Abstract
The solitary pulmonary nodule (SPN) is frequently seen on chest radiographs and computed tomography (CT). The finding of a SPN usually provokes a flurry of clinical and imaging activity as an SPN in at-risk population is an alert signal of possible lung cancer. The frequency of malignant nodules in a given population is variable and depends on the endemicity of granulomatous disease. The percentage of malignant nodules also rises when dealing with at-risk population. The problem is compounded by the fact that with the present generation of CT scanners, 1–2 mm nodules are discovered in approximately half of the smokers aged 50 years or older scanned. A variety of management approaches are applied in the work-up of SPN often requiring evaluation over a long period of time to establish a benign or malignant diagnosis. Comparison with previous imaging studies and morphologic evaluation of the size, margins, and internal characteristics are usually the first step in the evaluation of these nodules. It is often necessary to use additional imaging techniques and occasionally invasive procedures such a percutaneous needle lung or a surgical biopsy. Until recently, the guidelines for follow-up of indeterminate noncalcified nodules detected on nonscreening CT was a minimum of 2 years. However, during the past few years due to further refinements in CT technology and better understanding of tumor behavior, it has prompted a revision of the guidelines of the follow-up of small indeterminate nodules. These guidelines have been endorsed by the Fleischner Society.
Keywords: Benign lung nodules, CT, lung cancer, PET/CT, pulmonary nodules
INTRODUCTION
A lung nodule has been defined by the Nomenclature Committee of the Fleischner Society as a rounded opacity, well or poorly defined on a conventional radiograph, measuring up to 3 cm in diameter. Further subdivisions of nodules are defined as acinar, which usually measures 5–8 mm in diameter and is presumed to represent consolidation in an acinus. On computed tomography (CT) scan, a nodule appears as a rounded or irregular opacity, well or poorly defined, measuring up to 3 cm in diameter.[1] Solitary pulmonary nodule (SPN) is found incidentally on imaging studies unrelated to the respiratory system in 0.09–0.2% of all chest radiographs. Opacity less than 3 mm is defined as a micronodule. Mimics of pulmonary nodules include pseudonodules, which represent a rib fracture, a skin lesion, a device outside the patient, anatomic variants, or composite areas of increased opacity.[2] SPN is seen more often on CT scans. The overall reported incidence of SPN is 8–51%.[3,4] In one study of CT screening for lung cancer in smokers, 13% of patients had pulmonary nodules larger than 5 mm at baseline.[5] The initial step after discovery of a SPN is to determine its cause and characterize it as definitely benign, equivocal, or definitely malignant on radiologic features. Benign nodules include infectious granulomas and hamartomas, whereas common malignant causes include primary lung cancer, carcinoid tumors, and lung metastases.[6] Radiologic features, such as size, morphology, and rate of growth, help to determine the likelihood of malignancy in a majority of patients.[7–10] Depending on their appearance and radiologic context, certain nodular opacities may be judged sufficiently typical on scanning that follow-up is not warranted [Figure 1].
Figure 1.
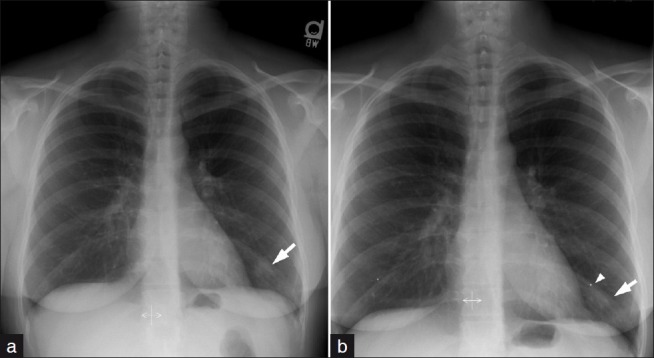
One of the most common SPN mimics are from prominent nipples both in men and women, which may be unilateral due to projection factors. A lateral radiograph is not always helpful. Therefore, application of nipple markers is important to separate a nodule from the nipple shadow. (a) An irregular opacity seen projecting over the left lower lobe was suspected to be related to a nipple shadow. (b) Repeat film after application of nipple markers separated the opacity from the nipple shadow. This opacity completely resolved following a course of antibiotics consistent with pneumonia.
The American College of Physicians[11] guidelines recommend that the assessment be based on nodule size and the patient's risk of cancer.[11] Ost and associates in a Review on Clinical Practice[12] recommended CT follow-up at 3, 6, 12, 18, and 24 months for all “low-probability” indeterminate nodules, regardless of size. The recommendations from the American College of Physicians[11] for management of indeterminate solitary nodules were similar, with the proposal for 3-, 6-, 12-, and 24-month CT follow-up intervals, also without any specified lower size limit for a nodule that would qualify for this protocol.[11] The issue of small incidental nodules detected on CT has not been addressed. However, monitoring an incidentally found nodule might lead to better outcomes by detecting early cancers.[13] The ACCP does not endorse screening for lung cancer for the general population including smokers as it does not prevent mortality.[14] The ACCP in their guidelines address the issue of risk assessment, the choice of imaging, and the frequency of follow-up.
DISCUSSION
Nodule characterization is the first important step to determine whether the lesion is benign or malignant. Features, such as size, morphology, and rate of growth, often help to determine the likelihood of malignancy.[6–9] Common benign causes of benign lung nodules include infectious granulomas and hamartomas, whereas common malignant causes include primary lung cancer, carcinoid tumors, and lung metastases.[6]
The nodule: Risk assessment
The risk of lung cancer in male smokers is 10 times that of nonsmokers and 15–35 times greater in heavy smokers.[15,16] Other risk factors for lung cancer are known. A lung cancer susceptibility gene has been described recently.[17,18] Exposure to asbestos, uranium, and radon are other known lung cancer risks.[19–21]
Several mathematical models have been formulated to estimate the odds of malignancy in SPNs, based on patient age, smoking habits,; history of primary cancer, and nodule morphology and location. One such the Mayo Clinic model is based on a history of extrathoracic cancer, spiculated morphology, current or past smoking, location in an upper lung, increased nodule diameter, and increased patient age.[22] Another model is based on data derived from the Veterans Affairs system for nodules larger than 7 mm in diameter and is based on: smoking history, patient age, nodule size, and time since quitting smoking.[22,23] Another study specifies the age and suggests that patients older than 40 years are associated with an increased risk of lung cancer.[24]
Only 1% of SPN smaller than 5 mm in patients with no history of cancer show a malignant potential on a follow-up of 2 or more years.[3,13,25]
Despite this available data, follow-up of small pulmonary nodules undergo multiple imaging procedures over a 2-year period as guided by American College of Chest Physicians that issued guidelines in 2003.[11]
Based on earlier screening studies of general population using conventional radiology, only 3–6% of SPNs proved to be malignant, while in patients referred for nodule evaluation the lung prevalence rate was 70%.[26,27]
Sone et al[28] evaluated a semi-automated volumetric method involving measurement by CT stratum and found it promising for evaluation of lung tumor progress and aggressiveness.
Calcification within a Nodule [Figures 2 and 3]
Figure 2.
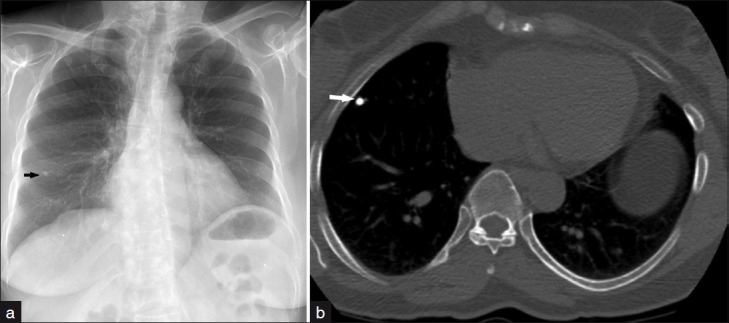
(a) Frontal chest radiograph demonstrates a 5-mm dense pulmonary nodule projecting in the right mid lung zone. (b) Axial CT image at the level of the heart displayed in bone settings reveals a calcified pulmonary nodule in the right middle lobe consistent with calcified granuloma.
Figure 3.
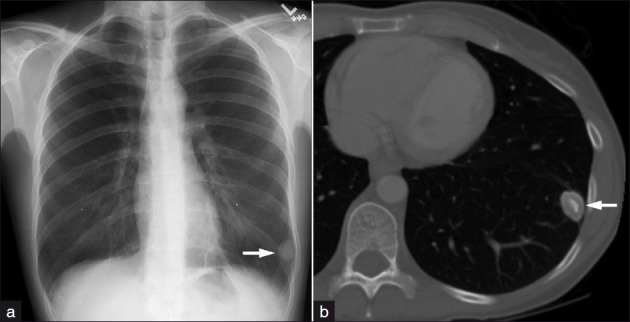
(a) Frontal chest radiograph demonstrates a sharply demarcated left lower lobe nodule. (b) Axial CT image at the level of the heart displayed in bone settings shows the well-defined nodule with central calcification and peripheral hypodensity consistent with pulmonary hamartoma.
Calcification in an SPN on imaging indicates a high probability that the lesion is benign. Six different patterns are known: (1) central dense nidus (Figure 3), (2) diffuse solid, (3) laminated, (4) popcorn, (5) punctuate, and (6) dendriform. The first three types have been described with granulomatous disease. Popcorn-like calcification typically occurs in a lung hamartoma. Diffuse, central, laminated, or popcorn calcifications are considered benign and usually seen in granulomas and pulmonary hamartomas. All other patterns of calcification should not be regarded as a sign of benignity. Calcification has been described in primary central lung carcinoid, metastasis, and a primary bronchogenic carcinoma. Radiological demonstration of calcification in primary lung cancers is rare; however, the widespread use of CT in lung imaging has increased the sensitivity of detecting calcification in malignant tumors. Amorphous, punctate, and reticular patterns of calcification have been described in lung cancer. Calcification in malignant tumors may result from a tumor engulfing a preexisting granuloma, or tumor necrosis causing dystrophic calcification. Calcification in a mucinous adenocarcinoma may occur as a primary phenomenon. In malignant SPN, calcification appears in the form of larger lesions and is usually stippled or eccentric. To classify calcification in a benign SPN. certain criteria need to be fulfilled. Benign calcification should encompass over 10% of the SPN and calcification should be central, diffuse, popcorn type. or laminated.[29–34]
Fat within a nodule [Figure 4]
Figure 4.
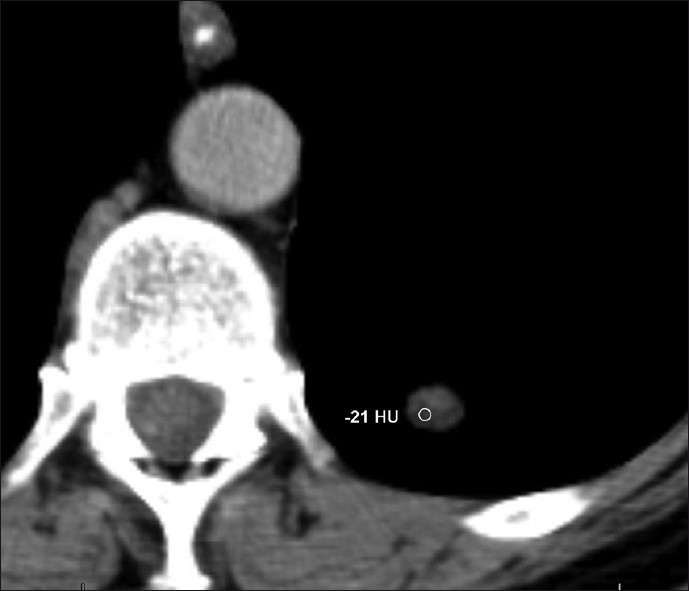
Axial CT images at the level of the left lung base demonstrates a well-defined 9-mm nodule with fat density (-21 HU) consistent with pulmonary hamartoma
The presence of fat within a SPN is a reliable sign of benignity.[35] Fat-containing lung lesions include pulmonary hamartoma, lipoid pneumonia, and lipoma. Endobronchial pulmonary hamartoma usually appears at CT as a lesion with a smooth edge, focal collections of fat that alternate with foci of calcification. An air cleft on the side or the inside is characteristic of pulmonary hamartoma. Pulmonary artery branches connect beyond half of pulmonary hamartoma. This finding suggests close relations in the bronchus along the artery. It is important that there is no connection of the pulmonary vein, to differentiate it from a bronchogenic carcinoma.[36,37] Magnetic resonance imaging may further characterize a discrete pulmonary nodule that demonstrates neither fat nor calcification on CT, in detecting a typical cleft-like structure in a pulmonary hamartoma providing further diagnostic confidence.[36,37] The majority of pulmonary hamartomas present an SPN. Peripheral tumors are usually simply observed after the definitive diagnosis; central tumors may be excised.[38–42] About 20% of (mainly large size) pulmonary hamartomas have uptake characteristics suggesting malignancy on PET/CT.[43]
Nodule size
Wahidi et al[10] analyzed seven studies comparing nodule size and frequency of malignancy. The study revealed that SPN with a diameter of less than 5 mm, 5–10 mm, and greater than 2 cm had malignancy rates of less than 1%, 6–28%, and 64–82%, respectively.
Nodule growth rate
A standard technique to assess growth rate in SPN is to compare the nodule with a previous radiograph or CT. Growth rate is calculated in terms of “doubling time,” which refers to doubling of tumor volume. Bronchial carcinomas generally have a doubling time of 1 and 18 months.[44] However, Schultz and associates[45] believe that there is substantial variability in experts’ beliefs about the natural history of untreated, malignant SPN. Different beliefs may be partly responsible for variation in management practices. Doubling times less than a month may indicate, infection, infarction, or a lymphoma or fast growing metastases.[46–49] Conversely, growth rates over 18 months usually indicate benign processes. Quint and associated[50] also showed a substantial range in lung cancer doubling times, and different volume determination methods gave considerably different doubling times. Absence of detectable growth over a 2-year period has been widely accepted as a sign of benignity. This belief is based on the work carried out in the 50s.[51–53] This concept has challenged more recently[54] on the basis that the data from the original work by Good et al had predictive value for benignity of 65%. Therefore, some investigators will follow-up an SPN beyond 2 years when previous imaging is not available. Sone et al[28] evaluated a semi-automated volumetric method involving measurement by CT stratum and found it promising for evaluation of lung tumor progress and aggressiveness.
Nodule location
Most primary malignant nodules are located in the upper lobes, especially on the right, while two thirds of metastatic pulmonary nodules affect the lower lobes.[55] Sixty per cent of lung nodules are placed in the periphery of the lung most in subpleural location.[55,56] The latter finding tends not to be so useful in the differential diagnosis as granulomas and intrapulmonary lymph nodes also have predilection for a subpleural location.[57]
Nodule margins [Figure 5]
Figure 5.
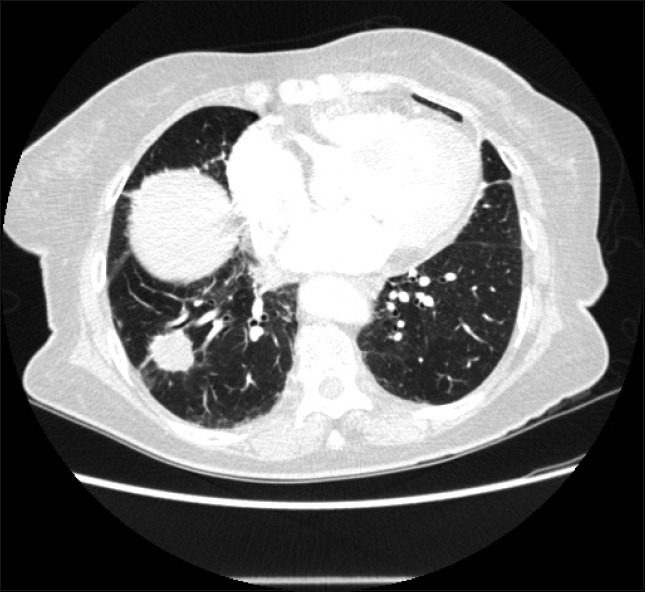
Axial CT image at the level of the right base reveals a spiculated right lower lobe nodule suggesting a malignant neoplasm. A CT-guided biopsy confirmed the diagnosis of primary bronchogenic carcinoma.
Zwirewich and associates[58] classified lung nodules according to the contours of the nodules and subdivided them into (1) sharp and smooth, (2) moderately smooth, (3) undulated borders or minimal spiculation, and (4) gross marginal spiculation. Benign nodules usually show a well-defined smooth edge. However, over a third of malignant nodules and lung metastases show similar characteristics.[59] Most malignant nodules present with irregular and spiculated and ill-defined margins.[59] However, the usefulness of reliance on marginal irregularity as limited as benign nodules may occasionally present with speculation. Pathologically, spiculation is related to desmoplastic reaction. Spiculation may also be the result of infiltration of interstitial planes and lymphatics by tumor. Pleural tags seen in association with malignant nodules have similar connotations. Unfortunately, neither spiculation of tumor margins or pleural tags are specific findings with malignant nodules and are seen with benign lesions[60]
Nodule cavitation [Figure 6]
Figure 6.
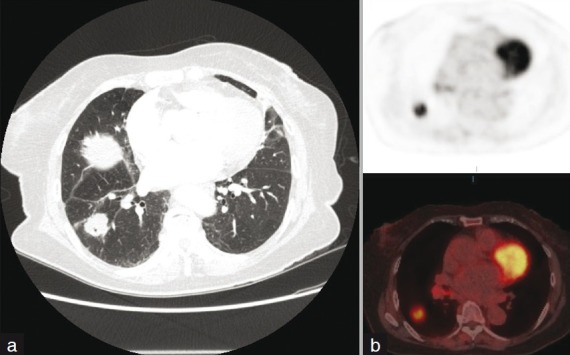
(a) Axial CT image at the level of the right lung base demonstrates a spiculated right lower lobe nodule with a peripheral cavity. (b) Corresponding 18-F FDG PET/fused PET CT image demonstrates intense FDG uptake in the nodule in keeping with bronchogenic carcinoma.
The most important factor in the assessment of cavitation within a lung mass is the wall thickness. A study by Naidich et al[61] found that all lesions with a wall thickness of 1 mm were benign. Among tumors with wall thickness between 5 and 15 mm, 51% were benign, whereas with wall thickness over 15 mm, 95% were malignant. The conclusions of another earlier study indicated that although thin wall cavities were always benign, thick wall cavities were usually indeterminate.[62]
Cavitation within a lung tumor is not specific for bronchial neoplasms. Görich J et al[63] examined 100 cavitating lung masses by CT with histopathological correlation. Three pathological subsets were identified: bronchial carcinomas, lung metastases, and benign lesions. The incidence of cavitation was significantly higher in malignant tumors. Cavitation in malignant tumors was thick walled and often associated with radiation of tumor tissue, mediastinal lymphadenopathy, ipsilateral displacement of the mediastinum, and intrapulmonary satellite foci and infiltration of the thoracic wall. Primary tumors were distinguished from metastases by their ill-defined outer contours. Eighty per cent of cavitating lung tumors were squamous cell carcinomas, the remaining 20% consisted of adenocarcinomas and large cell carcinomas. Small cell carcinomas practically never show cavitation.[63]
Miura H et al[64] studied 47 resected lung adenocarcinomas and found cavitation in 7 cases (14.9%). The tumor size varied from less than 3 cm in diameter to more than 5 cm. Some cavities were multiple. Histological examination revealed four types of cavities: (1) central necrosis due to suspected central ischemia; (2) the inner wall of the cavity was lined by viable cancer cells without necrosis presumably secondary to detachment of the central portion of a papillary growth tumor without necrosis; (3) the inner wall was composed of cancer cells and bronchus, probably caused by ectatic change of peripheral bronchi following tumor invasion to more central bronchi; and (4) the alveolar expansion type where the inner wall was composed of cancer cells and alveoli. Where the cause was presumed to be detachment of destroyed alveoli or invasion along the wall of cavities, a honeycomb lung was suspected as a possible cause. Cavities can occur in small adenocarcinomas. The prognosis of the central necrosis type is poor, suggesting rapid tumor growth.
CT densitometry
High-density SPNs demonstrated on CT, which appeared noncalcified on conventional tomography, are considered benign. Siegelman et al[65] suggested 164 Hounsfield units as threshold above which the nodules are considered benign. It was presumed that diffuse calcification likely accounts for the higher CT numbers of some benign lesions.
However, the usefulness of quantitative CT measurement has been questioned. Zerhouni et al[66] conducted a series of experiments on six different scanners to study the factors that affect the applicability of this technique. In their study, the type of reconstruction algorithm, the design of the CT system, the true slice thickness, and the beam kilovoltage were factors that produced large variations in the CT numbers of SPNs, preventing direct comparison of results from scanner to scanner. However, they suggested that a better standardization of technique may produce more consistent results.
Xu DM et al[67] retrospectively evaluated 372 SPNs to investigate whether baseline nodule density or changes in density or nodule features could be used to discriminate between benign and malignant solid indeterminate nodules. They concluded that baseline nodule density and changes in nodule features cannot be used to discriminate between benign and malignant solid indeterminate pulmonary nodules, but an increase in density is suggestive for malignancy and required a shorter follow-up or a biopsy.
Nodule attenuation
SPNs may be homogeneously solid or ground-glass or heterogeneously solid or ground-glass density. A lesion is said to be solid when it completely obscures the underlying lung parenchyma. Nonsolid nodules (ground-glass nodules) have lung parenchyma visible through them.[68,69]
In the Early Lung Cancer Action Project, both solid and part solid nodules were seen in patients at both baseline and repeat CT screening for lung cancer. A part-solid, nodule is more likely to be malignant than a solid one, even when nodule size is taken into account.[68]
Contrast nodule enhancement
Contrast enhancement in a SPN is an indicator of malignancy and vascularity. Malignant neoplasms enhance (median, 40.0 HU; range, 20 to 108 HU) significantly more than granulomas and benign neoplasms (median, 12.0 HU; range, -4 to 58 HU). The degree of enhancement is significantly related to the amount of central vascular staining.[70,71] Less than 15 HU after contrast injection as criteria for benignity has a sensitivity of 98%, specificity of 58%, and accuracy of 77%.[72]
Yi CA et al[73] studied 131 patients with SPNs to evaluate enhancement dynamics of SPNs at multidetector row CT and to correlate results with extent of tumor angiogenesis in pathologic specimens. Their study revealed that dynamic enhancement with multidetector row CT shows high sensitivity and negative predictive values for diagnosis of malignant nodules but low specificity because of highly enhancing benign nodules. They showed that the extent of enhancement reflected the underlying angiogenesis, which is considered an indicator of malignancy in SPNs.
Jeong et al[74] studied 107 patients with SPNs by analyzing combined wash-in and wash-out characteristics at dynamic contrast-enhanced multidetector row CT showed 92% accuracy for distinguishing benign nodules from malignant nodules
Bayraktaroglu et al[75] drew a similar conclusion in their study of 45 SPNs where there was a significantly greater enhancement in malignant nodules than in benign ones. Lung nodule enhancement of 15 HU or less strongly indicates benignity.
Jiang and associates[76] examined dynamic enhancement pattern in the differential diagnosis of SPNs. The study involved 50 pathologically and 1 clinically confirmed patients with SPNs (diameter £ 4 cm) undergoing multidetector row CT. The study revealed that net enhancement value is an important indicator for differential diagnosis of malignant and benign SPNs. When net enhancement of 20 HU was set as the cutoff point to differentiate malignant nodules from benign ones, the sensitivity, specificity, positive predictive value, negative predictive value and accuracy were 96.43%, 69.57%, 79.41%, 94.12%, and 84.31%, respectively. The study also showed that peak enhancement and net enhancement values are positively correlated with the expression of vascular endothelial growth and microvessel density, both of which reflect the extent of angiogenesis in SPNs to some extent.
Magnetic resonance imaging contrast enhancement
Dynamic contrast-enhanced magnetic resonance imaging (MRI) scanning has been used in the assessment of a SPN. It has been shown that a malignant SPN depicts a much higher enhancement associated with a higher maximum peak and a faster slope than the SPN, while a significant wash-out was seen only in malignant SPNs. In one study, a sensitivity of 100% was recorded with a combination of curve profiles and morphological enhancement patterns.[77]
A further study has shown that when using enhancement curves, malignant and active inflammatory nodules can be distinguished with a sensitivity of 93% and a specificity of 100%.[78]
Role of 18-FDG PET [Figures 6–9]
Figure 9.
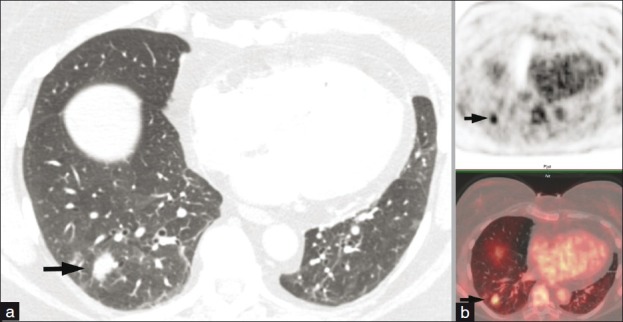
(a) Axial CT image displayed in lung settings reveals an irregular pulmonary nodule in the right lower lobe. (b) Corresponding 18-FDG PET CT images show increased FDG uptake with the nodule exceeding the background with SUVmax of 4.5 as compared to the mediastinal blood pool activity average of 2. Surgical pathology confirmed the diagnosis of organizing pneumonia with no evidence of malignant neoplasm.
Figure 7.
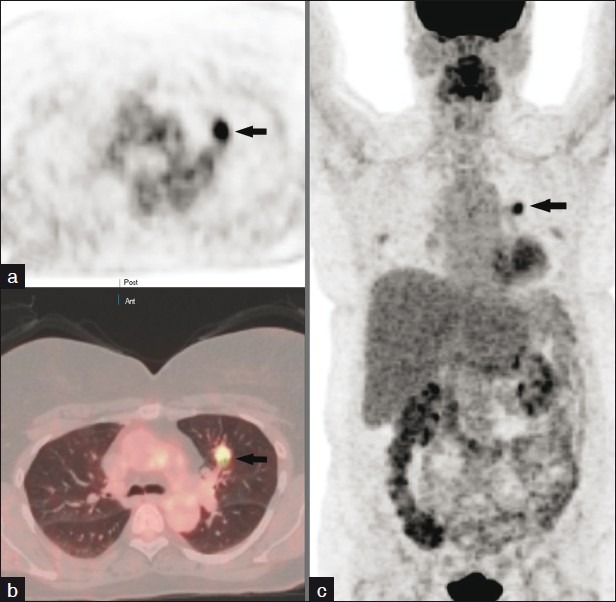
Axial 18-F FDG PET image (a) and corresponding PET-CT image (b) demonstrate an intensely hypermetabolic left upper lobe nodule with SUVmax of 7.4 as compared to the mediastinal blood pool activity average of 1.9 in keeping with malignant neoplasm. (c) No evidence of nodal or distant metastasis as displayed on the maximum intensity projection of the PET images.
Figure 8.
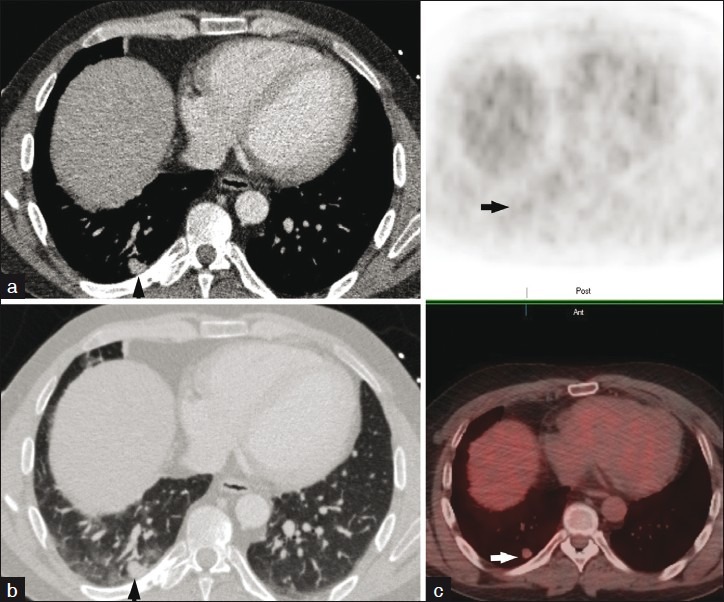
Axial CT images at the level of the right lower lobe displayed in mediastinal (a) and lung (b) windows demonstrate an approximately 1 cm pleural based right lower lobe pulmonary nodule. 18-F FDG PET/fused PET-CT images (c) reveal minimal uptake within the nodule not exceeding the background suggesting a benign etiology. Surgical pathology confirmed the diagnosis of carcinoid tumor.
Tumors grow, multiply, divide, and invade surrounding tissues and metastasize. Glucose provides the basic energy source for both normal and abnormal cells. F-18 Deoxy-2-Fluoro-D-Glucose analogue when administered to a patient would follow a similar metabolic pathway to glucose. Since cancer cells have a higher metabolic rate than normal body cells, cancer would show enhanced uptake and detected by 18 FDG PET imaging. 18 FDG PET has a sensitivity of 96%, specificity of 78%, and accuracy of 92%. SPNs are assessed by either visual method or SUV measurement; both are comparable and have similar accuracy. However, all that glows is not cancer and false positive may occur with lung infections, sarcoidosis, talc pleurodehesis, postirradiation pneumonitis, and postsurgical inflammation. False-negative examinations may occur with bronchoalveolar carcinoma, adenocarcinoma, and carcinoids. Other limitations include negative studies in SPNs close to the diaphragm and adjacent to the normally hot myocardium.
Christensen et al have shown that PET has a much higher specificity and only a slightly reduced sensitivity and is preferable to CT in evaluating indeterminate pulmonary nodules. However, CT remains useful and is usually the first choice because of the high NPV, more widespread availability higher patient through put, and lower cost.[79]
The positive bronchus sign
A positive bronchus sign is a CT concept where a hypoattenuating tube (bronchus) leads directly to a lung nodule. The hypoattenuating tube may extend into the tumor. Although the sign is not specific for a malignant lesion, its presence indicates that a high yield would be obtained by a transbroncialbiopsy (TBB). Gaeta and associates[81] suggest that thin-section CT should be used routinely to evaluate SPNs, and TBB should be attempted when a third- to fifth-order bronchus sign can be seen. Conversely, they suggest that if it is not advisable to perform TBB in patients with absence of bronchus sign, transthoracic biopsy should be considered as a first diagnostic approach.[80–84]
Bubble lucencies
Bubble-like lucencies/low attenuation areas are often seen in malignant SPN, especially in bronchoalveolar carcinomas. These lucencies may represent either patent bronchi or cystic glandular spaces formed by the tumor.[83]
The halo sign
The CT halo sign indicates ground-glass attenuation surrounding a pulmonary nodule.[85–87] The halo of ground-glass attenuation pathologically represents pulmonary hemorrhage, tumor infiltration, or nonhemorrhagic inflammatory processes.[88] When identified in an immune compromised patient, the halo sign has proved invasive pulmonary aspergillosis.[89] Initially, the sign was regarded as a specific sign of invasive pulmonary aspergillosis, but it has a wider differential diagnosis and can be caused by a variety of other conditions such as infection, neoplastic, and inflammatory diseases. Hemorrhagic pulmonary nodules may occur due to number of causes: (1) infective etiology such as mucormycosis, candidiasis, tuberculosis, viral pneumonia, and invasive aspergillosis and (2) noninfectious origin such as Wegener granulomatosis, Kaposi sarcoma, bronchioloalveolar carcinoma, lymphoma, and metastasis with intra-alveolar tumor growth. A halo sign has also been described with nonhemorrhagic lesions such as sarcoidosis and organizing pneumonia. Diagnosis must therefore be based on careful consideration of all the CT chest findings within the context of the patient's clinical state.[90,91]
The halo sign can be used to differentiate hemorrhagic from nonhemorrhagic pulmonary nodules. Most hemorrhagic pulmonary nodules have a characteristic CT appearance consisting of a central area of soft-tissue attenuation with a surrounding halo of ground-glass attenuation that allows distinction from nonhemorrhagic nodules.[87] Despite the nonspecific nature of the sign, the sign is important because the clinical setting and associated radiological features may give a clue to the diagnosis.[88]
The feeding vessel sign
A feeding vessel sign can sometimes be identified where a branch of pulmonary artery is seen leading into a center of a SPN. Two studies have shown that this sign is frequently associated with metastatic nodules.[92,93] However, this finding has not been substantiated using HRCT.[94,95] Nevertheless, when this sign is encountered it is helpful in distinguishing hematogenous and vascular causes such as metastasis and septic emboli.[91,96,97]
Algorithm for management [Algorithm 1]
Algorithm 1.
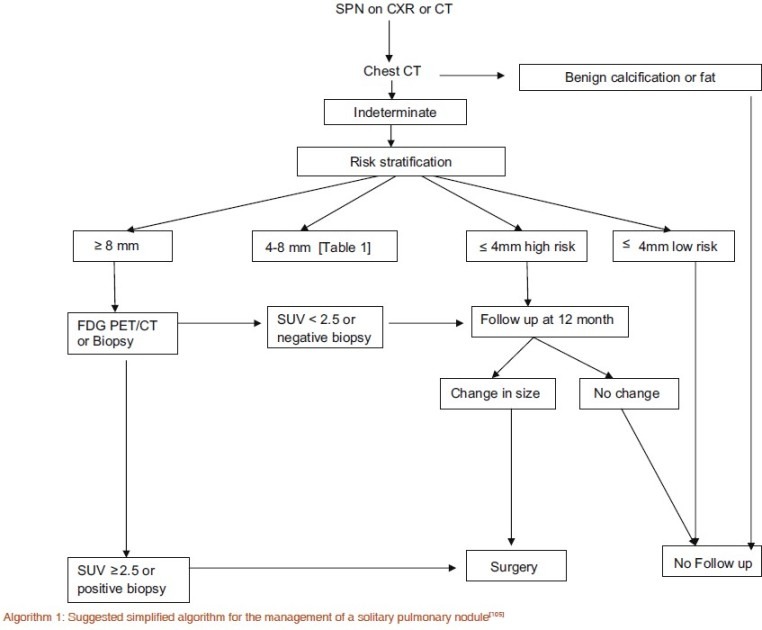
Suggested simplified algorithm for the management of a solitary pulmonary nodule[105]
The management of a SPN begins with evaluation of the patient's history and risk assessment followed by morphological review of the SPN. Depending on the appearance and radiologic context, certain SPNs are judged sufficiently typical of benign masses that follow-up is not warranted. Important factors that suggest benignity is the presence of characteristic calcification, the presence of fat with the SPN, the size, rate of growth certain characteristics such as SPN margins, and ancillary signs described above.[98] CT evaluation is the imaging of choice but in selected cases where the nodule is indeterminate MRI and 18-FDG PET may aid diagnosis. As 99% smaller than 4 mm are benign, CT follow-up should be pursued in selected cases.[99] Henschke et al their paper conclude that in modern CT screening for lung cancer at baseline, detected noncalcified nodules smaller than 5.0 mm in diameter do not justify immediate work-up but only annual repeat screening to determine whether interim growth has occurred.[3] It should also be borne in mind that there is no conclusive evidence, that serial CT studies with early intervention for detected cancers can reduce disease-specific mortality, even in high-risk patients.[3] Therefore, the Fleischner society does not recommend follow-up CT for every small indeterminate nodule.[100]
MacMahon et al in a statement from the Fleischner society (2005) recommend altering the existing practice of four to five follow-up CT scans of all indeterminate SPNs, regardless of size and morphology, before being designated benign and the patient being reassured.[11,12] This recommendation is primarily based on the work of Swensen[101] on an on-going MDCT screening program. Swensen's finding shows that approximately 50% of smokers over the age of 50 years show at least one SPN on an initial scan, while 10% of the screened develop new nodules over a 1-year period, and in additional 12% show nodules that were missed on the initial scan. Taking these findings into account, it is expected that the strict application of existing recommendations would result in multiple follow-up studies over 2 or more years for a large proportion of all patients who undergo thoracic CT.
In SPNs 8 mm or larger, additional imaging with 18FDG PET, percutaneous needle biopsy, and thoracoscopic resection can be considered.[101–104]
The Fleischner society recommendations apply only to adults with an incidental SPN unrelated to known underlying disease. These recommendations do not apply to a number of clinical situations, which include the following:[105]
Patients with known primary cancer known to metastasize to lungs.
Patient younger than 35 years because of the risks from radiation exposure. Fleischner society guidelines recommend that, unless there is a known primary cancer, multiple follow-up CT studies for small incidentally detected nodules should be not be instituted. In such cases, a single low-dose follow-up CT scan in 6–12 months should be considered.
Patients with clinical signs of infection such as the immune compromised, the presence of a nodule may indicate active infection, and short-term imaging follow-up or intervention may be appropriate.
Comparison with previous imaging if available may serve to demonstrate either stability or interval growth of the SPN.
When a follow-up of an SPN is the only clinical indication for CT, then a low-dose, thin-section, unenhanced technique should be used, with limited longitudinal coverage.
CONCLUSION
The management of SPNs involves both clinical and imaging assessment including risk assessment and morphology of the nodule. Emphasis should be placed on accurate diagnosis using the least possible resources avoiding surgical intervention where possible and the judicious use of biopsy procedures. Full use of newer techniques should play a part where available. Flow charts are for guidance only and one should remember that there are always exceptions as illustrated with several examples above. As a guide see flow chart and Table 1.
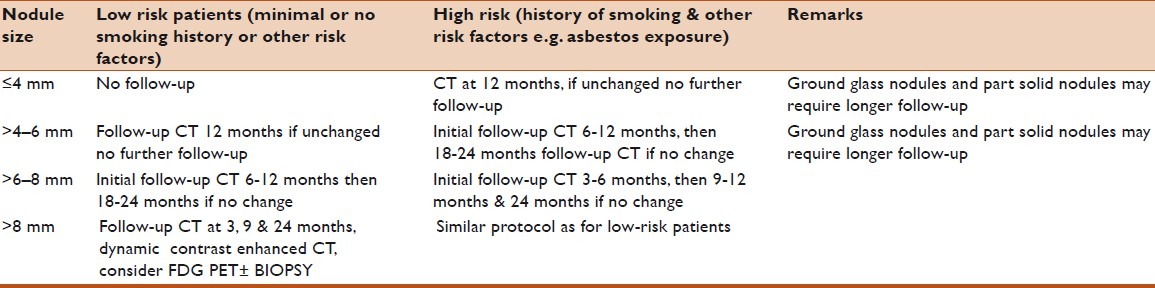
Table 1.
Fleischner Society recommendations for follow-up and management of nodules detected incidentally at nonscreening CT
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 2.Holin SN, Dwork RE, Glaser S, Rikli AE, Stocklen JB. Solitary pulmonary nodules found in a community-wide chest roentgenographic survey; a five-year follow-up study. Am Rev Tuberc. 1959;79:427–39. doi: 10.1164/artpd.1959.79.4.427. [DOI] [PubMed] [Google Scholar]
- 3.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sloan JA, Sykes AM, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226:756–61. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 4.Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P Writing Committee, Lung Screening Study Research Group. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest. 2004;12:114–21. doi: 10.1378/chest.126.1.114. [DOI] [PubMed] [Google Scholar]
- 5.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–71. doi: 10.1056/NEJMoa060476. International Early Lung Cancer Action Program Investigators. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines 2nd ed. Chest. 2007;132(3 suppl):108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 7.Midthun DE, Swensen SJ, Jett JR. Approach to the solitary pulmonary nodule. Mayo Clin Proc. 1993;68:378–85. doi: 10.1016/s0025-6196(12)60136-0. [DOI] [PubMed] [Google Scholar]
- 8.Takashima S, Sone S, Li F, Maruyama Y, Hasegawa M, Matsushita T, et al. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: Reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol. 2003;180:955–64. doi: 10.2214/ajr.180.4.1800955. [DOI] [PubMed] [Google Scholar]
- 9.Tozaki M, Ichiba N, Fukuda K. Dynamic magnetic resonance imaging of solitary pulmonary nodules: utility of kinetic patterns in differential diagnosis. J Comput Assist Tomogr. 2005;29:13–9. doi: 10.1097/01.rct.0000153287.79730.9b. [DOI] [PubMed] [Google Scholar]
- 10.Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. American College of Chest Physicians. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines 2nd ed. Chest. 2007;132(3 suppl):94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 11.Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. American College of Chest Physicians. The solitary pulmonary nodule. Chest. 2003;123(1 Suppl):89S–96S. doi: 10.1378/chest.123.1_suppl.89s. [DOI] [PubMed] [Google Scholar]
- 12.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348:2535–42. doi: 10.1056/NEJMcp012290. [DOI] [PubMed] [Google Scholar]
- 13.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 14.Henschke CI, Naidich DP, Yankelevitz DF, McGuinness G, McCauley DI, Smith JP, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer. 2001;92:153–9. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1982. U.S. Department of Health and Human Services. [Google Scholar]
- 16.Guyatt GH, Newhouse MT. Are active and passive smoking harmful? Determination causation. Chest. 1985;88:445–1. doi: 10.1378/chest.88.3.445. [DOI] [PubMed] [Google Scholar]
- 17.Mayne ST, Buenconsejo J, Janerich D. Familial cancer history and lung cancer risk in United States nonsmoking men and women. Cancer Epidemiol Biomarkers Prev. 1999;8:1065–9. [PubMed] [Google Scholar]
- 18.Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, et al. A major lung cancer susceptibility locus maps to chromosome 6q2325. Am J Hum Genet. 2004;75:460–74. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb LS, Husen LA. Lung cancer among Navajo uranium miners. Chest. 1982;81:449–52. doi: 10.1378/chest.81.4.449. [DOI] [PubMed] [Google Scholar]
- 20.Field RW, Steck DJ, Smith BJ, Brus CP, Fisher EL, Neuberger JS, et al. Residential radon gas exposure and lung cancer: the Iowa Radon Lung Cancer Study. Am J Epidemiol. 2000;151:1091–102. doi: 10.1093/oxfordjournals.aje.a010153. [DOI] [PubMed] [Google Scholar]
- 21.Lee PN. Relation between exposure to asbestos and smoking jointly and the risk of lung cancer. Occup Environ Med. 2001;58:145–53. doi: 10.1136/oem.58.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revel MP, Lefort C, Bissery A, Bienvenu M, Aycard L, Chatellier G, et al. Pulmonary nodules: preliminary experience with three-dimensional evaluation. Radiology. 2004;231:459–66. doi: 10.1148/radiol.2312030241. [DOI] [PubMed] [Google Scholar]
- 23.Revel MP, Bissery A, Bienvenu M, Aycard L, Lefort C, Frija G. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology. 2004;231:453–8. doi: 10.1148/radiol.2312030167. [DOI] [PubMed] [Google Scholar]
- 24.Yankelevitz DF, Gupta R, Zhao B, Henschke CI. Small pulmonary nodules: evaluation with repeat CT—preliminary experience. Radiology. 1999;212:561–6. doi: 10.1148/radiology.212.2.r99au33561. [DOI] [PubMed] [Google Scholar]
- 25.Henschke CI, Yankelevitz DF, Naidich DP, McCauley DI, McGuinness G, Libby DM, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231:164–8. doi: 10.1148/radiol.2311030634. [DOI] [PubMed] [Google Scholar]
- 26.Holin SM, Dwork RE, Glaser S, Rikli AE, Stocklen JB. Solitary pulmonary nodules found in a community-wide chest roentgenographic survey; a five-year follow-up study. Am Rev Tuberc. 1959;79:427–39. doi: 10.1164/artpd.1959.79.4.427. [DOI] [PubMed] [Google Scholar]
- 27.Gurney JW. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part I. Theory. Radiology. 1993;186:405–13. doi: 10.1148/radiology.186.2.8421743. [DOI] [PubMed] [Google Scholar]
- 28.Sone S, Tsushima K, Yoshida K, Hamanaka K, Hanaoka T, Kondo R. Pulmonary nodules: Preliminary experience with semiautomated volumetric evaluation by CT stratum. Acad Radiol. 2010;17:900–11. doi: 10.1016/j.acra.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Grewal RG, Austin JH. CT demonstration of calcification in carcinoma of the lung. J Comput Assist Tomogr. 1994;18:867–71. doi: 10.1097/00004728-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ledor K, Fish B, Chaise L, Ledor S. CT diagnosis of pulmonary hamartomas. J Comput Tomogr. 1981;5:343–4. doi: 10.1016/0149-936x(81)90075-8. [DOI] [PubMed] [Google Scholar]
- 31.Siegelman SS, Khouri NF, Scott WW, Jr, Leo FP, Hamper UM, Fishman EK, et al. Pulmonary hamartoma: CT findings. Radiology. 1986;160:313–7. doi: 10.1148/radiology.160.2.3726106. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney MC, Shipley RT, Corcoran HL, Dickson BA. CT demonstration of calcification in carcinoma of the lung. AJR Am J Roentgenol. 1990;154:255–8. doi: 10.2214/ajr.154.2.2153329. [DOI] [PubMed] [Google Scholar]
- 33.Oldham HN, Jr, Young WG, Jr, Sealy WC. Hamartoma of the lung. J Thorac Cardiovasc Surg. 1967;53:735–42. [PubMed] [Google Scholar]
- 34.Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: What does it mean? Ann Thorac Med. 2010;5:67–79. doi: 10.4103/1817-1737.62469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caskey CI, Templeton PA, Zerhouni EA. Current evaluation of the solitary pulmonary nodule. Radiol Clin North Am. 1990;28:511–20. [PubMed] [Google Scholar]
- 36.Kanauchi T, Hoshi T, Kato A. CT findings of pulmonary hamartoma with special reference to epithelial-lined clefts and connection with pulmonary arteries. Nippon Igaku Hoshasen Gakkai Zasshi. 2004;64:300–4. [PubMed] [Google Scholar]
- 37.Park KY, Kim SJ, Noh TW, Cho SH, Lee DY, Paik HC, et al. Diagnostic efficacy and characteristic features of MRI in pulmonary hamartoma: comparison with CT, MRI and pathology. J Comput Assist Tomogr. 2008;32:919–25. doi: 10.1097/RCT.0b013e31815abed4. [DOI] [PubMed] [Google Scholar]
- 38.Lien YC, Hsu HS, Li WY, Wu YC, Hsu WH, Wang LS, et al. Pulmonary hamartoma. J Chin Med Assoc. 2004;67:21–6. [PubMed] [Google Scholar]
- 39.Zakharov V, Schinstine M. Hamartoma of the lung. Diagn Cytopathol. 2008;36:331–2. doi: 10.1002/dc.20790. [DOI] [PubMed] [Google Scholar]
- 40.Jin MS, Ha HJ, Baek HJ, Lee JC, Koh JS. Adenomyomatoushamartoma of lung mimicking benign mucinous tumor in fine needle aspiration biopsy: a case report. Acta Cytol. 2008;52:357–60. doi: 10.1159/000325523. [DOI] [PubMed] [Google Scholar]
- 41.Wood B, Swarbrick N, Frost F. Diagnosis of pulmonary hamartoma by fine needle biopsy. Acta Cytol. 2008;52:412–7. doi: 10.1159/000325545. [DOI] [PubMed] [Google Scholar]
- 42.Guo W, Zhao YP, Jiang YG, Wang RW, Ma Z. Surgical treatment and outcome of pulmonary hamartoma: Retrospective study of 20-year experience. J Exp Clin Cancer Res. 2008;27:8. doi: 10.1186/1756-9966-27-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Cicco C, Bellomi M, Bartolomei M, Carbone G, Pelosi G, Veronesi G, et al. Imaging of lung hamartomas by multidetector computed tomography and positron emission tomography. Ann Thorac Surg. 2008;86:1769–72. doi: 10.1016/j.athoracsur.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Garland LH, Coulson W, Wollin E. The rate of growth and apparent duration of untreated primary bronchial carcinoma. Cancer. 1963;16:694–707. doi: 10.1002/1097-0142(196306)16:6<694::aid-cncr2820160603>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Schultz EM, Silvestri GA, Gould MK. Variation in experts’ beliefs about lung cancer growth, progression, and prognosis. J Thorac Oncol. 2008;3:422–6. doi: 10.1097/JTO.0b013e318167146b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins VP, Loeffler RK, Tivey H. Observations on growth rates of human tumors. Am J Roentgenol Radium Ther Nucl Med. 1956;76:988–1000. [PubMed] [Google Scholar]
- 47.Nathan MH, Collins VP, Adams RA. Differentiation of benign and malignant pulmonary nodules by growth rate. Radiology. 1962;79:221–32. doi: 10.1148/79.2.221. [DOI] [PubMed] [Google Scholar]
- 48.Dunnick NR, Parker BR, Castellino RA. Rapid onset of pulmonary infiltration due to histiocytic lymphoma. Radiology. 1976;118:281–5. doi: 10.1148/118.2.281. [DOI] [PubMed] [Google Scholar]
- 49.Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics. 2000;20:43–58. doi: 10.1148/radiographics.20.1.g00ja0343. [DOI] [PubMed] [Google Scholar]
- 50.Hood RT, Jr, Good CA, Clagett OT, MCdonald JR. Solitary circumscribed lesions of the lung; study of 156 cases in which resection was performed. J Am Med Assoc. 1953;152:1185–91. doi: 10.1001/jama.1953.03690130001001. [DOI] [PubMed] [Google Scholar]
- 51.Quint LE, Cheng J, Schipper M, Chang AC, Kalemkerian G. Lung lesion doubling times: values and variability based on method of volume determination. Clin Radiol. 2008;63:41–8. doi: 10.1016/j.crad.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Good CA, Wilson TW. The solitary circumscribed pulmonary nodule; study of seven hundred five cases encountered roentgenologically in a period of three and one-half years. J Am Med Assoc. 1958;166:210–5. doi: 10.1001/jama.1958.02990030008003. [DOI] [PubMed] [Google Scholar]
- 53.Good CA, Hood RT, Jr, McDonald JR. Significance of a solitary mass in the lung. Am J Roentgenol Radium Ther Nucl Med. 1953;70:543–54. [PubMed] [Google Scholar]
- 54.Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol. 1997;168:325–8. doi: 10.2214/ajr.168.2.9016198. [DOI] [PubMed] [Google Scholar]
- 55.Libby DM, Henschke CI, Yankelevitz DF. The solitary pulmonary nodule: update 1995. Am J Med. 1995;99:491–6. doi: 10.1016/s0002-9343(99)80225-3. [DOI] [PubMed] [Google Scholar]
- 56.Scholten ET, Kreel L. Distribution of lung metastases in the axial plane. A combined radiological-pathological study. Radiol Clin (Basel) 1977;46:248–65. [PubMed] [Google Scholar]
- 57.Bankoff MS, McEniff NJ, Bhadelia RA, Garcia-Moliner M, Daly BD. Prevalence of pathologically proven intrapulmonary lymph nodes and their appearance on CT. AJR Am J Roentgenol. 1996;167:629–30. doi: 10.2214/ajr.167.3.8751667. [DOI] [PubMed] [Google Scholar]
- 58.Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–76. doi: 10.1148/radiology.179.2.2014294. [DOI] [PubMed] [Google Scholar]
- 59.Hirakata K, Nakata H, Haratake J. Appearance of pulmonary metastases on high-resolution CT scans: comparison with histopathologic findings from autopsy specimens. AJR Am J Roentgenol. 1993;161:37–43. doi: 10.2214/ajr.161.1.8517317. [DOI] [PubMed] [Google Scholar]
- 60.Gaeta M, Pandolfo I, Volta S, Russi EG, Bartiromo G, Girone G, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol. 1991;157:1181–5. doi: 10.2214/ajr.157.6.1950861. [DOI] [PubMed] [Google Scholar]
- 61.Naidich DP, Webb WR, Muller NL, Vlahos I, Krinsky GA. Focal lung disease, in Computed Tomography and Magnetic Resonance of the Thorax. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1999. pp. 296–329. [Google Scholar]
- 62.O’keefe ME, Jr, Good CA, MCdonald JR. Calcification in solitary nodules of the lung. Am J Roentgenol Radium Ther Nucl Med. 1957;77:1023–33. [PubMed] [Google Scholar]
- 63.Görich J, Gamroth A, Beyer-Enke S, Kayser K, van Kaick G. Differential computed tomographic diagnosis of cavity-forming space-occupying lesions of the lung. Rofo. 1987;147:479–85. doi: 10.1055/s-2008-1048684. [DOI] [PubMed] [Google Scholar]
- 64.Miura H, Taira O, Hiraguri S, Hagiwara M, Kato H. Cavitating adenocarcinoma of the lung. Ann ThoracCardiovascSurg. 1998;4:154–8. [PubMed] [Google Scholar]
- 65.Siegelman SS, Zerhouni EA, Leo FP, Khouri NF, Stitik FP. CT of the solitary pulmonary nodule. AJR Am J Roentgenol. 1980;135:1–13. doi: 10.2214/ajr.135.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Zerhouni EA, Spivey JF, Morgan RH, Leo FP, Stitik FP, Siegelman SS. Factors influencing quantitative CT measurements of solitary pulmonary nodules. J Comput Assist Tomogr. 1982;6:1075–87. doi: 10.1097/00004728-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Xu DM, van Klaveren RJ, de Bock GH, Leusveld AL, Dorrius MD, Zhao Y, et al. Role of baseline nodule density and changes in density and nodule features in the discrimination between benign and malignant solid indeterminate pulmonary nodules. Eur J Radiol. 2009;70:492–8. doi: 10.1016/j.ejrad.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 68.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G. [Last accessed on 2011 Aug 29]. Available from: http://www.ncbi.nlm.nih.gov/pubmed?term=%22McCauley%20D%22%5BAuthor%5D. Miettinen OS; ELCAP Group. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7 . [DOI] [PubMed]
- 69.Lee HJ, Goo JM, Lee CH, Yoo CG, Kim YT, Im JG. Nodular ground-glass opacities on thin-section CT: size change during follow-up and pathological results. Korean J Radiol. 2007;8:22–31. doi: 10.3348/kjr.2007.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swensen SJ, Brown LR, Colby TV, Weaver AL. Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology. 1995;194:393–8. doi: 10.1148/radiology.194.2.7824716. [DOI] [PubMed] [Google Scholar]
- 71.Potente G, Iacari V, Caimi M. The challenge of solitary pulmonary nodules: HRCT evaluation. Comput Med Imaging Graph. 1997;21:39–46. doi: 10.1016/s0895-6111(96)00071-7. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997;205:471–8. doi: 10.1148/radiology.205.2.9356631. [DOI] [PubMed] [Google Scholar]
- 73.Yi CA, Lee KS, Kim EA, Han J, Kim H, Kwon OJ, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology. 2004;233:191–9. doi: 10.1148/radiol.2331031535. [DOI] [PubMed] [Google Scholar]
- 74.Jeong YJ, Lee KS, Jeong SY, Chung MJ, Shim SS, Kim H, et al. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology. 2005;237:675–83. doi: 10.1148/radiol.2372041549. [DOI] [PubMed] [Google Scholar]
- 75.Bayraktaroglu S, Savaş R, Basoglu OK, Cakan A, Mogulkoc N, Cagirici U, et al. Dynamic computed tomography in solitary pulmonary nodules. J Comput Assist Tomogr. 2008;32:222–7. doi: 10.1097/RCT.0b013e318136e29d. [DOI] [PubMed] [Google Scholar]
- 76.Jiang NC, Han P, Zhou CK, Zheng JL, Shi HS, Xiao J. Dynamic enhancement patterns of solitary pulmonary nodules at multi-detector row CT and correlation with vascular endothelial growth factor and microvessel density. Ai Zheng. 2009;28:164–9. [PubMed] [Google Scholar]
- 77.Lee KS, Yi CA, Jeong SY, Jeong YJ, Kim S, Chung MJ, et al. Solid or partly solid solitary pulmonary nodules: their characterization using contrast wash-in and morphologic features at helical CT. Chest. 2007;131:1516–25. doi: 10.1378/chest.06-2526. [DOI] [PubMed] [Google Scholar]
- 78.Schaefer JF, Vollmar J, Schick F, Vonthein R, Seemann MD, Aebert H, et al. Solitary pulmonary nodules: dynamic contrast enhanced MR imaging – perfusion differences in malignant and benign lesions. Radiology. 2004;232:544–53. doi: 10.1148/radiol.2322030515. [DOI] [PubMed] [Google Scholar]
- 79.Zou Y, Zhang M, Wang Q, Shang D, Wang L, Yu G. Quantitative investigation of solitary pulmonary nodules: dynamic contrast-enhanced MRI and histopathologic analysis. AJR Am J Roentgenol. 2008;191:252–9. doi: 10.2214/AJR.07.2284. [DOI] [PubMed] [Google Scholar]
- 80.Gaeta M, Russi EG, La Spada F, Barone M, Casablanca G, Pandolfo I. Small bronchogenic carcinomas presenting as solitary pulmonary nodules. Bioptic approach guided by CT-positive bronchus sign. Chest. 1992;102:1167–70. doi: 10.1378/chest.102.4.1167. [DOI] [PubMed] [Google Scholar]
- 81.Naidich DP, Sussman R, Kutcher WL, Aranda CP, Garay SM, Ettenger NA. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest. 1988;93:595–8. doi: 10.1378/chest.93.3.595. [DOI] [PubMed] [Google Scholar]
- 82.Zacharopoulos G, Adam A, Ind PW. The positive bronchus sign in patients with known lung cancer. Eur J Radiol. 1990;10:130–3. doi: 10.1016/0720-048x(90)90121-q. [DOI] [PubMed] [Google Scholar]
- 83.Gaeta M, Pandolfo I, Volta S, Russi EG, Bartiromo G, Girone G, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol. 1991;157:1181–5. doi: 10.2214/ajr.157.6.1950861. [DOI] [PubMed] [Google Scholar]
- 84.Singh SP. The positive bronchus sign. Radiology. 1998;209:251–2. doi: 10.1148/radiology.209.1.9769839. [DOI] [PubMed] [Google Scholar]
- 85.Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–4. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 86.Kuhlman JE, Fishman EK, Burch PA, Karp JE, Zerhouni EA, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia. The contribution of CT to early diagnosis and aggressive management. Chest. 1987;92:95–9. doi: 10.1378/chest.92.1.95. [DOI] [PubMed] [Google Scholar]
- 87.Primack SL, Hartman TE, Lee KS, Müller NL. Pulmonary nodules and the CT halo sign. Radiology. 1994;190:513–5. doi: 10.1148/radiology.190.2.8284408. [DOI] [PubMed] [Google Scholar]
- 88.Mori M, Galvin JR, Barloon TJ, Gingrich RD, Stanford W. Fungal pulmonary infections after bone marrow transplantation: evaluation with radiography and CT. Radiology. 1991;178:721–6. doi: 10.1148/radiology.178.3.1994408. [DOI] [PubMed] [Google Scholar]
- 89.Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol. 2005;78:862–5. doi: 10.1259/bjr/77712845. [DOI] [PubMed] [Google Scholar]
- 90.Parrón M, Torres I, Pardo M, Morales C, Navarro M, Martínez-Schmizcraft M. The halo sign in computed tomography images: differential diagnosis and correlation with pathology findings. Arch Bronconeumol. 2008;44:386–92. [PubMed] [Google Scholar]
- 91.Kuhlman JE, Fishman EK, Teigen C. Pulmonary septic emboli: diagnosis with CT. Radiology. 1990;174:211–3. doi: 10.1148/radiology.174.1.2294550. [DOI] [PubMed] [Google Scholar]
- 92.Milne EN, Zerhouni EA. Blood supply of pulmonary metastases. J Thorac Imaging. 1987;2:15–23. doi: 10.1097/00005382-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Meziane MA, Hruban RH, Zerhouni EA, Wheeler PS, Khouri NF, Fishman EK, et al. High resolution CT of the lung parenchyma with pathologic correlation. Radiographics. 1988;8:27–54. doi: 10.1148/radiographics.8.1.3353534. [DOI] [PubMed] [Google Scholar]
- 94.Hirakata K, Nakata H, Nakagawa T. CT of pulmonary metastases with pathological correlation. Semin Ultrasound CT MR. 1995;16:379–94. doi: 10.1016/0887-2171(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 95.Hirakata K, Nakata H, Haratake J. Appearance of pulmonary metastases on high-resolution CT scans: comparison with histopathologic findings from autopsy specimens. AJR Am J Roentgenol. 1993;161:37–43. doi: 10.2214/ajr.161.1.8517317. [DOI] [PubMed] [Google Scholar]
- 96.Dodd JD, Souza CA, Müller NL. High-resolution MDCT of pulmonary septic embolism: evaluation of the feeding vessel sign. AJR Am J Roentgenol. 2006;187:623–9. doi: 10.2214/AJR.05.0681. [DOI] [PubMed] [Google Scholar]
- 97.Lee SJ, Cha SI, Kim CH, Park JY, Jung TH, Jeon KN, et al. Septic pulmonary embolism in Korea: Microbiology, clinicoradiologic features, and treatment outcome. J Infect. 2007;54:230–4. doi: 10.1016/j.jinf.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 98.Jeong YJ, Yi CA, Lee KS. Solitary pulmonary nodules: detection, characterization, and guidance for further diagnostic workup and treatment. AJR Am J Roentgenol. 2007;188:57–68. doi: 10.2214/AJR.05.2131. [DOI] [PubMed] [Google Scholar]
- 99.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231:440–5. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 100.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 101.Swensen SJ. CTscreening for lung cancer. AJR Am J Roentgenol. 2002;179:833–6. doi: 10.2214/ajr.179.4.1790833. [DOI] [PubMed] [Google Scholar]
- 102.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–32. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 103.Yamagami T, Iida S, Kato T, Tanaka O, Nishimura T. Combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? AJR Am J Roentgenol. 2003;180:811–5. doi: 10.2214/ajr.180.3.1800811. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules. Chest. 1999;115:563–8. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 105.Jones YM, Irion KL, Holemans JA. A review of the imaging and clinical management of solitary pulmonary nodules. Imaging. 2008;20:303–11. [Google Scholar]


