Abstract
Background:
Epilepsy is a rare neurologic disorder during pregnancy. Despite its rarity, it could cause different clinical problems in this natural phenomenon of a woman's life. The aim of this study was to evaluate and compare the course of pregnancy and labor and their outcome in epileptic and healthy women.
Materials and Methods:
This study was performed during years 2009--2011 in Alzahra and Beheshti hospitals affiliated to Isfahan University of Medical Sciences. A total of 51 pregnant women, who were known cases of epilepsy and were on antiepileptic drugs treatment for at least 3 months, were compared with 47 matched healthy pregnant women without epilepsy. They were followed before and during their pregnancy in several visits and all of their neurologic and obstetric information were collected. For statistical analysis of continuous variables, the t-test was used. The chi-square test was used for dichotomous variables.
Results:
The rate of monotherapy was more than polytheraphy especially during the pregnancy. The epileptic attacks stopped in majority of patients during the pregnancy. Vaginal bleeding (P=0.020) and abortion (P=0.015) were significantly more frequent among epileptic mothers. The gestational age was lower meaningfully (P= 0.010) in epileptic patients’ neonates and the first minute Apgar score was lower in these babies too (P=0.028).
Conclusions:
Antiepileptic drugs could have some unsuitable effects on pregnancy course especially by increasing the rate of abortion, preterm labor, and vaginal bleeding. Their adverse effects on neonates’ health could not be neglected.
Keywords: Anticonvulsants, epilepsy, labor, newborn, pregnancy
INTRODUCTION
Epilepsy is a rare neurologic disorder among pregnant women which is estimated to occur in about 0.3-0.7% of pregnancies.[1,2] Despite its rarity, it could cause different clinical problems in this natural phenomenon of a woman's life. Some congenital disorders have been known to be more frequent in these patients’ newborns and antiepileptic drugs (AEDs) seem to have an important role in this process.[2,3] It seems that the impact of epileptic disorder itself as a causative factor is insignificant.[3–6]
Because of potential teratogenicity of many of the AEDs like valproate,[7] balancing between the advantages and side effects of these class of medications for the mother and her neonate seems to be important during the pregnancy.[4,6,8] Monotherapy has been recognized to be more harmless during pregnancy.[9–11]
Greater part of pregnancies takes place without important complications, but some obstetric problems have been found to be more common during this period. Also because of some effects of pregnancy and labor on seizure control and metabolism of AEDs, the risk of attacks is 10-fold higher during labor and delivery so consulting about pregnancy and management of delivery is essential.[12,13]
Several studies were done before in other countries but as far as we know no study was performed in our country for evaluation of the relation between pregnancy and epilepsy.
So the aim of this study was to evaluate the course of pregnancy and labor and their outcome in epileptic women and to comparison of their situation with control pregnant subjects.
MATERIALS AND METHODS
This was a cohort study which was performed during years 2009--2011 in Beheshti and Alzahra University Hospitals affiliated to Isfahan University of Medical Sciences.
A total of 51 pregnant women, who were known cases of epilepsy and were on antiepileptic drugs treatment for at least 3 months, were chosen as the case group by nonprobable convenient methods. Also 47 matched healthy pregnant women without epilepsy were recruited as the control group. Both of the groups were recruited from general population who referred to neurology and obstetrics clinics.
Pregnancies with the evidence of neonatal chromosomal abnormalities, seizure-free women who were not under antiepileptic drugs treatment, noncooperative patients and patients whose epilepsy was due to brain tumors, stroke, and other gross brain insults were excluded from the study.
After achieving the written informed consent from the subjects, collection of the data was started. All of the collected data were registered in questionnaires and forms.
In the case and control groups the baseline visit was done before the pregnancy by the neurologist and obstetrician. In this visit the demographic data were collected. A complete epilepsy history (in the case group) and gynecologic/obstetric and medical history (in both groups) were taken and all of the subjects were completely examined. The neurologic examination was done more precisely in the case group. The prenatal care was done as much as possible. Information about the AED treatment and frequency and types of seizures and the course of epilepsy were obtained from epileptic women.
The epileptic and the control groups were visited at least four times during pregnancy by the neurologist and, when possible, by the obstetrician in the same day.
According to the predefined protocol, the first control visit was done at the end of the first trimester (11--12 weeks of pregnancy). In this visit data about the treatment and its changes and also the neurologic examination were collected. Also patients’ vital signs and weights were registered.
The second control visit took place in the second trimester (18--20 weeks), including a routine structural ultrasound performed by the obstetrician. The same data as at the first visit were gathered. Fetal heart rate (FHR) and fundal height were assessed too.
The third control visit occurred in the 28th week of gestation, and the fourth, in the 36th week, with an assessment of the mode of delivery. Vital signs, weight, fetal movements, FHR, uterine contraction, and fundal height were evaluated in both of these visits.
Folate substitution was tried to be used in controls and patients who were under the treatment with phenytoin, carbamazepine (CBZ), or valproate (VPA) before conception and during the first trimester systematically. The patients who were under our observation were treated with 5 mg Folate daily from at least 1 month before pregnancy and the subjects who were not under our inspection were advised to begin or continue the mentioned dose as exactly as possible. During the entire study period, all newborns receive intramuscular injections of 1 mg vitamin K at birth to avoid coagulation defects.
Outcomes of pregnancies were collected with a questionnaire during maternity care unit visits. Detailed information of delivery and the health status of the newborn after delivery were evaluated.
The infants were evaluated after delivery by a pediatrician. Information about their gestational age, body weight, head circumference, length, first- and fifth-minute Apgar score, general examination, and neonatal bleeding due to AEDs were assessed and registered.
Premature delivery was defined as delivery before 37th gestational week, and prolonged pregnancy was known as lasting longer than 42 weeks. Small for gestational age newborns were mentioned as the babies below the 10th percentile according to the normal tables of population when adjusted to the gestational age and sex. Infants with birth weight lower than 2500 g were considered to be low birth weight.
For statistical analysis with continuous variables, the t-test was used. The chi-square test was used for dichotomous variables, and Fisher's exact test was used when the expected count is <5. P-values less than 0.05 were considered as significant and 95% confidence intervals were assumed. All of the results were expressed as mean±SD.
RESULTS
In this study, 51 pregnant epileptic women were recruited as the case group and the mean of their ages was 27.01±4.88 years (with a range of 20--41 years) and 47 healthy pregnant women were enrolled as the control group with a mean of 27.42±4.88 years (with a range of 21--45 years).
The comparison of two groups about their demographic features is summarized in Table 1.
Table 1.
The comparison of demographic features between two groups

The epileptic women have suffered from seizure attacks about 10.74±6.27 years. The prevalence of different seizure types was as below:A total of 29 patients (56.9%) generalized tonic clonic seizure, 15 patients (29.4%) complex partial seizure, 4 patients (7.8%) simple partial seizure, 3 patients (5.9%) juvenile myoclonic seizure.
The etiology of seizure attacks in all of the patients was idiopathic.
The patients experienced about 3.71±6.56 attacks per month. The neurologic examination was normal in all of the epileptic subjects.
A total of 24 patients were on polytherapy treatment and 27 subjects were on monotherapy and carbamazepine was used more than the others in the monothrapy group (10 patients). The combination of valproate and carbamazepine was used more in the polytherapy group.
Two patients (3.9%) received no treatment during their pregnancy.
The data about patients’ treatment are summarized in Table 2.
Table 2.
The course of treatment and its changes during four visits in epileptic patients
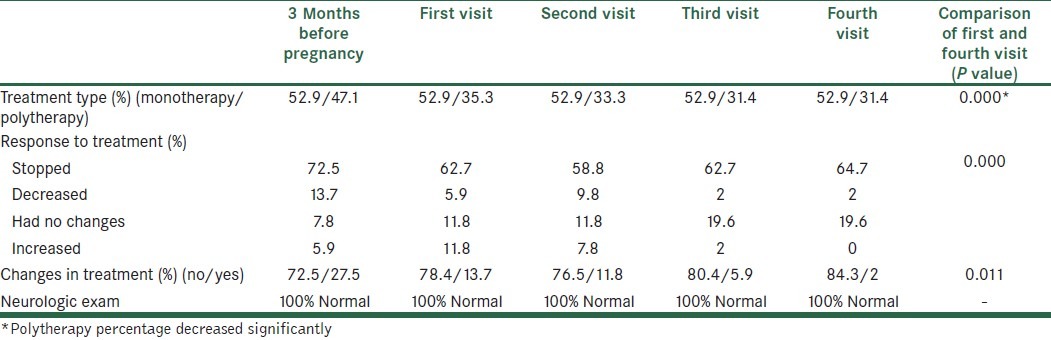
Among these patients 46 used folic acid tablets (27 patients began to use before pregnancy) and the mean of their used doses was 2.64±2.09 mg.
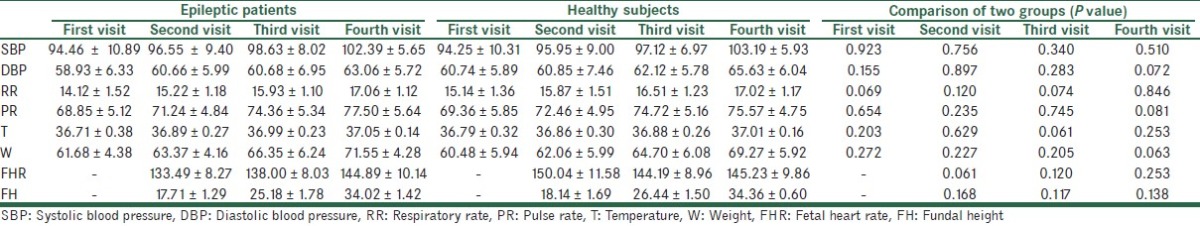
The data which were collected during prenatal examinations in both groups are shown in Table 3. There were no significant differences between these parameters. The fetal movements were normal in all cases and no uterine contraction was detected.
Table 3.
The prenatal examinations data in both groups
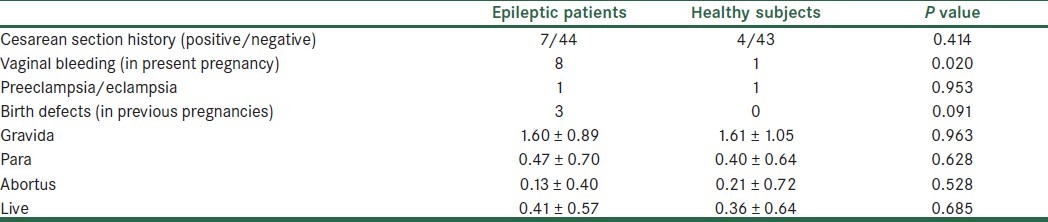
The obstetric past history of groups is summarized in Table 4. The vaginal bleeding rate during their pregnancy was significantly higher in epileptic patients (P=0.020). In the epileptic group one person had a history of placenta previa, one had a history of pre-eclampsia, one had a history of cervical incompetence, and one had a history of low birth weight and in the control group one person had a history of myoma and one had a history of pre-eclampsia.
Table 4.
The obstetric history of groups
About their medical history, in the epileptic group the history was as this: Two had gestational diabetes mellitus (GDM), one had diabetes mellitus, three had hypothyroidism, two had nephrolithiasis, one had deep vein thrombosis, one had pseudo tumor cerebri, and one had toxoplasmosis infection history. In the healthy subjects one person had GDM, one had endometriosis, and one had mitral valve prolapse (MVP).
In the epileptic group, 21 subjects had NVD (normal vaginal delivery), 12 had C/S (caesarean section) without indication, 11 had C/S with indication, 6 had abortion, and 1 had ectopic pregnancy. In the control group, 16 had NVD, 21 had C/S without indication, and 10 had C/S with indication. So there was a significant difference between groups in the abortion rate (P=0.015).
The rate of C/S was higher than NVD among the subjects whether in epileptic or in healthy women but the difference was not significant (P=0.206).
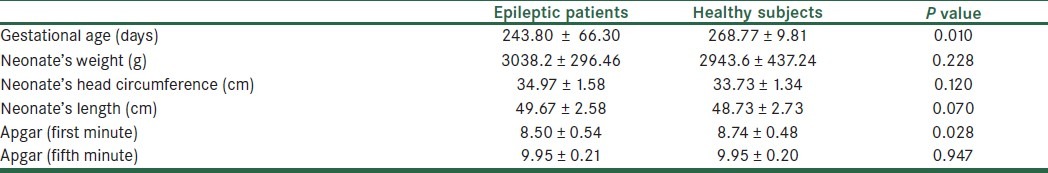
All of the neonates were normal in physical examination and none of them had bleeding due to coagulation defects. Their information is summarized in Table 5.
Table 5.
Comparison of neonates’ features between groups
DISCUSSION
This prospective study was done to evaluate the effect of epilepsy itself and also the AED treatment on pregnancy, delivery, and neonatal status and comparison of their situation with healthy pregnant women as control.
The majority of epileptic disorders are continuing during the patient's life and because of this, long-term or lifelong management with AEDs is frequent.[14] Despite this idea that the complications are common during pregnancy, their rates differ meaningfully between the studies.[15]
Although both of the seizures and AEDs seem to be risk factors for complications during pregnancy, but there are some controversies about their importance. Some studies shown a little role for epileptic attacks,[3–6] while the others reported that uncontrolled tonic--clonic seizures could be harmful for the mother and the fetus than are AEDs.[16]
Some previous studies showed that mothers’ neurological problems took place in about 0.3--0.6% of all pregnancies.[17–19] The outcome of pregnancy is good in majority of gestations, but some studies have reported that the rate of some complications such as hyperemesis gravidarum, preterm labor, pregnancy induced hypertension, preeclampsia, cesarean delivery, placental abruption, and perinatal mortality is higher in these women.[20–22] Also anemia, vaginal bleeding, and stillbirth were thought to be more frequent in these women.[23] In this study the rate of vaginal bleeding was significantly higher in epileptic patients. But the rates of preeclampsia/eclampsia, placenta abruption, cervical incompetence, placenta previa incidence were not significantly different in epileptic women in comparison with healthy subjects.
The same as other studies, the incidence of abortion was significantly higher among our epileptic subjects. According to previous studies this could be due to deficiencies in folate concentration. The cause of this deficiency is using of cytochrome P450 enzyme-inducing antiepileptic drugs,[24] or defects in the absorption due to drugs like phenytoin, carbamazepine, and barbiturates.[25]
Overlay, the rate of C/S have been reported to be higher than the accepted rate (35--40% in comparison to accepted 15%).[26] In this study the rate of C/S was higher than NVD in both groups and in healthy subjects its rate was higher. Our epileptic patients were collected from the general population but a larger part of our healthy controls were recruited from Alzahra and Beheshti hospitals as the referral obstetric hospitals of Isfahan province and the rate of C/S was higher in them. Also because of some different effects of anesthesia, surgery procedure, and using of parenteral AEDs during C/S, in most of cases, it was advised by the neurologist to terminate the pregnancy in the NVD method.
Some of the fetal complications such as fetal death, neonatal hemorrhage, congenital malformations, low birth weight, and childhood epilepsy may be more frequent among these women's children. Birth defects were more common in infants who exposed to AEDs during their mothers’ pregnancy (4--6%) in comparison to infants who did not expose (1--2%) to these drugs.[27] In this study no birth defects were found among the neonates but three of epileptic mothers had a history of birth defects, especially cardiac problems, in their children. Different probable mechanisms have been reported for AED-induced teratogenicity and the most important of them include folic acid antagonism, fetal tissue binding and toxic effects of metabolic products.[28] In this study, the majority of epileptic patients as well as healthy subjects had used folic acid tablets. But the mean of their used doses was lower than the necessary dose.
Unfortunately, some of the patients became pregnant without any previous planning so we were obligated to continue their previous AED (especially valproate). Also the used dose of folic acid was not enough and we advised the right dose at our first visit.
Gestational age was significantly lower in epileptic women's babies in our study so the chance of preterm labor is possibly higher in these women. It could support the previous studies which reported higher rates of preterm labor in these patients.[20–22]
When the first-minute Apgar score is low, it shows that the neonate needs more medical care but is not essentially an indicator for later problems, especially when it improves in the 5th minute assessment[29] so the score is evaluated in both times. The first minute Apgar score was significantly lower in these neonates which may be due to some background problems during pregnancy or some problems in their cardiac, respiratory, and neurologic systems.
Some previous studies showed that monotherapy is more frequent among epileptic pregnant women during pregnancy.[30] In our study, rates of mono and polytherapy methods were less different before beginning of pregnancy but after that monotherapy was more frequent. The most approved advice for these patients is continuing treatment during pregnancy in the monotherapy method and at the lowest dose required to attain seizure control. Polytherapy should not be used where possible for achievement of best outcomes.[31] Congenital problems were reported to be more frequent among patients who used more than one AED.[8,9] So in this study we tried to shift the treatment method to monotherapy as much as possible and to modulate the dose of AEDs.
Also sometimes a hemorrhagic phenomenon occurs in the infants of epileptic mothers and seems to be the result of a deficiency of vitamin K-dependent clotting factors.[28] But in this study none of the neonates experienced this type of hemorrhages.
The epileptic attacks stopped in majority of the epileptic patients in this study and in some of them decreased and it could probably mention the effect of sexual hormones on epilepsy especially high concentrations of them during pregnancy. The effects of sexual steroids on neurons, neuroreceptors and also the psychiatric features as well as their role in mechanism of action of AEDs have been studied and some molecular relationships were reported.[32]
In conclusion, our study showed that antiepileptic drugs could have some unsuitable effects on pregnancy course especially by increasing the rate of abortion, preterm labor, and vaginal bleeding. Their adverse effects on neonates’ health could not be neglected. The best treatment during the pregnancy is monotherapy with the lowest possible dose.
ACKNOWLEDGMENT
We thank Drs. Z. Allameh (Gynecologist) and R. Iranpoor (Pediatrician, Neonatologist) for their assistance and cooperation in patients’ physical examination.
This study was supported by Isfahan University of Medical Sciences with the grant number 388548.
Footnotes
Source of Support: Isfahan University of Medical Sciences with the grant number 388548
Conflict of Interest: None declared.
REFERENCES
- 1.Borthen I, Eide MG, Veiby G, Daltveit AK, Gilhus NE. Complications during pregnancy in women with epilepsy: Population-based cohort study. BJOG. 2009;116:1736–42. doi: 10.1111/j.1471-0528.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 2.Brosh K, Matok I, Sheiner E, Koren G, Wiznitzer A, Gorodischer R, et al. Teratogenic determinants of first-trimester exposure to antiepileptic medications. J Popul Ther Clin Pharmacol. 2011;18:e89–98. [PubMed] [Google Scholar]
- 3.Fried S, Kozer E, Nulman I, Einarson TR, Koren G. Malformation rates in children of women with untreated epilepsy: A meta-analysis. Drug Saf. 2004;27:197–202. doi: 10.2165/00002018-200427030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–8. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 5.Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–8. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- 6.Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: A prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–8. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–83. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meador KJ. Neurodevelopmental effects of antiepileptic drugs. Curr Neurol Neurosci Rep. 2002;2:373–8. doi: 10.1007/s11910-002-0013-6. [DOI] [PubMed] [Google Scholar]
- 10.Montouris G. Importance of monotherapy in women across the reproductive cycle. Neurology. 2007;69(24 Suppl 3):S10–6. doi: 10.1212/01.wnl.0000302371.01359.c6. [DOI] [PubMed] [Google Scholar]
- 11.Pennell PB. The importance of monotherapy in pregnancy. Neurology. 2003;60(11 Suppl 4):S31–8. doi: 10.1212/wnl.60.11_suppl_4.s31. [DOI] [PubMed] [Google Scholar]
- 12.Tomson T, Danielsson BR, Winbladh B. [Epilepsy and pregnancy.Balancing between risks to the mother and child] Lakartidningen. 1997;94:2827–32. 35. [PubMed] [Google Scholar]
- 13.Yerby MS. The use of anticonvulsants during pregnancy. Semin Perinatol. 2001;25:153–8. doi: 10.1053/sper.2001.24900. [DOI] [PubMed] [Google Scholar]
- 14.Steinhoff BJ. Pregnancy, epilepsy, and anticonvulsants. Dialogues Clin Neurosci. 2008;10:63–75. doi: 10.31887/DCNS.2008.10.1/bjsteinhoff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow JI, Craig JJ. Anti-epileptic drugs in pregnancy: Current safety and other issues. Expert Opin Pharmacother. 2003;4:445–56. doi: 10.1517/14656566.4.4.445. [DOI] [PubMed] [Google Scholar]
- 16.Battino D, Tomson T. Management of epilepsy during pregnancy. Drugs. 2007;67:2727–46. doi: 10.2165/00003495-200767180-00007. [DOI] [PubMed] [Google Scholar]
- 17.Harden CL, Sethi NK. Epileptic disorders in pregnancy: An overview. Curr Opin Obstet Gynecol. 2008;20:557–62. doi: 10.1097/GCO.0b013e3283184059. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KB, Ellenberg JH. Maternal seizure disorder, outcome of pregnancy, and neurologic abnormalities in the children. Neurology. 1982;32:1247–54. doi: 10.1212/wnl.32.11.1247. [DOI] [PubMed] [Google Scholar]
- 19.Nulman I, Laslo D, Koren G. Treatment of epilepsy in pregnancy. Drugs. 1999;57:535–44. doi: 10.2165/00003495-199957040-00006. [DOI] [PubMed] [Google Scholar]
- 20.Pschirrer ER, Monga M. Seizure disorders in pregnancy. Obstet Gynecol Clin North Am. 2001;28:601–11. doi: 10.1016/s0889-8545(05)70221-7. vii. [DOI] [PubMed] [Google Scholar]
- 21.Yerby M, Koepsell T, Daling J. Pregnancy complications and outcomes in a cohort of women with epilepsy. Epilepsia. 1985;26:631–5. doi: 10.1111/j.1528-1157.1985.tb05703.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm J, Morris D, Hotham N. Epilepsy and pregnancy--a review of 98 pregnancies. Aust N Z J Obstet Gynaecol. 1990;30:290–5. doi: 10.1111/j.1479-828x.1990.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 23.Yerby MS. Contraception, pregnancy and lactation in women with epilepsy. Baillieres Clin Neurol. 1996;5:887–908. [PubMed] [Google Scholar]
- 24.Kampman MT. Folate status in women of childbearing age with epilepsy. Epilepsy Res. 2007;75:52–6. doi: 10.1016/j.eplepsyres.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Morrell MJ. The new antiepileptic drugs and women: Efficacy, reproductive health, pregnancy, and fetal outcome. Epilepsia. 1996;37(Suppl 6):S34–44. doi: 10.1111/j.1528-1157.1996.tb06037.x. [DOI] [PubMed] [Google Scholar]
- 26.Yazdizadeh B, Nedjat S, Mohammad K, Rashidian A, Changizi N, Majdzadeh R. Cesarean section rate in Iran, multidimensional approaches for behavioral change of providers: A qualitative study. BMC Health Serv Res. 2011;11:159. doi: 10.1186/1472-6963-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meador KJ, Zupanc ML. Neurodevelopmental outcomes of children born to mothers with epilepsy. Cleve Clin J Med. 2004;71(Suppl 2):S38–41. doi: 10.3949/ccjm.71.suppl_2.s38. [DOI] [PubMed] [Google Scholar]
- 28.Yerby MS. Problems and management of the pregnant woman with epilepsy. Epilepsia. 1987;28(Suppl 3):S29–36. doi: 10.1111/j.1528-1157.1987.tb05775.x. [DOI] [PubMed] [Google Scholar]
- 29.Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344:467–71. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- 30.Kulaga S, Sheehy O, Zargarzadeh AH, Moussally K, Bérard A. Antiepileptic drug use during pregnancy: Perinatal outcomes. Seizure. 2011;20:667–72. doi: 10.1016/j.seizure.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Adab N, Tudur SC, Vinten J, Williamson P, Winterbottom J. Common antiepileptic drugs in pregnancy in women with epilepsy. Cochrane Database Syst Rev. 2004;3:CD004848. doi: 10.1002/14651858.CD004848. [DOI] [PubMed] [Google Scholar]
- 32.Pack AM, Reddy DS, Duncan S, Herzog A. Neuroendocrinological aspects of epilepsy: Important issues and trends in future research. Epilepsy Behav. 2011;22:94–102. doi: 10.1016/j.yebeh.2011.02.009. [DOI] [PubMed] [Google Scholar]


