Abstract
Background:
Rheumatoid arthritis (RA) is one of the most frequent autoimmune diseases in the world which affect about 1% of people. Measurement of the anti-CCP1 antibody titer in these patients is one of the new tests that is available in our country and in the multiple studies has been shown to be more specific than rheumatoid factor in the diagnosis of RA (97%). This test becomes positive in early stage of disease and it has a high predictive value. The aim of our study was to determine the relationship between anti-CCP1 antibody and disease activity score 28 (DAS-28) in patients with rheumatoid arthritis.
Materials and Methods:
A cross-sectional study was performed in 90 patients with RA for evaluating the relation between anti-CCP1 antibody titer and DAS-28, patients were selected by consecutive method; serum samples were collected from patients. Anti-CCP1 antibody was measured by the corresponding enzyme-linked immunosorbent assay. Additionally, erythrocyte sedimentation rates (ESR), rheumatoid factor (RF), DAS-28, visual analog scale (VAS) were determined in patients with RA. Statistical analysis performed with t-test and Pearson's correlations coefficient.
Results:
Anti-CCP1 level was associated with DAS-28 (P<0.001, r=0.35). The largest linear correlation was between anti-CCP1 antibody levels and VAS; it means that higher titers of anti-CCP1 antibody are associated with more painful joints in our patients. The average of DAS-28 in our positive and negative anti-CCP1 antibody patients was 5.07±1.1 and 3.5±1.5, respectively (P<0.05).
Conclusion:
There was moderate correlation between anti-CCP1 titer and DAS-28.
Keywords: Anti-CCP1, disease activity score 28 (DAS-28), rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by chronic and erosive polyarthritis caused by abnormal growth of synovial tissue or pannus and causes irreversible joint disability. It is the most common inflammatory arthritis, affecting from 0.5% to 1% of the general population worldwide, with a female/male ratio of 2.5:1. The disease may appear at any age, but it is most common among those aged from 40 to 70 years and its incidence increases with age.[1] Apart from pain, RA is associated with reduction of functional capacity and increased comorbidity and mortality.[2,3] Because of the highly variable and unpredictable course of the disease, current therapeutic strategies in RA are increasingly aggressive regimens early in the course of the disease. Therefore, diagnostic tests with high specificity are desirable for deciding on the optimal treatment.[4] So far, serological support in the diagnosis of RA was mainly based on the presence of rheumatoid factors (RF).[5] RF can be detected in up to 70–80% of RA patients.[6] The ACR criteria for RA diagnosis include the presence of RF, a decision that has contributed to the widely routine use of this test as a diagnostic marker for RA in most clinical laboratories. However, these antibodies are not very specific for RA and can also be detected in other rheumatic diseases, infectious diseases, and even in 3–5% of apparently healthy individuals.[5,7]
In recent years, many studies on antibodies against cyclic citrullinated peptide (CCP) have demonstrated that these antibodies are highly specific and predictive for RA[8] that they can be detected many years before onset of disease[9] and so that they are associated with joint destruction.[10] Furthermore, the presence or absence of these antibodies seems to be a stable trait.[11] Anti-CCP antibodies are detected in seropositive as well as in seronegative RA patients. Antibodies to CCP as detected by enzyme-linked immunosorbent assays (ELISA) are found in most patients with RA but less often in other diseases.[12] The sensitivity of ELISA for anti-CCP1 antibodies is similar to that of testing for IgM RF, but the specificity of a positive anti-CCP1 antibody assay is higher, in the range of 90% to 96%. Anti-CCP1 antibodies predict erosive disease more effectively among patients with RA than do assays for RF.[13,14] ELISA test for anti-CCP1 may be useful in the differential diagnosis of early stage of RA, particularly the ability of distinguishing RA from primary Sjögren's syndrome or SLE. It may also be valuable in identifying those patients with early RA who are at increased risk of progressive joint damage among patients with early oligo- or polyarthritis. Anti-CCP1 test and so has predictive value in the IgM-RF negative subgroup of RA patients. The anti-CCP1 test is a useful tool for diagnosing or exclusion of RA in patients with polyarthritis. In contrast to RF, anti-CCP1 antibodies are rarely present in the serum of patients with HCV infections.
The aim of our study was to determine the relationship between anti-CCP1 and DAS- 28 in patients with RA.
MATERIALS AND METHODS
Ninety RA patients (median age, 49.5 years ± 15.5 range, 21–84) who were being evaluated at the Rheumatology Department of Isfahan University of Medical Sciences Center in Alzahra Hospital, Iran, and who fulfilled four or more of the American College of Rheumatology (ACR) 1987 criteria for RA were asked to participate.
Ninety patients during the period November 2007 and November 2008 were randomly enrolled in this study.
Those patients with RA overlap with other rheumatic disease, RF negative patients, and pregnant women's or unable to give valid consent were excluded from the study.
Protocol was approved by the Research Ethics Committee of Isfahan University of Medical Sciences. Serum samples were obtained from patients with RA and were aliquoted and stored at –80°C until assayed. Additionally, erythrocyte sedimentation rate (ESR), RF, disease activity score 28 (DAS-28), visual analog scale (VAS) were recorded in patients with RA. Serum antibodies directed to the cyclic citrullinated peptide (anti-CCP) were assessed with a commercial enzyme-linked immunosorbent assay (ELISA), (Euroimmun, Germany) and it was considered as positive if the antibody titer was greater than 5 relative units (RU). These results were analyzed by SPSS software (version 15). We used t-test and Pearson's correlations coefficient. P-values less than 0.05 were regarded as significant.
RESULTS
We evaluate 90 patients with RA (median age, 49.5 years ± 15.5 range, 21–84). Characteristics of the patients with rheumatoid arthritis were summarized in Table 1. Fifteen patients were anti-CCP1 negative (16.7%) and 75 persons were positive (83.3%) . The mean ± SD titer of the anti-CCP1 was 196.3 ± 188.1 RU range (1.4-780 ). Eight (8.9%) patients were in remission. In 3 (3.4 %) patients, DAS-28 was low, in 36 (40%) patients it was medium, and in 43 (47.7%) patients it was high. With using of Pearson's correlation coefficient, we checked the correlation between anti-CCP1 and DAS-28 includes ESR, VAS, swelling joints, and tender joints [Table 2]. There was an intermediate significant correlation between DAS-28, VAS, and tender joints with anti-CCP1 titer (P<0.001). The largest linear correlation was between anti-CCP1 antibody levels and VAS; it means that higher titers of the anti-CCP1 antibody are associated with more painful joints in our patients. There was a meaningful difference in DAS-28 between two groups of anti-CCP1 positive and negative patients 5.07 ± 1.1 and 3.5 ± 1.5 respectively (P<0.001) [Figure 1].
Table 1.
Descriptive characteristic of patients with rheumatoid arthritis (anti-CCP1 positive and negative)
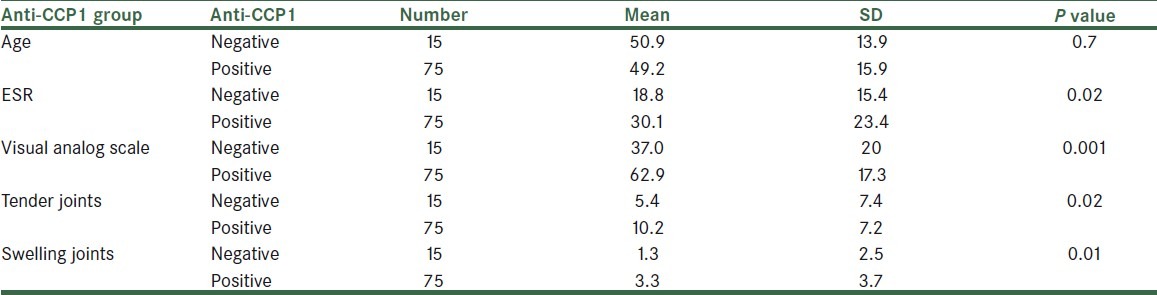
Table 2.
Linear correlation between Anti-CCP1 and disease activity index

Figure 1.
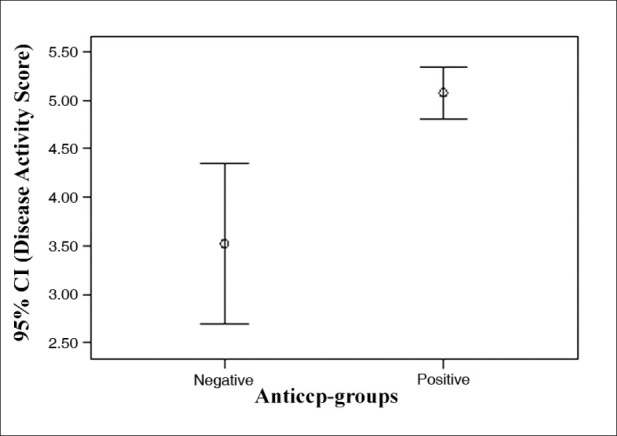
Comparison of mean and 95% confidence interval of the disease activity index in two groups of anti-CCP1 positive and negative patients.
We used multiple regression (Inter model) for predicting DAS-28 as a dependent variable; therefore, we interred some variables like ESR, VAS, tender joints and swelling joints, age and anti-CCP as independent variables to the model. After running the model age and anti-CCP1 were omitted from the final model and the following formula was obtained: DAS-28 = 1.72 + 0.022 ESR + 0.022 VAS + 0.089 tender joints + 0.093 swelling joints. Linear correlation between anti-CCP1 and DAS-28 is depicted in Figure 2.
Figure 2.
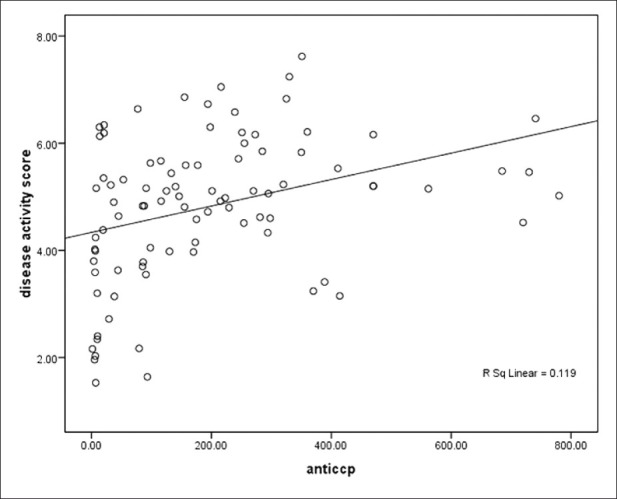
Linear correlation between anti-CCP1 and DAS-28 Y (DAS- 28) = 4.3+ 0.34(anti-CCP).
DISCUSSION
RA is one of the most prevalent autoimmune diseases that affect about 1% of people in the world.[15] Anti-CCP is from IgG class and they have specificity about 97% for RA.[16,17] They become positive in early stages of disease and have high predictive value.[18–20] Patients who are anti-CCP positive have more radiologic destruction and joint erosion than anti-CCP negative patients.[21,22]
In a study by Glasnovic et al. on 211 patients with RA, they evaluated an anti-CCP titer as a predictive factor for erosive changes and disease activity. They evaluate disease activity by DAS-28 and radiological change by Steinbrocker score. In this study, anti-CCP had 100% specificity and 65% sensitivity and so there was an intermediate correlation between anti-CCP and Steinbrocker score. Pearson's coefficient was 0.479 and ranking correlation coefficient of spearman's was 0.614 (P<0.0001). But they didn’t find any significant correlation between anti-CCP and DAS-28.[23] In our study, there was a significant correlation between this two components (r=0.35).
In a study by Papadopoulos et al. on 135 patients with early stage of RA, those patients who had positive anti-CCP had tenderer and swelling joints and so higher DAS-28 (P<0.001).[24] In our study, we found similar results.
In a study by Greiner et al., there wasn’t any significant correlation between specificity and sensitivity of (anti-CCP, IgA, and IgM RF) tests and so there wasn’t any significant correlation between anti-CCP, IgA and IgM, RF with WBC, ESR, and CRP.[25]
Diagram 1 depicts that DAS-28 in anti-CCP1 positive patients was two-fold greater than anti-CCP1 negatives. This means that higher levels of anti-CCP1 titers are associated, with higher DAS-28 (P<0.001).
In our study, we found a significant correlation between anti-CCP1 and disease activity (P<0.001) and so there was high correlation between VAS and disease activity index.
In Table 2, there was the most linear correlation between anti-CCP and VAS; it means that an increase in the titer of anti-CCP can increase patient feeling of pain and patient who had more anti-CCP titer had more feeling of pain than others. In next ranking, tender joints had statistically significant linear correlation with anti-CCP; it means that increased titer of anti-CCP can increase joint tenderness.
The regression model showed opposite results, thus we can’t use anti-CCP as a predictive marker of disease activity.
In general we can find a significant correlation between anti-CCP and disease activity (P<0.001). But this correlation was not enough powerful to use it as a surrogate of disease activity. Instead other factors such as VAS, ESR, and tender joints had more correlation with disease activity.
ACKNOWLEDGMENT
This research has been supported by Isfahan University of medical sciences.
Footnotes
Source of Support: Isfahan University of medical sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–72. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 3.Goodson N. Coronary artery disease and rheumatoid arthritis. Curr Opin Rheumatol. 2002;14:115–20. doi: 10.1097/00002281-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Olsen NJ, Stein CM. New drug for rheumatoid arthritis. N Engl J Med. 2004;350:2167–79. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 5.Eggeland T, Munthe E. The role of the laboratory in rheumatology, rheumatoid factors. Clin Rheum Dis. 1983;9:135–60. [PubMed] [Google Scholar]
- 6.Smolen JS. Autoantibodies in rheumatoid arthritis. In: Van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease, Section C1.1/1–C1.1/18. Dordrecht: Kluwer; 1996. [Google Scholar]
- 7.Thorsteinsson J, Bjfrnsson OJ, Kolbeinsson A, Allander E, Sigfusson N, Olafsson O. A population study of rheumatoid factor in Iceland.A 5 year follow-up of 50 women with rheumatoid factor (RF) Ann Clin Res. 1975;7:183–94. [PubMed] [Google Scholar]
- 8.Van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undiVerentiated arthritis: A prospective cohort study. Arthritis Rheum. 2004;50:709–15. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 9.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic Citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 10.Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting Wve year radiographic damage. Ann Rheum Dis. 2003;62:120–6. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project) Ann Rheum Dis. 2004;63:1085–9. doi: 10.1136/ard.2003.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003;62:870. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 14.Finckh A, Liang MH. Anti-cyclic citrullinated peptide antibodies in the diagnosis of rheumatoid arthritis: Bayes clears the haze. Ann Intern Med. 2007;146:816–7. doi: 10.7326/0003-4819-146-11-200706050-00011. [DOI] [PubMed] [Google Scholar]
- 15.Emery P. The optimal management of early rheumatoid disease: The key to preventing disability. Br J Rheumatol. 1994;33:765–8. doi: 10.1093/rheumatology/33.8.765. [DOI] [PubMed] [Google Scholar]
- 16.Lindqvist E, Eberhardt K, Bendtzen K, Heinegård D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;4:196–201. doi: 10.1136/ard.2003.019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser H. Early diagnosis of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2005;19:55–72. doi: 10.1016/j.berh.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Kaarela K, Kauppi MJ, Lehtinen KE. The value of the ACR 1987 criteria in very early rheumatoid arthritis. Scand J Rheumatol. 1995;24:279–81. doi: 10.3109/03009749509095163. [DOI] [PubMed] [Google Scholar]
- 19.Saraux A, Berthelot JM, Chales G, Le HenaV C, Thorel JB, Hoang S, et al. Ability of the American College of Rheumatology 1987 criteria to predict rheumatoid arthritis in patients with early arthritis and classiWcation of these patients two years later. Arthritis Rheum. 2001;44:2485–91. doi: 10.1002/1529-0131(200111)44:11<2485::aid-art428>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Van Venrooij WJ, Hazes JM, Visser H. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med. 2002;60:383–8. [PubMed] [Google Scholar]
- 21.Kirwan JR, Quilty B. Prognostic criteria in rheumatoid arthritis: Can we predict which patients will require specific antirheumatoid treatment? Clin Exp Rheumatol. 1997;15(Suppl 17):S15–25. [PubMed] [Google Scholar]
- 22.Quinn MA, Gough AK, Green MJ, Devlin J, Hensor EM, Greenstein A, et al. Anti-CCP antibodies measured at disease onset help identify seronegative rheumatoid arthritis and predict radiological and functional outcome. Rheumatology. 2006;45:478–80. doi: 10.1093/rheumatology/kei203. [DOI] [PubMed] [Google Scholar]
- 23.Glasnović M, Bosnjak I, Vcev A, Soldo I, Glasnović-Horvatić E, Soldo-Butković S, et al. Anti-citrullinated antibodies, radiological joint damages and their correlations with disease activity score (DAS28) Coll Antropol. 2007;31:345–8. [PubMed] [Google Scholar]
- 24.Papadopoulos NG, Tsiaousis GZ, Pavlitou-Tsiontsi A, Giannakou A, Galanopoulou VK. Does the Presence of Anti-CCP Auto antibodies and Their Serum Levels Influence the Severity and Activity in Rheumatoid Arthritis Patients? Clin Rev Allergy Immunol. 2008;34:11–5. doi: 10.1007/s12016-007-8018-1. [DOI] [PubMed] [Google Scholar]
- 25.Greiner A, Plischke H, Kellner H, Gruber R. Association of anti-cyclic citrullinated peptide antibodies, anti-citrullin antibodies, and IgM and IgA rheumatoid factors with serological parameters of disease activity in rheumatoid arthritis. Ann N Y Acad Sci. 2005;1050:295–303. doi: 10.1196/annals.1313.031. [DOI] [PubMed] [Google Scholar]


