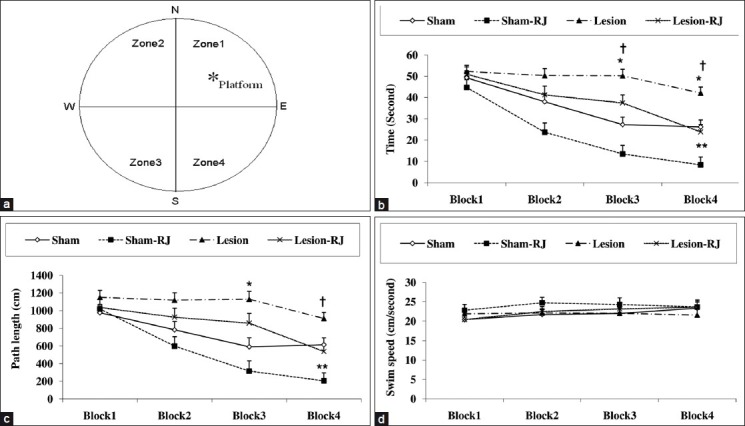
Effects of Royal jelly (RJ) contained food on performance during the spatial acquisition of Morris water maze test in rats with intracerebroventricular injection of streptozotocin. Schematic diagram of tank and site of the platform (a). The escape latencies (b), the path length (c), and the swim speed (d) at different days to reach the platform. Each point represents the day mean ± SEM of 4 swims. For latency and path length, lower numbers indicate better performance (*P < 0.05 and **P < 0.01 with respect to the sham group, †P < 0.05 significant difference between the lesion and the lesion-RJ groups; n = 9).
In the retention phase, 1 day and 1 week after the spatial acquisition phase, 60-s probe trial was conducted to examine how well the rats had learned the exact location of the platform. During this trial, the platform was removed from the tank. The quadrant time (percent time spent in the training quadrant) was recorded during the probe trial.[14] To test possible deficits in sensory–motor processes, rats were tested in the water maze with a visible platform on a new location on the final day of training.[15]
Statistical analysis
Data were analyzed using the SPSS 16 for Windows. Results are given as mean ± S.E.M. The escape latencies, path length, and swim speed were analyzed with three-factor mixed ANOVA for between-subjects differences between sham and lesion (“ICV-STZ” effect), between non-RJ and RJ (“RJ” effect), and ICV-STZ*RJ effect interaction and repeated measures (within subjects) effects across block interval 1 to 4 (“BLOCK” effect). The probe trial data for percentage of time spent in each of the four zones were analyzed by multivariate ANOVA.
RESULTS
All rats except lesion group showed a reduction in escape latencies (BLOCK effect, F(3,96) = 46.157, P < 0.001) and a reduction in the distance swam to locate the platform (BLOCK effect, F(3,96)=25.259, P < 0.001) across blocks of trials, indicating spatial acquisition. Sham groups found the platform more quickly than lesion groups [28.9 ±1.8 s and 43.54 ± 1.6 s, respectively; ICV-STZ effect, F(1,32) = 36.265, P < 0.001] and took shorter paths to the platform [637.7 ± 48.1 cm and 958.6 ± 43.6 cm, respectively; ICV-STZ effect, F(1,32) = 24.46, P < 0.001]. The rats that received RJ contained food, found the platform more quickly than the rats that received regular food [30.48 ± 1.85 s and 41.94 ± 1.58 s, respectively; RJ effect, F(1,32) = 22.2, P < 0.001] and took shorter paths to the platform [687.4 ± 49.4 cm and 908.86 ± 42.9 cm, respectively; RJ effect, F(1,32) = 11.65, P <0.01]. Also, RJ contained food maintained both the escape latencies [ICV-STZ*RJ effect interaction, F(1,32) = 0.22, P = 0.64] and the pathlengths [ICV-STZFNx01RJ effect interaction, F(1,32) = 0.06, P = 8] in lesion rats [Figures 1b and 1c].
Swim speed did not show any change during continuous day [BLOCK effect, F(3,96) = 2.01, P = 0.118] and there wasn’t any difference between the groups [Figure 1d].
Results from the probe trial as measured by the mean percentage (%) time spent in each of the four zones indicated that 1 day after acquisition phase the sham groups spent more time in zone 1, where the platform was previously located, than the ICV-STZ groups [38.14 ± 2.58% and 23.41 ± 2.33%, respectively; ICV-STZ effect, F(1,32) = 31.02, P < 0.001]. The mean percentage (%) time spent in zone 1 was not significantly different between the rats that received RJ-contained food and the rats that received regular food [36.71 ± 2.65% and 24.83 ± 2.26%, respectively; RJ effect, F(1,32) = 3.564, P = 0.068]; however, RJ-contained food maintained that in lesion rats [ICV-STZ*RJ effect interaction, F(1,32) = 0.85, P = 0.36] [Figure 2a].
Figure 2.
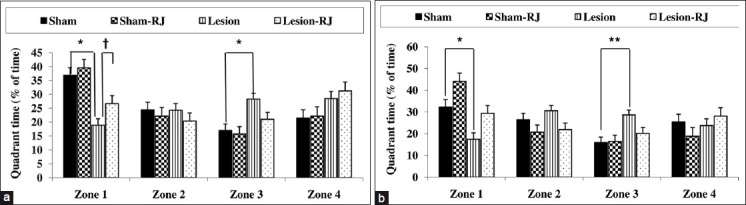
Effects of Royal jelly (RJ) contained food on performance during the probe trial in rats with intracerebroventricular injection of streptozotocin, quadrant time, as measured by mean percentage (%) time spent in each of the four zones, 1 day (a) and 1 week (b) after spatial acquisition phase. Zone 1 was the training quadrant that previously platform was located (*P < 0.05 and **P < 0.01 with respect to the sham group, †P < 0.05 significantly difference between the lesion and the lesion-RJ groups; n = 9).
One week after acquisition phase, the sham groups spent more time in zone 1 than the ICV-STZ groups [38.19 ± 2.05% and 22.78 ± 1.86%, respectively; ICV-STZ effect, F(1,32) = 17.94, P < 0.001]. The rats that received RJ-contained food spent more time in zone 1 than the rats that received regular food [33.1 ± 2.1% and 27.87 ± 1.8%, respectively; RJ effect, F(1,32) = 11.67, P < 0.01]; also, RJ contained food maintained that in lesion rats [ICV STZ*RJ effect interaction, F(1,32) = 0, < 0.01]; also, RJ-contained food maintained that in lesion rats [ICV-STZ*RJ effect interaction, F(1,32) = 0, P = 0.99) [Figure 2b].
DISCUSSION
The present results showed that use of RJ is effective in significant improvement of learning and memory defects that induced with icv-STZ in rats.
Like previous studies, our results demonstrated that ICV-STZ causes impairment of learning and memory in rats.[16] The study has been shown that the application of STZ in a subdiabetogenic dose in rats brain leads to damage to glucose's metabolism and causes reduction in adenosine triphosphate (ATP/ADP ratio). This may be calculated by the imbalance between intake and output of energy.[17] It has been shown that acetylcholine is necessary to formation and improvement of memory; its synthesis needs to glucose metabolism and insulin in order to control choline acetyltransferase (ChAT) activity.[1] Previous researches have demonstrated that icv-STZ causes reduced energy metabolism/oxidative stress leading to cognitive dysfunction by inhibiting the synthesis of acetyl-CoA and therefore acetyl-choline synthesis. Also, streptozotocin causes reduced ChAT activity in hippocampus and increased cholinesterase (ChE) activity in the rat's brains.[1]
In addition, like in AD, icv-STZ through prolonged impairment of brain energy metabolism and oxidative damage increases the inflammatory cytokines, such as interleukin-8 (IL-8) and interleukin-1 (IL-1). This inflammatory cytokines and severe oxidative stress lead to mitochondrial dysfunction and increase the risk of cell apoptosis in the brain, particularly in the hippocampus.[18,19]
As a secondary observation, our results demonstrated that use of RJ in rats enhanced learning and memory performance. Previous studies showed that RJ stimulates production of neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), and has neuroprotective effects in the adult brain, especially in hippocampus.[6] These studies are compatible with the results from our behavioral study.
One of unique components in RJ is 10-hydroxy-trans-2decanoic acid (HDEA), an unsaturated fatty acid. Because HDEA is a small unsaturated fatty acid, it can pass through blood–brain barrier. It has been demonstrated that HDEA mimics the effects of brain-derived neurotrophic factor (BDNF) and probably stimulate neurogenesis in the mature brain.[20] Other active component in RJ is adenosine monophosphate (AMP) N1-oxide that is effective in neuronal differentiation of PC12 cells.[7,20]
One of the factors which play an effective role in pathogenesis of ageing and neurodegenerative diseases is oxidative stress, which is an imbalance between free radicals and antioxidant system.[21,22] Oxygenate radicals can attack to proteins, nucleic acids, and lipid membranes and accordingly interrupt the integrity and performance of the cell.[23] The brain tissue contains a lot of unsaturated fatty acids which are especially vulnerable for free-radical attacks.[24] Therefore, antioxidant substances can play an important role in prevention and cure of neurodegenerative diseases.[10,16] Recently, studies have suggested that RJ has free-radical scavenging capacity and is a highly efficient antioxidant.[25,26] RJ has been shown that inhibits lipid proxidation both in vitro and in vivo.[27]
RJ has a potent ability to improve insulin resistance and this is a valuable effect in AD.[28] Same to AD or ageing brain, ICV-STZ in rats causes desensitization of insulin receptors[29] and it has demonstrated that ICV-STZ through damage of the neuronal insulin receptor induces progressive deteriorations in the mental capacities of learning, memory, and cognition.[1]
In conclusion, our findings show that RJ protects spatial learning and memory performance in AD and it has positive effects on neural functions and cognition.
ACKNOWLEDGEMENTS
This research was supported by the Applied Physiology Research Center of Isfahan University of Medical Sciences, Isfahan, Iran. Also, the authors wish to thank Isfahanhoney.com Corporation for preparation and standardization of royal jelly.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 2.Herring A, Ambree O, Tomm M, Habermann H, Sachser N, Paulus W, et al. Environmental enrichment enhances cellular plasticity in transgenic mice with Alzheimer-like pathology. Exp Neurol. 2009;216:184–92. doi: 10.1016/j.expneurol.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Robert R, Dolezal O, Waddington L, Hattarki MK, Cappai R, Masters CL, et al. Engineered antibody intervention strategies for Alzheimer's disease and related dementias by targeting amyloid and toxic oligomers. Protein Eng Des Sel. 2009;22:199–208. doi: 10.1093/protein/gzn052. [DOI] [PubMed] [Google Scholar]
- 4.Bothwell M, Giniger E. Alzheimer's disease: Neurodevelopment converges with neurodegeneration. Cell. 2000;102:271–3. doi: 10.1016/s0092-8674(00)00032-5. [DOI] [PubMed] [Google Scholar]
- 5.Smith JP, Lal V, Bowser D, Cappai R, Masters CL, Ciccotosto GD. Stimulus pattern dependence of the Alzheimer's disease amyloid-beta 42 peptide's inhibition of long term potentiation in mouse hippocampal slices. Brain Res. 2009;1269:176–84. doi: 10.1016/j.brainres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto M, Kanda M, Ikeno K, Hayashi Y, Nakamura T, Ogawa Y, et al. Oral administration of royal jelly facilitates mRNA expression of glial cell line-derived neurotrophic factor and neurofilament H in the hippocampus of the adult mouse brain. Biosci Biotechnol Biochem. 2005;69:800–5. doi: 10.1271/bbb.69.800. [DOI] [PubMed] [Google Scholar]
- 7.Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S. Royal jelly-induced neurite outgrowth from rat pheochromocytoma PC12 cells requires integrin signal independent of activation of extracellular signal-regulated kinases. Biomed Res. 2007;28:139–46. doi: 10.2220/biomedres.28.139. [DOI] [PubMed] [Google Scholar]
- 8.Kanbur M, Eraslan G, Silici S, Karabacak M. Effects of sodium fluoride exposure on some biochemical parameters in mice: Evaluation of the ameliorative effect of royal jelly applications on these parameters. Food Chem Toxicol. 2009;47:1184–9. doi: 10.1016/j.fct.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Hattori N, Ohta S, Sakamoto T, Mishima S, Furukawa S. Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid Based Complement Alternat Med. 2009 doi: 10.1093/ecam/nep029. doi:10.1093/ecam/nep029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, et al. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Reisi P, Babri S, Alaei H, Sharifi MR, Mohaddes G, Noorbakhsh SM, et al. Treadmill running improves long-term potentiation (LTP) defects in streptozotocin-induced diabetes at dentate gyrus in rats. Pathophysiology. 2010;17:33–8. doi: 10.1016/j.pathophys.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Narita Y, Nomura J, Ohta S, Inoh Y, Suzuki KM, Araki Y, et al. Royal jelly stimulates bone formation: Physiologic and nutrigenomic studies with mice and cell lines. Biosci Biotechnol Biochem. 2006;70:2508–14. doi: 10.1271/bbb.60240. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. 5th ed. San Diego: Academic Press; 2005. The rat brain in stereotaxic coordinates. [Google Scholar]
- 14.Hoveida R, Alaei H, Oryan S, Parivar K, Reisi P. Treadmill running improves spatial memory in an animal model of Alzheimer's disease. Behav Brain Res. 2011;216:270–4. doi: 10.1016/j.bbr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Reisi P, Alaei H, Babri S, Sharifi MR, Mohaddes G. Effects of treadmill running on spatial learning and memory in streptozotocin-induced diabetic rats. Neurosci Lett. 2009;455:79–83. doi: 10.1016/j.neulet.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, Yousuf S, et al. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Res. 2009;1281:117–27. doi: 10.1016/j.brainres.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Qu ZQ, Zeng YS, Lin YK, Li Y, Chung P, et al. Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp Toxicol Pathol. 2011 doi: 10.1016/j.etp.2010.12.011. Article in press. [DOI] [PubMed] [Google Scholar]
- 18.Vendramini AA, de Labio RW, Rasmussen LT, Dos Reis NM, Minett T, Bertolucci PH, et al. Interleukin-8-251T > A, Interleukin-1alpha-889C > T and Apolipoprotein E polymorphisms in Alzheimer's disease. Genet Mol Biol. 2011;34:1–5. doi: 10.1590/S1415-47572010005000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiese L, Hempel C, Penkowa M, Kirkby N, Kurtzhals JA. Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J. 2008;7:3. doi: 10.1186/1475-2875-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res. 2007;28:261–6. doi: 10.2220/biomedres.28.261. [DOI] [PubMed] [Google Scholar]
- 21.Holscher C, Gengler S, Gault VA, Harriott P, Mallot HA. Soluble beta-amyloid[25-35] reversibly impairs hippocampal synaptic plasticity and spatial learning. Eur J Pharmacol. 2007;561:85–90. doi: 10.1016/j.ejphar.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–9. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 23.Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–83. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 24.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- 25.El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, bdel-Wahhab MA. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon. 2007;50:256–69. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Jamnik P, Goranovic D, Raspor P. Antioxidative action of royal jelly in the yeast cell. Exp Gerontol. 2007;42:594–600. doi: 10.1016/j.exger.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Guo H, Ekusa A, Iwai K, Yonekura M, Takahata Y, Morimatsu F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J Nutr Sci Vitaminol. 2008;54:191–5. doi: 10.3177/jnsv.54.191. [DOI] [PubMed] [Google Scholar]
- 28.Zamami Y, Takatori S, Goda M, Koyama T, Iwatani Y, Jin X, et al. Royal jelly ameliorates insulin resistance in fructose-drinking rats. Biol Pharm Bull. 2008;31:2103–7. doi: 10.1248/bpb.31.2103. [DOI] [PubMed] [Google Scholar]
- 29.Kaur B, Singh N, Jaggi AS. Exploring mechanism of pioglitazone-induced memory restorative effect in experimental dementia. Fundam Clin Pharmacol. 2009;23:557–66. doi: 10.1111/j.1472-8206.2009.00708.x. [DOI] [PubMed] [Google Scholar]


