Abstract
Gene therapy is the process of introducing foreign genomic materials into host cells to elicit a therapeutic benefit. Although initially the main focus of gene therapy was on special genetic disorders, now diverse diseases with different patterns of inheritance and acquired diseases are targets of gene therapy. There are 2 major categories of gene therapy, including germline gene therapy and somatic gene therapy. Although germline gene therapy may have great potential, because it is currently ethically forbidden, it cannot be used; however, to date human gene therapy has been limited to somatic cells. Although numerous viral and nonviral gene delivery systems have been developed in the last 3 decades, no delivery system has been designed that can be applied in gene therapy of all kinds of cell types in vitro and in vivo with no limitation and side effects. In this review we explain about the history of gene therapy, all types of gene delivery systems for germline (nuclei, egg cells, embryonic stem cells, pronuclear, microinjection, sperm cells) and somatic cells by viral [retroviral, adenoviral, adeno association, helper-dependent adenoviral systems, hybrid adenoviral systems, herpes simplex, pox virus, lentivirus, Epstein–Barr virus)] and nonviral systems (physical: Naked DNA, DNA bombardant, electroporation, hydrodynamic, ultrasound, magnetofection) and (chemical: Cationic lipids, different cationic polymers, lipid polymers). In addition to the above-mentioned, advantages, disadvantages, and practical use of each system are discussed.
Keywords: Chemical delivery, gene therapy, non viral delivery systems, physical delivery, viral delivery systems
INTRODUCTION
Basically gene therapy is an intracellular delivery of genomic materials (transgene) into specific cells to generate a therapeutic effect by correcting an existing abnormality or providing the cells with a new function.[1] Different types of gene delivery systems may be applied in gene therapy to restore a specific gene function or turning off a special gene(s). The ultimate goal of gene therapy is single administration of an appropriate material to replace a defective or missing gene.[2] The first human gene transfer was utilized in 1989 on tumor-infiltrating lymphocytes[3,4] and the first gene therapy was done on ADA gene for treatment of patients with SCID (Severe Combined Immunodeficiency Defect) in 1990.[5] Although initially the main focus of gene therapy was on inherited genetic disorders, now diverse diseases, including autosomal or X-linked recessive single gene disorders (CF(Cystic Fibrosis), ADA (Adenosine Deaminase) –SCID, emphysema, retinitis pigmentosa, sickle cell anemia, phenylketonuria, hemophilia, DMD (Duchenne Muscular Dystrophy), some autosomal dominant disorders, even polygenic disorders, different forms of cancers, vascular disease, neurodegenerative disorders, inflammatory conditions, and other acquired diseases are targets of gene therapy. To date, thousands of disorders have been treated by more than hundreds of protocols of gene therapy.[1] There are 2 major categories of gene therapy: Germline gene therapy and somatic gene therapy. Although germline gene therapy may have a great potential, because it is currently ethically forbidden, it cannot be used.[6–8] To date, human gene therapy has been limited to somatic cell alterations and there is a remarkable development in the field. There are different viral and nonviral vectors for gene delivery, but all gene therapy applications depend on the fact that the genetic material needs to be delivered across the cell membrane and ultimately to the cell nucleus. Each of the delivery systems has some advantages and disadvantages, and in this review we explain about all types of gene delivery systems briefly [Figure 1].
Figure 1.
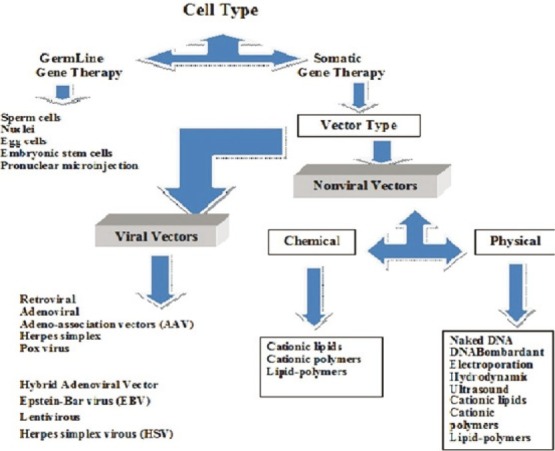
Different gene delivery systems
DIFFERENT METHODS OF GENE THERAPY
Germline gene therapy
The technology of this type of gene therapy is simple as genetic abnormalities can be corrected by direct manipulation of germline cells with no targeting, and not only achieve a cure for the individual treated, but some gametes could also carry the corrected genotype. Although it almost never has been tested on humans, some different transgenic techniques have been used on other species, which include the following:
-
(1)
Gene delivery to the nuclei taken from somatic cells at metaphase stage.[9,10]
-
(2)
Ex vivo alteration of egg cells, following in vitro fertilization.[11,12]
-
(3)
Manipulation of embryonic stem cells of mouse during in vitro culture by different gene delivery systems.[12–14]
-
(4)
Pronuclear microinjection of exogenous DNA solution by a glass needle.[15]
-
(5)
Transgenic delivery into sperm cells by direct or indirect injection to testis or other parts of the genital system.[16,17]
Somatic gene therapy
Somatic gene therapy involves the insertion of genes into diploid cells of an individual where the genetic material is not passed on to its progeny. Somatic cell therapy is viewed as a more conservative, safer approach because it affects only the targeted cells in the patient, and is not passed on to future generations; however, somatic cell therapy is short-lived because the cells of most tissues ultimately die and are replaced by new cells. In addition, transporting the gene to the target cells or tissue is also problematic. Regardless of these difficulties, however, somatic cell gene therapy is appropriate and acceptable for many disorders.
There are 3 types of somatic gene therapy
Ex vivo delivery
In this system the genetic material is explanted from the target tissue or bone marrow, cultivated and manipulated in vitro, and then transducted and/or transfected into the target tissue. There are no immunologic problems in this way but only the technique is used in cases where the target cells act as protein secretion resources (like the treatment of ADA or hemophilia) or as a vaccine for cancer treatment, so there are major limitations on the use of ex vivo delivery. In addition, at present only a small percentage of reimplanted cells remain viable.[18,19]
In situ delivery
The administration of the genetic material directly into the target tissue is in situ delivery. As most of the current delivery systems need no effective targeting, the way is proper. The system has been utilized in the delivery of CFTR gene by lipid and adenoviral vectors to a specific site in the respiratory tract and is also used in the treatment of different cancers. However, low efficiency of transduction is the main problem of this system, because in cancer therapy one malignant cell can re-establish the tumor again.[20–22]
In vivo delivery
The transfer of genetic material through an appropriate vector, which can be a viral or nonviral vector, into the target tissue is in vivo delivery. This technique is the least advanced strategy at present but potentially it might be the most useful. The problem of this way is insufficient targeting of vectors to the correct tissue sites; however, improvement in targeting and vector development will solve the problem.
DIFFERENT VECTOR SYSTEMS FOR GENE DELIVERY
Viral vectors
One of the successful gene therapy systems available today are viral vectors, such as retrovirus, adenovirus (types 2 and 5), adeno-associated virus, herpes virus, pox virus, human foamy virus (HFV), and lentivirus.[23] All viral vector genomes have been modified by deleting some areas of their genomes so that their replication becomes deranged and it makes them more safe, but the system has some problems, such as their marked immunogenicity that causes induction of inflammatory system leading to degeneration of transducted tissue; and toxin production, including mortality, the insertional mutagenesis; and their limitation in transgenic capacity size.[24,25] During the past few years some viral vectors with specific receptors have been designed that could transfer the transgenes to some other specific cells, which are not their natural target cells (retargeting).[26]
Retroviral vectors
Retroviral vectors are one of the most frequently employed forms of gene delivery in somatic and germline gene therapies. Retroviruses in contrast to adenoviral and lentiviral vectors, can transfect dividing cells because they can pass through the nuclear pores of mitotic cells; this character of retroviruses make them proper candidates for in situ treatment.[27,28] In addition, all of the viral genes have been removed, creating approximately 8 kb of space for transgenic incorporation. Retroviruses are useful for ex vivo delivery of somatic cells because of their ability to linearly integrate into host cell genome; for example, they have been used for human gene therapy of X-SCID successfully but incidence of leukemia in some patients occurred because of integration of retroviruses to the LMO2 gene and inappropriate activation of it.[29–34] Retroviral vectors also have been applied for familial hyperlipidemia gene therapy and tumor vaccination. However, the main limitations of retroviral vectors are their low efficiency in vivo, immunogenic problems, the inability to transduce the nondividing cells and the risk of insertion, which could possibly cause oncogene activation or tumor-suppressor gene inactivation.[27–34]
Adenoviral vectors
Adenoviral vectors have been isolated from a large number of different species, and more than 100 different serotypes have been reported. Most adults have been exposed to the adenovirus serotypes most commonly used in gene therapy (types 2 and 5). Adenoviruses type 2 and 5 can be utilized for transferring both dividing and nondividing cells and have low host specificity so can be used for gene delivery into large range of tissues.[35] Adenoviruses are able to deliver large DNA particles (up to 38 kb),[36] but in contrast to retroviruses, as they would not integrate into the host genome, their gene expression is too short term. Natural and acute immunologic responses against adenoviruses have made their clinical application limited to a few tissues, such as liver, lung (especially for CF(Cystic Fibrosis) treatment), or localized cancer gene therapy. Although the risk of serious disease following natural adenovirus infection is rare and the viral genome would not integrate into the host genome, gene therapy by adenoviral vectors has caused serious bad side effects and even death of some patients.[37–40] Recently, in addition to safety of these vectors, several essential genes have been deleted so that viral replication can only occur under control and also most of the viral genome is deleted to obtain sufficient space for 38 kb of transgene particles, this kind of adenoviruses are called “gutless” or “pseudo” adenoviruses.
Adeno-associated vectors
Adeno-associated vectors (AAV) are like adenoviral vectors in their features but because of having some deficiency in their replication and pathogenicity, are safer than adenoviral vectors.[41] In human, AAVs are not associated with any disease. Another special character of AAV is their ability to integrate into a specific site on chromosome 19 with no noticeable effects cause long-term expression in vivo. The major disadvantages of these vectors are complicated process of vector production and the limited transgene capacity of the particles (up to 4.8 kb). AAVs have been used in the treatment of some diseases, such as CF, hemophilia B, Leber congenital amaurosis, and AAT (Alpha-1 antitrypsine) deficiency.[41–44]
Helper-dependent adenoviral vector
Helper-dependent adenoviral vector (HdAd), called also as “gutless” or “gutted” vector, are last generation of adenovirus vectors.[35] The disadvantages of the first-generation AdV, such as a packaging capacity limitation (8 kb), immunogenicity, and toxicity, could be overcome, with the development of high-capacity “gutless” Advs (HC-AdV). In this helper-dependent vector system, one vector (the helper) contains all the viral genes required for replication but has a conditional gene defect in the packaging domain. The second vector contains only the ends of the viral genome, therapeutic gene sequences, and the normal packaging recognition signal, which allows selectively packaged release from cells.[46] Therefore, this helper-dependent system reduces toxicity but helps prolonged gene expression of up to 32 kb of foreign DNA in host cells. Nowadays, gutless adenovirus is administered in different organs, such as muscle, liver, and central nervous system.[45–51]
Hybrid adenoviral vectors
Hybrid adenoviral vectors are made of the high transduction efficiency of a gene-deleted adenoviral vector and the long-term genome-integrating potential of adeno-associated and retroviruses viruses. Such hybrid systems show stable transduction and limited integration sites.[52,53] Among integrating vectors, those derived from retroviruses are most common. One of the family of Retroviridae are called spuma retroviruses or foamy viruses (FVs). FVs are a group of apparently nonpathogenic nonhuman retroviruses, which have been developed only recently.[54,55] The potential advantages of FV vectors include a broad range of hosts, the largest packaging capacity of any retrovirus, and the ability to persist in quiescent cells. Because of these features, FVs have the unique potential to safely and efficiently deliver several genes into a number of different types of cells.[56,57]
Herpes simplex virus
Herpes simplex virus (HSV) is one of the recent viruses candidate in gene delivery. HSV systems include the development of the so-called disabled infectious single copy (DISC) viruses, which comprise a glycoprotein H defective mutant HSV genome. When the defective HSV propagated in complementing cells’ viral particles are generated, they can infect in subsequent cells permanently replicating their own genome but not producing more infectious particles.[58] Herpes vectors can deliver up to 150 kb transgenic DNA and because of its neuronotropic features, it has the greatest potential for gene delivery to nervous system,[59] tumors, and cancer cells.[60–64]
Lentiviruses
Lentiviruses are a subclass of retroviruses. They have recently been used as gene delivery vectors due to their ability to naturally integrate with nondividing cells, which is the unique feature of lentiviruses as compared with other retroviruses, which can infect only the dividing cells. Lentiviral vectors can deliver 8 kb of sequence. Because lentiviruses have strong tropism for neural stem cells, extensively used for ex vivo gene transfer in central nervous system with no significant immune responses and no unwanted side effects. Lentiviral vectors have the advantages of high-efficiency infection of dividing and nondividing cells, long-term stable expression of a transgene, low immunogenicity, and the ability to accommodate larger transgenes.[65–67]
There are numerous examples of effective long-term treatment of animal models of neurologic disorders, such as motor neuron diseases, Parkinson, Alzheimer, Huntington's disease, lysosomal storage diseases, and spinal injury.[68–73]
Poxvirus vectors
Poxvirus vectors are members of the Poxviridae family that are widely used for high-level cytoplasmatic expression of transgenes. The high stable insertion capacity (more than 25 KB) of this virus is the most advantageous feature of it for gene delivery. The insertion of the transgene sequences is somewhat different from the other vector systems and utilizes homologous recombination or in vitro ligation for construction of recombinant vaccinia virus vectors.[74–76] Poxviruses have been used for cancer therapy in various studies, such as prostate cancer, colorectal cancer, breast cancer, and lung cancer.[77,78] Recombinant vaccinia virus vectors were also used for expression of E6 and E7 genes of human papilloma virus types 16 and 18 in cervical cancer patients to induce tumor regression.[79]
There are some problems in utilizing poxviruses for gene delivery because of their complex structure and biology, so further studies are required to improve their safety and to reduce the risk of cytopathic effects.
Epstein–Barr virus
Epstein–Barr virus as a herpes virus can be used for the expression of large DNA fragments in target cells. Because Epstein–Barr virus (EBV) establishes itself in the host nucleus in a latent state as extrachromosomal circular plasmid, this virus is suitable for long-term retention in the target cell.[80–82] Because of the natural B-cell tropism of the virus, EBV-derived vectors, such as B-cell lymphoma, have been tested for immune therapy of cancer.[83]
However, other types of viruses are under investigation to date and recently, many more different virus vector systems are being developed. These are derived from vaccinia virus, human cytomegalovirus, EBV, but as mentioned earlier, problems, such as their mutagen and carcinogen properties and long-term maintenance, are major limitations in utilizing the viral vectors in gene therapy.
NONVIRAL DELIVERY SYSTEMS
Nonviral systems comprise all the physical and chemical systems except viral systems and generally include either chemical methods, such as cationic liposomes and polymers, or physical methods, such as gene gun, electroporation, particle bombardment, ultrasound utilization, and magnetofection. Efficiency of this system is less than viral systems in gene transduction, but their cost-effectiveness, availability, and more importantly less induction of immune system and no limitation in size of transgenic DNA compared with viral system have made them more effective for gene delivery than nonviral delivery systems to date.[84,85]
Physical methods of nonviral gene delivery
Physical methods applied for in vitro and in vivo gene delivery are based on making transient penetration in cell membrane by mechanical, electrical, ultrasonic, hydrodynamic, or laser-based energy so that DNA entrance into the targeted cells is facilitated.
Naked DNA
Naked DNA alone is able to transfer a gene (2–19 kb) into skin, thymus, cardiac muscle, and especially skeletal muscle and liver cells when directly injected,[86,87] also it has been applied directly.[87] Long-term expression has been observed in skeletal muscle following injection for more than 19 months. Single injection yields transgenic expression in less than 1% of total myofibers of the muscle but multiple injection would improve it. Although naked DNA injection is a safe and simple method, its efficiency for gene delivery is low so it is only proper for some applications, such as DNA vaccination.
DNA particle bombardant by gene gun
DNA particle bombardant by gene gun is an ideal alternative technique to injection of naked DNA. Gold or tungsten spherical particles (1–3 μm diameter) are coated with plasmid DNA and then accelerated to high speed by pressurized gas to penetrate into target tissue cells.[88] Actually it is a modification of a technique called “biolistic,” originally developed for plant transgenesis, but now used for in vitro and in vivo gene delivery into mammalian cells too,[89,90] such as skin, mucosa, or surgically exposed tissue and especially for DNA-based immunization or vaccination.[91]
Electroporation
Electroporation is temporary destabilization of the cell membrane targeted tissue by insertion of a pair of electrodes into it so that DNA molecules in the surrounding media of the destabilized membrane would be able to penetrate into cytoplasm and nucleoplasm of the cell[92,93] but unfortunately the trangene can integrate only to 0.01% of the treated cells.[94] Electroporation has been used in vivo for many types of tissues, such as skin, muscle, lung,[95–97] HPRT gene delivery,[98] and tumor treatment.[99] There are some problems in this method too that the more important are the difficulty in surgical procedure in the placement of electrodes into the internal tissues and that the high voltage applied to tissue might damage the organ and affect genomic DNA stability.[100]
Hydrodynamic
Hydrodynamic is a simple and highly efficient method for direct intracellular delivery of any water-soluble compounds and particles into internal organs.[101] The efficiency of this simple method in vivo is higher than any other nonviral system. This method has been successful for gene delivery into rodent liver and expression of hemophilia factors,[102] cytokines,[103] erythropoietin,[104] and hepatic growth factors,[105] in mouse and rat but it has been successful only in small animals and not in human.
Ultrasound
Ultrasound can make some nanomeric pores in membrane to facilitate intracellular delivery of DNA particles into cells of internal organs or tumors, so the size and concentration of plasmid DNA have great role in efficiency of the system.[106,107] The most important limitation of the system is low efficiency of it, especially in vivo.
Magnetofection
Magnetofection is a simple and efficient transfection method that has the advantages of the nonviral biochemical (cationic lipids or polymers) and physical (electroporation, gene gun) transfection systems in one system while excluding their inconveniences, such as low efficiency and toxicity. In this method the magnetic fields are used to concentrate particles containing nucleic acid into the target cells.[108,109] In this way, the magnetic force allows a very rapid concentration of the entire applied vector dose onto cells, so that 100% of the cells get in contact with a significant vector dose. Magnetofection has been adapted to all types of nucleic acids (DNA, siRNA, dsRNA, shRNA, mRNA, ODN,…), nonviral transfection systems (transfection reagents) and viruses. It has been successfully tested on a broad range of cell lines, hard-to-transfect and primary cells.[110,111]
Chemical nonviral delivery systems
Chemical systems are more common than physical methods and generally are nanomeric complexes, which include compaction of negatively charged nucleic acid by polycationic nanomeric particles, belonging to cationic liposome/micelle or cationic polymers. The nanomeric complex between a cationic liposome or micelle and nucleic acids is called lipoplex; but polyplex is the nanomeric complex formed between a cationic polymer and nucleic acids. These nanomeric complexes are generally stable enough to produce their bound nucleic acids from degradation and are competent to enter cells usually by endocytosis.[112] Cationic nonviral delivery systems have several advantages compared to other nonviral systems and especially viral vectors, such as low toxicity and antigenicity because they are made of only biological lipids, long-term expression with less risk of insertional oncogenesis but still low efficiency is the disadvantage of this system as well. Generally cationic lipids are included in 6 subcategories:
-
(1)
Monovalent cationic lipids
-
(2)
Polyvalent cationic lipids
-
(3)
Guanidine containing
-
(4)
Cholesterol derivative compounds
-
(5)
Cationic polymers: Poly(ethylenimine) (PEI)
Poly-l-lysine) (PLL)
Protamine
Other cationic polymers[113]
-
(6)
Lipid-polymer hybrid
Mechanism of gene delivery by cationic particles
The mechanism of gene delivery by cationic systems includes 4 steps:
-
(1)
Nonspecific interaction between cationic particles and cell surface
-
(2)
Endocytosis into endocytosis vesicles (endosomes)
-
(3)
Compaction and release of the DNA particle from endosomes
-
(4)
Translocation of the DNA particle to nucleus by membrane receptors and transgenic expression of it.[114]
For targeting of cationic particles various cell-targeting legends are covalently attached to a lipid anchor (in lipoplexes) or a DNA-binding cationic polymer (in polyplexes),[115] including proteins,[116–118] antibodies,[119,120] small chemical compounds,[121] carbohydrates,[122] peptide ligands,[123] and vitamins,[124] some of these ligands have enhanced the vector efficiency from 10- to 1000-folds. When lipoplex or polyplex particles made association with cell surface, they would enter the cell by endocytosis. It seems more of the lipid particles in early endosomes become trapped in lysosomes and degenerate by nucleases so the interaction of endosome with lysosome is a consensus and lipoplex or polyplex particles should be released before contraction of lysosome to endosome, so fugenic peptides can help it, these peptides originating from viruses can cut off the endosomal membrane to release the genomic DNA leading to increase of genetic translocation efficiency of the liposome.[125]
In this section we focus mainly on the 2 most common cationic particles: Cationic lipids and cationic polymers:
Cationic liposomes
Cationic liposomes are the more important current nonviral polycationic systems, which compact negatively charged nucleic acids lead to the formation of nanomeric complexes. Cationic liposomes have unique characteristics, such as capability to incorporate hydrophilic and hydrophobic drugs, low toxicity, no activation of immune system, and targeted delivery of bioactive compounds to the site of action.[126–129] But the rapid degradation of liposomes due to the reticuloendothelial system and the inability to achieve sustained drug delivery over a prolonged period of time are 2 drawbacks of these delivery systems that have been overcome by modification of the surface of liposomes with hydrophilic polymers, such as polyethylene glycol (PEG)[128] and integration of the pre-encapsulated drug-loaded liposomes within depot polymer-based systems.[130]
All liposomes have 1 or 2 fatty acids and alkyl moieties that are 12–18 carbons in length, in addition to a positively charged polar head group hydrophobic groups, this hydrophobic structure causes the cationic lipids. Since the first monovalent cationic lipid, DOTAP, was synthesized by Felgner et al. in 1987,[131] hundreds of new cationic liposome/micelle systems have been reported for gene delivery in vitro or in vivo. The routine way to prepare a lipoplex is mixing the solution of plasmid DNA and liposome in a proper buffer. The gene delivery efficiency of liposomes is dependent on the size, structure, and even the amount of the liposome, the charge ratio between transgenic DNA and cationic liposome, presence of helper lipid, and the structure and proportion of it and cell type.
As mentioned earlier, cationic systems are mad of either a single synthetic cationic amphiphile (cytofectin), such as DOTAP, DOTMA, DOSPA, DOGS, or more commonly of a combination of a cationic amphiphile and a neutral lipid, such as DOPE and cholesterol, these neutral helper lipids unstabilize the endosomal membrane to facilitate lipid exchange and membrane fusion between lipoplexes and endosomal membrane leading to more gene expression.[132,133] Cationic liposome-mediated delivery of DNA materials is optimal in vivo when the mol ratio of cationic liposome to nucleic acid in the lipoplex mixture is such that the positive/negative charge ratio is around 1 or greater[134–136] and in vitro the optimal ratio is closer to 1.[137–140] However, multivalent lipids with long and unsaturated hydrocarbon chains are more efficient than monovalent cationic lipids with the same hydrophobic chains.[141]
Cationic liposomes are being used in gene delivery into lung, skeletal muscles, spleen, kidney, liver, testis, heart, and skin cells.[141–148]
For gene transfer in vivo, many complexes (in equimolar ratios) are used that the more general ones are Chol/DOPE (1:1), DOTMA/DOPE (1:1), and DOTAP/DOPE (1:1).
Liposome-based technology has progressed from the first-generation conventional vesicles to stealth liposomes, targeted liposomes, and more recently stimuli-sensitive liposomes.[149,150] These new generation of liposomes overcome most of the challenges encountered by conventional liposomes, such as the inability to escape from immune system, toxicity due to charged liposomes, and low half-life stability.[151–153]
Cationic polymers
Cationic polymers at first were introduced by Wu et al. 1987[184] as PLL, the same year of synthesizing the first cationic lipids, and were further expanded by a second generation, PEI by Behr et al. in 1995.[154] To date a variety of linear or branched cationic polymers have been synthesized, including PLL-containing peptides, endosomolytic peptides (histidine-rich peptides), fusogenic peptides, nuclear localization peptides (mono partite NLS(Nuclear localization signal), bipartite NLS, nonclassical NLS), proteosomes.[155] However, PLL is still the most widely studied cationic polymer and has been used in a variety of polymerizations of lysine ranging from 19 to 1116 amino acid residues (3.97–233.2 kDa). While the molecular weight of the polymer increases, the net positive charge of it also increases and are therefore able to bind DNA tighter and form more stable complexes, totally. There is a relationship between the length of the polymer, gene delivery efficiency, and toxicity as the length of the polymer increases, so does its efficiency and its toxicity.[155,156] However, the efficiency of PLL-mediated polyplexes are low when the PLL is used alone so some conjugation agents are used to facilitate cellular uptake in vitro (as EGF(fibroblast growth factor) or transferring) or endosomal escape in vivo (as fusogenic peptides or defective viruses). Also the attachment of PEG to the polymer can prevent plasma protein binding and increase circulation of half-life of the complex.[157,158] Different homogenous PLL-conjugated peptides have been developed that have low toxicity, higher efficiency, and site-specific attachment of ligands used for cell targeting.[159–162] The optimal peptide sequence contains 18 lysines followed by a tryptophan and alkylated cysteine (AlkCWK18). A variety of branched forms of cationic peptides with a lysine as branching point have been explored.[162] PEI is the most important cationic polymer next to PLL. PEI is one of the most positively charged dense polymers, synthesized in linear (LPEI) or branched (BPEI) form, which have high transfection activity in vitro and moderate activity in vivo but the linear forms have low toxicity and high efficiency than branched forms.[163] As PLL, conjugation of some agents, such as galactose, anti-CD3 antibodies and RGD motif-containing peptides can facilitate PEI polyplex cellular uptake.[164–166] Two advantages of PEI is that it forms toroidal polyplex particles, which are stable to aggregation in physiological buffer conditions, PEI also has a strong buffering capacity at almost any pH because of the great number of primary, secondary, and tertiary amino groups.[167] One disadvantage of PEI is its nonbiodegradable nature[168] and its serious toxicity in vivo (in contrast to cationic liposome/micelle). There are conflicting associations between the gene delivery efficiency and PEI toxicity, such as PLL, the most active PEI is 25 k for BPEI and 22 k for LPEI.[169] Unfortunately, due to this property there are some limitations in the application of PEI in nonviral vector in vivo delivery. More biodegradable cationic polymers, such as aminoesters have been explored that have less toxicity than PEI and PLL.[170] However, as mentioned earlier, there are a variety of new cationic polymer groups but each of them have some advantages and disadvantages.[155] The notable factors for in vivo application are toxicity and transfection efficiency.
Lipid–polymer systems
Lipid–polymer systems are 3-part systems in which DNA is first precondensed with polycations and then coated with either cationic liposomes, anionic liposomes, or amphiphilic polymers with or without helper lipids.[171–174]
CONCLUSION
Although numerous viral and nonviral gene delivery systems have been developed in the last 3 decades, all of them have some disadvantages that have made some limitations in their clinical application and yet no delivery system has been designed that can be applied in gene therapy of all kinds of cell types in vitro and in vivo with no limitation and side effects; however, some delivery systems has been explored, which can be efficient for gene delivery to specific cells or tissues. So it seems that the process of developing successful delivery systems, especially nonviral systems, for use in in vivo is still in its adolescence and more efforts are needed. Totally, key steps effective in improving the currently available systems include the following: (1) improving extracellular targeting and delivery, (2) enhancing intracellular delivery and long-time expression, and (3) reducing toxicity and side effects on human body. However, clinical successes in 2009–2011 have bolstered new optimism in the promise of gene therapy. These include successful treatment of patients with the retinal disease Leber congenital amaurosis,[175–178] X-linked SCID,[179] ADA–SCID,[180] adrenoleukodystrophy,[180] and Parkinson's disease.[181]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stone D. Novel viral vector systems for gene therapy. Viruses. 2010;2:1002–7. doi: 10.3390/v2041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katare DP, Aeri V. Progress in gene therapy: A review. I.J.T.P.R. 2010;1:33. [Google Scholar]
- 3.Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. Gene transfer into humans immunotherapy of patients with advanced melanoma, using tumor- infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–8. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 4.Culver K, Cornetta K, Morgan R, Morecki S, Aebersold P, Kasid A, et al. Lymphocytes as cellular vehicles for gene therapy in mouse and man. Proc Natl Acad Sci USA. 1991;88:3155–9. doi: 10.1073/pnas.88.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. T lymphocyte-directed gene therapy for ADA-SCID: Initial trial results after 4 years. Science. 1995;270:475–80. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 6.Resnik B, Langer PJ. Human germline gene therapy reconsidered. Hum Gene Ther. 2001;12:1449–58. doi: 10.1089/104303401750298607. [DOI] [PubMed] [Google Scholar]
- 7.McDonough PG. The ethics of somatic and germline gene therapy. Ann N Y Acad Sci. 1997;816:378–82. doi: 10.1111/j.1749-6632.1997.tb52165.x. [DOI] [PubMed] [Google Scholar]
- 8.Resnik DB, Langer PJ. Human germline gene therapy reconsidered. Hum Gene Ther. 2001;12:1449–58. doi: 10.1089/104303401750298607. [DOI] [PubMed] [Google Scholar]
- 9.Wolf E, Schernthaner W, Zakhartchenko V, Prelle K, Stojkovic M, Brem G. Transgenic technology in farm animals-progress and perspectives. Exp Physiol. 2000;85:615–25. [PubMed] [Google Scholar]
- 10.Johnson-Saliba M, Jans DA. Gene therapy: Optimising DNA delivery to the nucleus. Curr Drug Targets. 2001;2:371–99. doi: 10.2174/1389450013348245. [DOI] [PubMed] [Google Scholar]
- 11.Jaenisch R. Transgenic animals. Science. 1988;240:1468–74. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- 12.Smith KR. Gene Therapy: The Potential Applicability of Gene Transfer Technology to the Human Germline. Int J Med Sci. 2004;1:76–91. doi: 10.7150/ijms.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirabayashi M, Takahashi R, Ito K, Kashiwazaki N, Hirao M, Hirasawa K, et al. A comparative study on the integration of exogenous DNA into mouse, rat, rabbit, and pig genomes. Exp Anim. 2001;50:125–31. doi: 10.1538/expanim.50.125. [DOI] [PubMed] [Google Scholar]
- 14.Torres M. The use of embryonic stem cells for the genetic manipulation of the mous. Curr Topics Dev Biol. 1998;36:99–114. doi: 10.1016/s0070-2153(08)60497-4. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by micro-injection of purified DNA. Proc Natl Acad Sci USA. 1980;77:7380–4. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John PL, Kevin C, Joaquin G. Sperm and testis mediated DNA transfer as a means of gene therapy. Syst Biol Reprod Med. 2011;57:35–42. doi: 10.3109/19396368.2010.514022. [DOI] [PubMed] [Google Scholar]
- 17.Kevin S, Corrado S. Sperm-mediated gene transfer: Applications and implications. BioEssays. 2005;27:551–62. doi: 10.1002/bies.20211. [DOI] [PubMed] [Google Scholar]
- 18.Herrero MJ, Sabater L, Guenechea G, Sendra L, Montilla AI, Abargues R, et al. DNA delivery to ‘ex vivo’ human liver segments. Gene Ther. 2011 doi: 10.1038/gt.2011.144. in press. [DOI] [PubMed] [Google Scholar]
- 19.Suhonen J, Ray J, Blömer U, Gage FH, Kaspar B. Ex vivo and in vivo gene delivery to the brain. Curr Protoc Hum Genet. 2006;13 doi: 10.1002/0471142905.hg1303s51. Unit 13.3. [DOI] [PubMed] [Google Scholar]
- 20.Hu WW, Wang Z, Hollister SJ, Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14:891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 21.Takefumi S, Akira I, Shin E, Shiro B. In Situ Gene Therapy for Prostate Cancer. Curr Gene Ther. 2005;5:111–9. doi: 10.2174/1566523052997523. [DOI] [PubMed] [Google Scholar]
- 22.Davis PB, Cooper MJ. Vectors for airway gene delivery. AAPS J. 2007;9:2. doi: 10.1208/aapsj0901002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Liu X, Dong L, Liu Z, He X, Liu W. Development of Viral Vectors for Gene Therapy for Chronic Pain. Pain Res Treat. 2011;2011:968218. doi: 10.1155/2011/968218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardlik R, Palffy R, Hodosy J, Lukacs J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11:110–21. [PubMed] [Google Scholar]
- 25.Katare DP, Aeri V. Progress in gene therapy: A Review. I.J.T.P.R. 2010;1:33–41. [Google Scholar]
- 26.Wickham TJ. Ligand-directed targeting of genes to the site of disease. Nat Med. 2003;9:135–9. doi: 10.1038/nm0103-135. [DOI] [PubMed] [Google Scholar]
- 27.Anson DS. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet Vaccines Ther. 2004;2:9. doi: 10.1186/1479-0556-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frederic D. Bushman.Retroviral integration and human gene therapy. J Clin Invest. 2007;117:2083–6. doi: 10.1172/JCI32949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laufs S, Gentner B, Nagy KZ, Jauch A, Benner A, Naundrof S, et al. Retroviral vector integration occurs in preferred genomic targets of human bone marrow-repopulating cells. Blood. 2003;101:2191–8. doi: 10.1182/blood-2002-02-0627. [DOI] [PubMed] [Google Scholar]
- 30.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–93. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 31.Fischer A, Hacein-Bey-Aina S, Lagresle C, Garrigue A, Cavazanna-Calvo M. Gene therapy of severe combined immunodeficiency disease: Proof of principle of efficiency and safety issues.Gene therapy, primary immunodeficiencies, retrovirus, lentivirus, genome. Bull Acad Natl Med. 2005;189:779–85. [PubMed] [Google Scholar]
- 32.Buckly RH. Gene therapy for SCID: A complication after remarkable progress. Lancet. 2003;360:1185–6. doi: 10.1016/S0140-6736(02)11290-6. [DOI] [PubMed] [Google Scholar]
- 33.Fox JL. US authorities uphold suspension of SCID gene therapy. Nat Biotechnol. 2003;21:217. doi: 10.1038/nbt0303-217. [DOI] [PubMed] [Google Scholar]
- 34.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-XI. Science. 2003;302:415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 35.Thrasher AJ, Gaspar HB, Baum C, Modlich U, Schambach A, Candotti F, et al. Gene therapy: X-SCID transgene leukaemologenicity. Nature. 2006;443:E5–7. doi: 10.1038/nature05219. [DOI] [PubMed] [Google Scholar]
- 36.Vorburger SA, Hunt KK. Adenoviral Gene Therapy. Oncologist. 2002;7:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- 37.Bett AJ, Prevec L, Graham FL. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67:5911–21. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002;9:979–86. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–5. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 41.Teramato S, Ishii T, Matsuse T. Crisis of adenoviruses in human gene therapy. Lancet. 2000;355:1911–2. doi: 10.1016/S0140-6736(05)73358-4. [DOI] [PubMed] [Google Scholar]
- 42.Lai CM, Lai YK, Rakoczy PE. Adenovirus and adeno-associated virus vectors. DNA Cell Biol. 2002;21:895–913. doi: 10.1089/104454902762053855. [DOI] [PubMed] [Google Scholar]
- 43.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, et al. A phase study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–59. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 44.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–61. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 45.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration Molecular therapy. J Am Soc Gene Ther. 2010;18:643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–7. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crane B, Luo X, Demaster A, Williams KD, Kozink DM, Zhang P, et al. Rescue administration of a helper dependent adenovirus vector with long-term efficacy in dogs with glycogen storage disease type Ia. Gene Ther. 2011 doi: 10.1038/gt.2011.86. in press. [DOI] [PubMed] [Google Scholar]
- 48.Alba R, Bosch A, Chillon M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005;12:18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- 49.Croyle MA, Le HT, Linse KD, Cerullo V, Toietta G, Beaudet A, et al. PEGylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–87. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 50.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–33. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morral N, O’Neal W, Rice K, Leland M, Kaplan J, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–21. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balagué C, Zhou J, Dai Y, Alemany R, Josephs SF, Andreason G, et al. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood. 2000;95:820–8. [PubMed] [Google Scholar]
- 53.Morral N, Parks RJ, Zhou H, Langston C, Schiedner G, Quinones J, et al. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum Gene Ther. 1998;9:2709–16. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 54.Ehrhardt A, Yant SR, Giering JC, Xu H, Engler JA, Kay MA. Somatic Integration From an Adenoviral Hybrid Vector into a Hot Spot in Mouse Liver Results in Persistent Transgene Expression Levels In Vivo. Mol Ther. 2007;15:146–56. doi: 10.1038/sj.mt.6300011. [DOI] [PubMed] [Google Scholar]
- 55.Shuji K, Kazunori H, Atsuko T, Donna JP, Philip N, Haruki O, et al. Adenovirus–retrovirus hybrid vectors achieve highly enhanced tumor transduction and antitumor efficacy in vivo. Mol Ther. 2011;19:76–82. doi: 10.1038/mt.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Liu Z, Cao X, Cao Z, Xue L, Zhu F, et al. Recombinant human foamy virus, a novel vector for neurological disorders gene therapy, drives production of GAD in cultured astrocytes. Mol Ther. 2007;15:1834–41. doi: 10.1038/sj.mt.6300224. [DOI] [PubMed] [Google Scholar]
- 57.Mergia A, Heinkelein M. Foamy virus vectors. Curr Topics Microbiol Immunol. 2003;277:131–59. doi: 10.1007/978-3-642-55701-9_6. [DOI] [PubMed] [Google Scholar]
- 58.Trobridge GD. Foamy virus vectors for gene transfer. Exp Opin Biol Ther. 2009;9:1427–36. doi: 10.1517/14712590903246388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W, Liu Z, Liu L, Xiao Z, Cao X, Cao Z, et al. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett. 2008;432:13–8. doi: 10.1016/j.neulet.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 60.Burtton EA, Wechuck JB, Wendell SK, Goins WF, Fink DJ, Glorioso JC. Multiple applications for replication-defective Herpes simplex virus vectors. Stem Cell. 2001;19:358–77. doi: 10.1634/stemcells.19-5-358. [DOI] [PubMed] [Google Scholar]
- 61.Berto E, Bozac A, Marconi P. Development and application of replication incompetent HSV-1-based vectors. Gene Ther. 2005;12:98–102. doi: 10.1038/sj.gt.3302623. [DOI] [PubMed] [Google Scholar]
- 62.Goins WF, Goss JR, Chancellor MB, de Groat WC, Glorioso JC, Yoshimura N. Herpes simplex virus vectormediated gene delivery for the treatment of lower urinary tract pain. Gene Ther. 2009;16:558–69. doi: 10.1038/gt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfe D, Mata M, Fink DJ. A human trial of HSVmediated gene transfer for the treatment of chronic pain. Gene Ther. 2009;16:455–60. doi: 10.1038/gt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X, et al. Herpes vectormediated expression of proenkephalin reduces bone cancer pain. Ann Neurol. 2002;52:662–5. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 65.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8:551–6. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 66.Lachmann RH, Efstathiou S. The use of Herpes simolex virus-based vectors for gene delivery to the nervous system. Mol Med Today. 1997;3:404–11. doi: 10.1016/S1357-4310(97)01106-4. [DOI] [PubMed] [Google Scholar]
- 67.Federici T, Kutner R, Zhang XY, Kuroda H, Tordo N, Boulis NM, et al. Comparative analysis of HIV-1-based lentiviral vectors bearing lyssavirus glycoproteins for neuronal gene transfer. Genet Vaccines Ther. 2009;7:1–9. doi: 10.1186/1479-0556-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 69.Kafri T. Gene delivery by lentivirus vectors an overview. Methods Mol Biol. 2004;246:367–90. doi: 10.1385/1-59259-650-9:367. [DOI] [PubMed] [Google Scholar]
- 70.Balaggan KS, Ali RR. Ocular gene delivery using lentiviral vectors. Gene Ther. 2012;19:145–53. doi: 10.1038/gt.2011.153. [DOI] [PubMed] [Google Scholar]
- 71.Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND. Lentivirus-Mediated Gene Transfer to the Central Nervous System: Therapeutic and Research Applications. Human Gen Ther. 2006;17:1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 72.Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, et al. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease. J Neurosci. 2002;22:10302–12. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Betchen SA, Kaplitt M. Future and current surgical therapies in Parkinson's disease. Curr Opin Neurol. 2003;16:487–93. doi: 10.1097/01.wco.0000084227.82329.ae. [DOI] [PubMed] [Google Scholar]
- 74.Blesch A, Ynski MH. Gene therapy and cell transplantation for Alzheimer's disease and spinal cord injury. Yonsei Med J. 2004;45:28–31. doi: 10.3349/ymj.2004.45.Suppl.28. [DOI] [PubMed] [Google Scholar]
- 75.Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–9. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 76.Moroziewicz D, Kaufman HL. Gene therapy with poxvirus vectors. Curr Opin Mol Ther. 2005;7:317–25. [PubMed] [Google Scholar]
- 77.Gómez CE, Nájera JL, Krupa M, Esteban M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr Gene Ther. 2008;8:97–120. doi: 10.2174/156652308784049363. [DOI] [PubMed] [Google Scholar]
- 78.Pastoret PP, Vanderplasschen A. Poxviruses as vaccine vectors. Comp Immunol Microbiol Infect Dis. 2003;26:343–55. doi: 10.1016/S0147-9571(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 79.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination and safety. Proc Natl Acad Sci U S A. 1996;93:11341–8. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McAneny D, Ryan CA, Beazley RM, Kaufman HL. Results of a phase I trial of a recombinant vaccinia virus that expresses carcinoembryonic antigen in patients with advanced colorectal cancer. Ann Surg Oncol. 1996;3:395–500. doi: 10.1007/BF02305769. [DOI] [PubMed] [Google Scholar]
- 81.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 82.Mecsas J, Sugden B. Replication of plasmids derived from bovine papilloma virus type 1 and Epstein-Barr virus in cell in culture. Annu Rev Cell Biol. 1987;3:87–108. doi: 10.1146/annurev.cb.03.110187.000511. [DOI] [PubMed] [Google Scholar]
- 83.Kishida T, Shin-Ya M, Imanishi J, Mazda O. Dept. of Microbiol., Kyoto Prefectural Univ. of Medicine Application of EBV.based artificial chromosome to genetic engineering of mammalian cells and tissues. Micro Nano Mechatron Hum Sci. 2005;7-9:133–8. [Google Scholar]
- 84.Hirai H, Satoh E, Osawa M, Inaba T, Shimazaki C, Kinoshita S, et al. Use of EBV-based vector/ HVJ-liposome complex vector for targeted gene therapy of EBV-associated neoplasms. Biochem Biophys Res Commun. 1997;241:112–8. doi: 10.1006/bbrc.1997.7776. [DOI] [PubMed] [Google Scholar]
- 85.Robertson ES, Ooka T, Kieff ED. Epstein-Barr virus vectors for gene delivery to B lymphocytes. Proc Natl Acad Sci U S A. 1996;93:11334–40. doi: 10.1073/pnas.93.21.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Audouny SA, Deleij LF, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res. 2002;19:1599–6005. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 87.Varga CM, Hong K, Lanf Furburger DA. Quantitative analysis of synthesis gene delivery vector design properties. Mol Ther. 2001;4:438–46. doi: 10.1006/mthe.2001.0475. [DOI] [PubMed] [Google Scholar]
- 88.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–9. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 89.Knapp JE, Liu D. Hydrodynamic delivery of DNA. Methods Mol Biol. 2004;245:245–50. doi: 10.1385/1-59259-649-5:245. [DOI] [PubMed] [Google Scholar]
- 90.Herweijer H, Wolff JA. Progress and prospects: Naked DNA gene transfer and therapy. Gen Ther. 2003;10:453–8. doi: 10.1038/sj.gt.3301983. [DOI] [PubMed] [Google Scholar]
- 91.Yang NS, Burkhorder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci USA. 1990;87:9568–72. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klein TM, Arentzen R, Lewis PA, Fitzpatrick McElligoutt S. Transformation of microbes, plants and animals by particle bombardment. Biotechnology. 1992;10:286–91. doi: 10.1038/nbt0392-286. [DOI] [PubMed] [Google Scholar]
- 93.Cheng L, Ziegelhoffer PR, Yang NS. In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci USA. 1993;90:4455–9. doi: 10.1073/pnas.90.10.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahvi DM, Sheehy MJ, Yang NS. DNA cancer vaccines: A gene gun approach. Immunol Cell Biol. 1997;75:456–60. doi: 10.1038/icb.1997.70. [DOI] [PubMed] [Google Scholar]
- 95.Heller LC, Ugen K, Heller R. Electroporation for targeted gene transfer. Expert Opin Drug Deliv. 2005;2:255–68. doi: 10.1517/17425247.2.2.255. [DOI] [PubMed] [Google Scholar]
- 96.Lurquin PF. Gene transfer by electroporation. Mol Biotechnol. 1997;7:5–35. doi: 10.1007/BF02821542. [DOI] [PubMed] [Google Scholar]
- 97.Potter H, Cooke SW. Gene transfer into adherent cells growing on microbeads. In: Change DC, editor. Guide to electroporation and electrofusion. San Diego, CA, USA: Academic Press; 1992. pp. 201–8. [Google Scholar]
- 98.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transferin to mouse lyoma cells by electroporation in high electric fields. EMBOJ. 1982;1:841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dean DA, Machado Aranda D, Blair Parks K, Yeldandi AV, Young JL. Electroporation as a method for high level nonviral gene transfer to the lung. Gene Ther. 2003;10:1608–15. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mc Mahon JM, Wells DJ. Electroporation for gene transfer to skeletal muscles: Current status. Biol Drugs. 2004;18:155–65. doi: 10.2165/00063030-200418030-00002. [DOI] [PubMed] [Google Scholar]
- 101.Hatada S, Nikkuni K, Bentley SA, Kirby S, Smithies O. Gene correction in hematopoietic progenitor cells by homologous recombination. Proc Natl Acad Sci USA. 2000;97:13807–11. doi: 10.1073/pnas.240462897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hofmann GA, Dev SB, Nanda GS, Rabusssay D. Electroporation therapy of solid tumors. Crit Rev Ther Drug Carrier Syst. 1999;16:523–69. [PubMed] [Google Scholar]
- 103.Gissel H, Clausen T. Excitation -induced Ca influx and skeletal muscle cell damage. Acta Physiol Scand. 2001;171:327–34. doi: 10.1046/j.1365-201x.2001.00835.x. [DOI] [PubMed] [Google Scholar]
- 104.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;10:1258–66. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 105.Miao CH, Ye X, Thompson AR. High level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum Gen Ther. 2003;14:1297–305. doi: 10.1089/104303403322319381. [DOI] [PubMed] [Google Scholar]
- 106.Jiang J, Yamato E, Miyazaki J. Intravenous delivery of naked plasmid DNA for in vivo cytokine expression. Biochem Biophys Res Commun. 2001;289:1088–92. doi: 10.1006/bbrc.2001.6100. [DOI] [PubMed] [Google Scholar]
- 107.Maruyama H, Higuchi N, Kameda S, Miyazaki J, Gejyo F. Rat liver targeted naked plasmid DNA transfer by tail vein injection. Mol Biotechnol. 2004;26:165–72. doi: 10.1385/mb:26:2:165. [DOI] [PubMed] [Google Scholar]
- 108.Yang J, Chen S, Huang L, Michalopoulos GK, Liu Y. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology. 2001;33:848–59. doi: 10.1053/jhep.2001.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim HJ, Greeenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther. 1996;7:1339–46. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- 110.Liang HD, Lu QL, Xue SA, Halliwell M, Kodama T, Cosgrove DO, et al. Optimisation of ultrasound mediated gene transfer (sonoporation) in skeletal muscle cells. Ultrasound Med Biol. 2004;30:1523–9. doi: 10.1016/j.ultrasmedbio.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 111.Plank C, Schillinger U, Scherer F, Bergemann C, Rémy JS, Krötz F, et al. The magnetofection method: Using magnetic force to enhance gene delivery. Biol Chem. 2003;384:737–47. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 112.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–9. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 113.Plank C, Anton M, Rudolph C, Rosenecker J, Krötz F. Enhancing and targeting nucleic acid delivery by magnetic force. Exp Opin Biol Ther. 2003;3:745–58. doi: 10.1517/14712598.3.5.745. [DOI] [PubMed] [Google Scholar]
- 114.Mair L, Ford K, Alam MR, Kole R, Fisher M, Superfine R, et al. Size-Uniform 200 nm Particles: Fabrication and Application to Magnetofection. J Biomed Nanotechnol. 2009;5:182–91. doi: 10.1166/jbn.2009.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu D, Ren T, Gao X. Cationic transfection lipids. Curr Med Chem. 2003;10:1735–7. doi: 10.2174/0929867033457386. [DOI] [PubMed] [Google Scholar]
- 116.Zhang S, Xu Y, Wang B, Qiao W, Liu D, Li Z. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J Controlled Release. 2004;100:165–80. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 117.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 118.Boeckle S, Wagner E. Optimizing targeted gene delivery: Chemical Modification of viral vectors and synthesis of artificial virus vector systems. AAPS J. 2006;8 doi: 10.1208/aapsj080483. Article 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim TG, Kang SY, Kang JH, Cho MY, Kim JI, Kim SH, et al. Gene transfer into human hepatoma cells by receptor-associated protein/polylysine conjugates. Bioconjug Chem. 2004;15:326–32. doi: 10.1021/bc0340262. [DOI] [PubMed] [Google Scholar]
- 120.Wolschek MF, Thallinger C, Kursa M, Rössler V, Allen M, Lichtenberger C, et al. Specific systemic nonviral gene delivery to human hepatocellular carcinoma xenografts in SCID mice. Hepatology. 2002;36:1106–14. doi: 10.1053/jhep.2002.36372. [DOI] [PubMed] [Google Scholar]
- 121.Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther. 1999;10:2941–52. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 122.Chiu SJ, Ueno NT, Lee RJ. Tumor-targeted gene delivery via anti-HER2 antibody (trastuzumab, Herceptin) conjugated polyethylenimine. J Control Release. 2004;97:357–69. doi: 10.1016/j.jconrel.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–77. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 124.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–7. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 125.Plank C, Zatloukal K, Cotton M, Mechtler K, Wagner E. Gene transfer into hepatocytes using asialoglycoprotein receptor-mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand1. Bioconjug Chem. 1992;3:533–9. doi: 10.1021/bc00018a012. [DOI] [PubMed] [Google Scholar]
- 126.Ziandy AG, Ferkol T, Dawson DV, Perlmutter DH, Davis PB. Chain length of the polylysine in receptor-targeted gene transfer complexes affects duration of reporter gene expression both in vitro and in vivo. 1999;274:4908–16. doi: 10.1074/jbc.274.8.4908. [DOI] [PubMed] [Google Scholar]
- 127.Hofland HE, Masson C, Iginla S, Osetinsky I, Reddy JA, Leamon CP, et al. Folate-targeted gene transfer in vivo. Mol Ther. 2002;5:739–44. doi: 10.1006/mthe.2002.0604. [DOI] [PubMed] [Google Scholar]
- 128.Son KK, Tkaeh D, Hall KJ. Efficient in vivo gene delivery by the negatively charged complexes of cationic liposome and plasmid. DNA Biochem Biophys Acta. 2000;1468:6–10. doi: 10.1016/s0005-2736(00)00311-4. [DOI] [PubMed] [Google Scholar]
- 129.Mastrobattista E, Koning GA, van Bloois L, Filipe AC, Jiskoot W, Storm G. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J Biol Chem. 2002;277:27135–43. doi: 10.1074/jbc.M200429200. [DOI] [PubMed] [Google Scholar]
- 130.Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2:99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Immordino ML, Dosio F, Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 132.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 133.Stenekes RJ, Loebis AE, Fernandes CM, Crommelin DJ, Hennink WE. Controlled release of liposomes from biodegradable dextran microspheres: A novel delivery concept. Pharm Res. 2000;17:690–5. doi: 10.1023/a:1007526114744. [DOI] [PubMed] [Google Scholar]
- 134.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cullis PR, Hope MJ, Tilcock CP. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipds. 1986;40:127–44. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- 136.Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochem Biophys Acta. 1995;1235:296–304. doi: 10.1016/0005-2736(95)80017-a. [DOI] [PubMed] [Google Scholar]
- 137.Schwartz B, Benoist C, Abdallah B, Scherman D, Behr JP, Demeneix BA. Liposopermine-based gene transfer into the newborn mous brain is optimized by a low lipospermine DNA charge ratio. Hum Gene Ther. 1995;6:1515–24. doi: 10.1089/hum.1995.6.12-1515. [DOI] [PubMed] [Google Scholar]
- 138.Liu F, Qi H, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997;4:517–23. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 139.Yang JP, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1995;92:1744–1748. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 140.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, et al. Enhanced gene delivery and mechanism studies with a novol series of cationic lipid formulations. J Biol Chem. 1994;269:2550–61. [PubMed] [Google Scholar]
- 141.Alton EW, Middleton PG, Caplen NJ, Smith SN, Steel DM, Munkonge FM, et al. Non-invasive liposome mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Gent. 1993;5:135–42. doi: 10.1038/ng1093-135. [DOI] [PubMed] [Google Scholar]
- 142.McQuillin A, Murray KD, Etheridge CJ, Stewart L, Cooper RG, Brett PM, et al. Optimization of liposome mediated transfection of a neuronal cell line. 1997;8:135–42. doi: 10.1097/00001756-199704140-00031. [DOI] [PubMed] [Google Scholar]
- 143.Fife K, Bower M, Cooper RG. Endothelial cell transfection with cationic liposomes and herpes simolex-thymidine kinase mediated killing. Gen Ther. 1998;5:614–20. doi: 10.1038/sj.gt.3300627. [DOI] [PubMed] [Google Scholar]
- 144.Birchall JC, Kellawy IW, Mills SN. Physicochemical characterization and transfection efficiency of lipid-based gene delivery complexes. Int J Pharm. 1999;183:195–207. doi: 10.1016/s0378-5173(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 145.Stribling R, Brunette E, Liggitt D, Gaensler K, Debs R. Aerosol gene delivery in vivo. Proc Natl Acad Sci USA. 1992;89:11277–81. doi: 10.1073/pnas.89.23.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 147.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, et al. Cationic lipid mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: A double blind placebo-controlled trial. Lancet. 1999;353:947–54. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 148.Zhu N, Liggitt D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209–11. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]
- 149.Rogy MA, Auffenberg T, Espat NJ, Philip R, Remick D, Wollenberg GK, et al. Human tumor necrosis factor receptor(p55) and interleukin 10 gene transfer in the mouse reduces mortality to the lethal endotoxemia and also attenuates local inflammatory responses. J Exp Med. 1995;181:2289–93. doi: 10.1084/jem.181.6.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Murray KD, McQuillin A, Stewart L, Etheridge CJ, Cooper RG, Miller AD, et al. Cationic liposome mediated DNA transfection in organotype explant cultures of the ventral mesencephalon. Gene Ther. 1999;6:190–7. doi: 10.1038/sj.gt.3300743. [DOI] [PubMed] [Google Scholar]
- 151.Thierry AR, Lunardiskander Y, Bryant JL, Rabinovich P, Gallo RC, Mahan LC. Systemic gene therapy: Biodistribution and long term expression of a transgene in mice. Proc Natl Acad Sci USA. 1995;92:9742–6. doi: 10.1073/pnas.92.21.9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Immordino ML, Dosio F, Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 153.Bharali DJ, Khalil M, Gurbuz M, Simone TM, Mousa SA. Nanoparticles and cancer therapy: A concise review with emphasis on dendrimers. International. J Nanomed. 2009;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 154.Kim ES, Lu C, Khuri FR, Tonda M, Glisson BS, Liu D, et al. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced nonsmall cell lung cancer. Lung Cancer. 2001;34:427–32. doi: 10.1016/s0169-5002(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 155.Goyal P, Goyal K, Kumar SG, Singh A, Katare OP, Mishra DN. Liposomal drug delivery systems—clinical applications. Acta Pharm. 2005;55:1–25. [PubMed] [Google Scholar]
- 156.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Döblinger M, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;1:108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 157.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenamine. Proc Natl Acad Sci USA. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Martin ME, Rice KG. Peptide-guided Gene Delivery. AAPS J. 2007;9 doi: 10.1208/aapsj0901003. Article 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wolfert MA, Saymour LW. Atomic force microscopic analysis of the influence of the molecular weight of poly(L)lysine on the size of polyelectrolyte complexes formed with DNA. Gen Ther. 1996;3:269–73. [PubMed] [Google Scholar]
- 160.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94:1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 161.Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–32. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 162.Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG. Peptide-mediated gene delivery: Influence of peptide structure on gene expression. Bioconjug Chem. 1997;8:81–8. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 163.McKenzie DL, Collard WT, Rice KG. Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J Pept Res. 1999;54:311–8. doi: 10.1034/j.1399-3011.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 164.Adami RC, Collard WT, Gupta SA, Kwok KY, Bonadio J, Rice KG. Stability of peptide-condensed Plasmid DNA formulations. J Pharm Sci. 1998;87:678–83. doi: 10.1021/js9800477. [DOI] [PubMed] [Google Scholar]
- 165.Plank C, Tang MX, Wolfe AR, Szoka FC. Branched cationic peptides for gene delivery: Role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther. 1999;10:319–32. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 166.Wightman L, Kircheis R, Rössler V, Carotta S, Ruzicka R, Kursa M, et al. Different behavior of the branched and linear polyethylenamine for gene delivery in vitro and in vivo. J Gene Med. 2000;3:362–72. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 167.Zanta MA, Boussif O, Adib A, Behr JP. In vitro gene delivery to hepatocytes with galactosylenimine. Bioconjugate Chem. 1997;8:839–44. doi: 10.1021/bc970098f. [DOI] [PubMed] [Google Scholar]
- 168.Kircheis R, Kichler A, Wallner G, Kursa M, Ogris M, Felzmann T, et al. Coupling of cell binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997;4:409–18. doi: 10.1038/sj.gt.3300418. [DOI] [PubMed] [Google Scholar]
- 169.Erbacher P, Remy JS, Behr JP. Gene transfer with synthetic virus like particles via the integrin mediated endocytosis pathway. Gene Ther. 1999;6:138–45. doi: 10.1038/sj.gt.3300783. [DOI] [PubMed] [Google Scholar]
- 170.Tang MX, Szoka FC. The influence of polymer structure on the interaction of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–32. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 171.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–31. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 172.Fischer D, Bieber T, Li Y, Elasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenamine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–9. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 173.Lim YB, Han SO, Kong HU. Biodegradable polyester, poly[alpha-(4-aminobutyl)-L-glycolic acid], as anon-toxic gene carrier. Pharm Res. 2000;7:811–6. doi: 10.1023/a:1007552007765. [DOI] [PubMed] [Google Scholar]
- 174.Gao X, Huang L. Potentiation of cationic liposome-mediated gene delivery by polycationics. Biochemistry. 1996;35:1027–36. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- 175.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–7. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 176.Lee LK, Williams CL, Devore D, Roth CM. Poly (eropylacrylicacid) enhances cationic lipid-mediated delivery of antisense oligonucleotides. Biomacromolecules. 2006;7:1502–8. doi: 10.1021/bm060114o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration Molecular therapy. J Am Soc Gene Ther. 2010;18:643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–7. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cartier N, Aubourg P. Hematopoietic Stem Cell Transplantation and Hematopoietic Stem Cell Gene Therapy in X-Linked Adrenoleukodystrophy. Brain Pathol. 2010;20:857–862. doi: 10.1111/j.1750-3639.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson's disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–19. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]


