Abstract
Sleep disordered breathing is a common chronic condition in the general population. This review will highlight the prevalence of different types of sleep apnea in general and obstructive type in particular in the United States and Middle East. Despite the extensive research studies on the sleep apnea pathogenesis, the exact mechanism is not well known. Obesity, however, is the leading risk factor to upper airway narrowing and obstruction and main contributor to the escalating prevalence of morbidity worldwide including the Arab countries. Due to the serious consequences of the untreated sleep disordered breathing, this article will emphasize on the importance of early recognition, key clinical manifestations, and how to treat and prevent the disease.
Keywords: Apnea, breathing, sleep
INTRODUCTION
Sleep disordered breathing is a common chronic condition in the general population characterized by repeated episodes of apnea and hypopnea during sleep. It can present as obstructive sleep apnea/hypopnea (OSAH) disorder, central sleep apnea (CSA), or mixed sleep disordered breathing. When OSAH disorder is associated with daytime sleepiness, it is called obstructive sleep apnea-hypopnea syndrome (OSAHS).
Historically, sleep apnea was first described in the early nineteenth century when Charles Dickens reported in his book “The Posthumous Papers of the Pickwick Club” the obese young man that was sleepy and snoring “Said the old gentleman, he's always asleep. Goes on errands fast asleep, and snores as he waits at table”. Since then the Pickwickian syndrome, known as obesity hypoventilation syndrome, is defined as a combination of obesity, snoring, and excessive sleepiness associated with hypoventilation resulting awake hypercapnia. OSAHS was not recognized as a clinical disorder until nearly 100 years later.
How common is OSAHS?
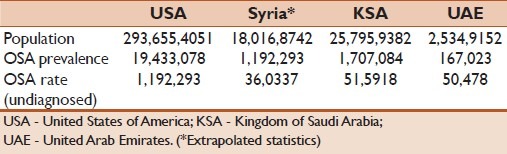
OSAHS is a common chronic disorder and the most common of all sleep disorders. It can occur on a similar frequency as type-I diabetes and twice that of asthma. It is estimated from the Wisconsin cohort that the prevalence of OSAHS in the United States of America is 9–24% for men and 4–9% for women who were not obese (body mass index <30 kg/m2) and aged 30–60 years old.[1] Although epidemiological data from the Arab countries are lacking, especially related to OSHAS prevalence, it is estimated that millions of patients suffer from OSAHS in the Middle East and Arab countries [Table 1].
Table 1.
Sleep apnea in the Middle East from US Census Bureau, International Data Base, 2004
What causes OSAHS?
The pathogenesis of sleep disordered breathing involves an interaction between unfavorable pharyngeal anatomy and ventilatory control instability.[2–5] Obstructive sleep apnea is due to anatomic factors that promote pharyngeal narrowing including large neck circumference, cervical soft tissue,[6] vessels, and bony structures. Many of these factors promote pharyngeal collapsibility by decreasing the caliber of the upper airway or by increasing the upper airway surrounding pressure.[7] Increased upper airway collapsibility during sleep has been linked to structural changes in the surrounding boney and soft tissues, which is best measured by determining the critical collapsing pressure or compliance under inhibited neuromuscular activity (passive Pcrit or passive Cua, respectively).[8,9]
CSH is due to an absent or reduced ventilatory motor output, which is an important determinant of upper airway patency during sleep, especially in individuals with high susceptibility to pharyngeal collapse such as patients with sleep apnea and snoring individuals.[10–12] When ventilatory motor output oscillates during periodic breathing, pharyngeal narrowing or obstruction occurs at the nadir of ventilatory motor output, especially in individuals with a highly collapsible airway.[13] Recent studies observed that when airflow obstruction occurred during sleep, it triggered an overshoot of ventilation with or without arousals from sleep. The events followed by arousals, however, had greater overshoot ventilation and ensuring obstructive events than those without arousals.[14] These findings indicate that when neuromuscular responses to airflow obstruction fail to compensate, it trigger breathing instability and perpetuate recurrent apnea and hypopneas. Nevertheless, the mechanism of pharyngeal narrowing or obstruction during sleep remains unclear.
Pharyngeal occlusion during obstructive apnea is often described as an inspiratory phenomenon, caused by negative collapsing pressure. However, several lines of evidence indicate that expiratory narrowing may be a significant contributor to pharyngeal obstruction during sleep.[13–16] More recently, it was found that reduced ventilatory motor output leads to pharyngeal narrowing during expiration.[17] The magnitude of expiratory narrowing correlated with body mass index, indicating that neuromuscular and anatomic factors contribute to upper airway patency during NREM sleep.
To ascertain the mechanisms of pharyngeal narrowing during sleep, it is useful to consider the hypotonic pharynx during apnea or hypopnea as a Starling Resistor with a collapsible segment governed by the principles of flow through collapsible tubes [Figure 1]. Accordingly, if the upstream pressure is below the critical closing pressure (Pcrit), the collapsible segment is closed, and no flow occurs (i.e., apnea). Flow commences when upstream pressure exceeds Pcrit and is determined by the gradient between the upstream segment and Pcrit. The critical closing pressure reflects the surrounding tissue pressure (extraluminal pressure) being negative in normal subjects and positive in patients with OSA. The aforementioned principles operate during both phases of respiration[14] because Pcrit and upstream pressure may change dynamically throughout the respiratory cycle.[18] Upstream pressure is the mask pressure during inspiration and the supraglottic pressure during expiration. Thus, the effects of decreased ventilatory motor output on upper airway patency may differ by the phase of respiration. Decreased ventilatory motor output diminishes the magnitude of negative supraglottic airway pressure, hence increasing downstream pressure with no adverse effects on inspiratory upper airway patency. In contrast, supraglottic pressure is the upstream pressure during expiration and is a major determinant of expiratory flow. Consequently, expiratory pharyngeal obstruction would occur if the supraglottic pressure falls below Pcrit. This may explain the occurrence of pharyngeal occlusion during central apnea or periods of very low drive (as depicted in Figure 2).[18]
Figure 1.
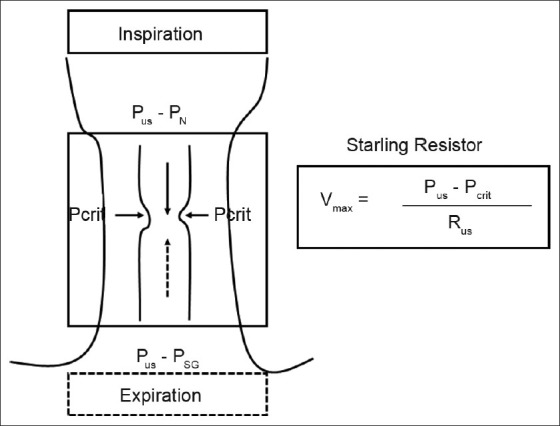
Schematic illustration for the collapsible segment of upper airway as a Starling Resistor. In this model, flow is determined by the gradient between the upstream segment and critical closing pressure (Pcrit). During inspiration, when upstream pressure (Pus) (i.e., PN: Nasal pressure) is below the Pcrit, the collapsible segment is closed, and no flow occurs. During expiration, when Pus in the supraglottic area (i.e., PSG: Supraglottic pressure) is below the Pcrit, the collapsible segment is closed, and no flow occurs. During hypopnea, inspiratory and expiratory flows are limited, correlating with the gradient between the upstream pressure and Pcrit. Hence, this pressure gradient is important determinant of pharyngeal narrowing. Rus, upstream resistance; Vmax, maximal flow
Figure 2.
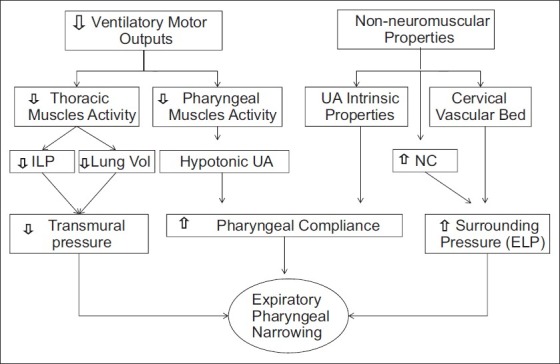
A diagram to illustrate the potential mechanisms responsible of pharyngeal narrowing during reduced ventilator motor output and how neuromuscular and non-neuromuscular factors influence upper airway patency. The reduced ventilatory drive inhibits thoracic and pharyngeal neuromuscular activity affecting upper airway transmural pressure and/or compliance, respectively. In addition non-neuromuscular factors influence the upper airway patency by increasing surrounding pressure. UA - Upper airway, NC - Neck circumference, ILP - Intra-luminal pharyngeal pressure, ELP - Extra-luminal pharyngeal pressure.
What predisposes to OSAHS?
A spectrum of risk factors predispose to OSAHS as depicted in Figure 3. The condition is more common overweight men. In fact obesity is considered the most important risk factor for OSAHS. Obesity as defined by body mass index (BMI) equal or greater than 30 kg/m2 has become a global epidemic throughout the world. According to the World Health Organization estimation performed in 2005, more than 30% of the general population in the United States has obesity. This high prevalence is now seen in countries in Europe and the Middle East such as Syria, Saudi Arabia, and United Arab Emirates [Table 2]. This increased prevalence of obesity is strongly linked to higher incidence of SDB and OSAHS; therefore screening this type of population for early detection of morbid conditions is vital in the clinical assessment by any clinician or health care provider. Other risk factors include chronic heart failure patients (estimated prevalence of 40–80%),[19] cervical spinal cord injury patients (estimated prevalence of 60%),[16] and stroke (estimated prevalence of 44–72%).[20–23]
Figure 3.
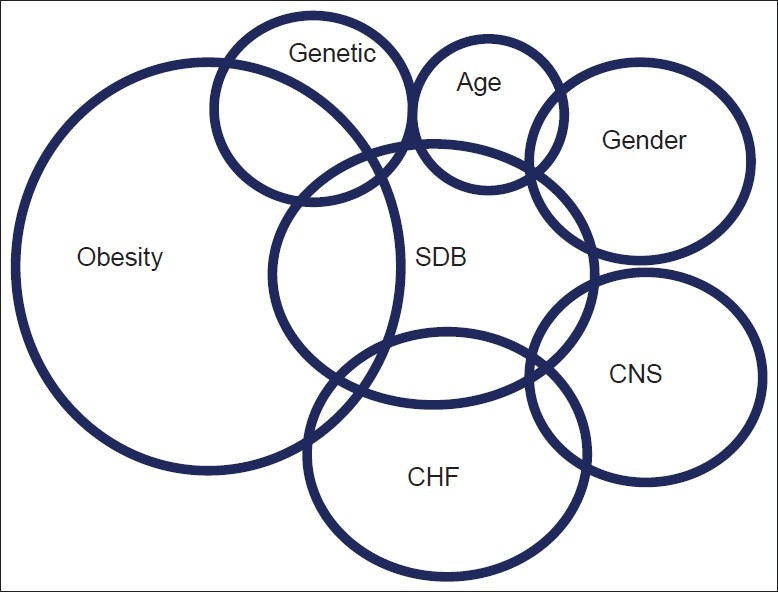
A diagram to illustrate the risk factors for sleep disordered breathing. Abbreviations: SDB - Sleep disordered breathing; CHF - Chronic heart failure; CNS - Central nervous system
Table 2.
Obesity in the Middle East from US Census Bureau, International Data Base, 2004

Why is it important to recognize OSAHS as early as possible?
Many people who have sleep apnea do not realize it. Drowsiness and lack of concentration contribute to increased traffic accidents.[24] OSAHS can lead to adverse outcomes on the public health, including hypertension, cardiovascular, metabolic, and neuropsychological consequences.[1,25–28] Hypopneas comprise a significant component of OSAHS and may also cause deleterious cardiovascular consequences, particularly when accompanied by desaturation (>4% from baseline).[27] More recently, it has been found in well-designed longitudinal study that moderate and severe sleep apnea are associated with three-fold increase in risk of ischemic stroke after 8 years of follow-up of community-dwelling men and women.[29,30]
OSAHS has been associated with several neuropsychological impairments thought to be due to structural changes in the brain.[31] Recurrent apnea and hypopnea events during sleep result in intermittent hypoxia, hypo-, and hypercapnia and sleep fragmentation. Intermittent hypoxia, hypo-, and hypercapnia are associated with altered vasomotor protection in the central nervous system which can contribute to the structural changes in the brain. A recent study demonstrated that cognitive impairment is associated with decreased gray-matter volume in specific areas of the brain such as frontal and hippocampal regions which associated with decreased executive function and short-term memory. Interestingly, these cognitive and structural changes were reversed after 3 months of OSA treatment.[31] Thus, early recognition of sleep disordered breathing is important step toward effective prevention of such serious consequences in near future.
What are the clinical manifestations of OSAHS?
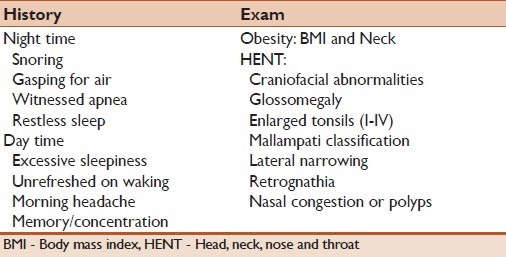
OSAHS is commonly associated with snoring and excessive daytime sleepiness. Snoring is the most common nocturnal symptoms that could be loud and disturbing to the bed partner's sleep. Bed partners may report also cessation of breathing (witnessed apnea) which ends by snorting sound or gasping for air. Other nocturnal symptoms are unexplained awakenings, nocturia, and restless sleep. During the day, patient commonly complain of excessive sleepiness despite long hours of night sleep. This may correlate with the severity of the OSAHS when there is no other precipitating factor to cause hypersomnia. Some patients will also complain of morning headache, not refreshed from sleep, memory problems, and difficulty concentrating.
OSAHS should be suspected clinically by presenting history, physical findings (as delineated in Table 3), and then confirmed by specific sleep tests. The severity of OSAHS can range from mild snoring to hypopnea and sleep apnea. Apnea is usually characterized as cyclical cessation of breathing (for a duration of at least 10 sec), which can be central, obstructive, or mixed in its etiology. Hypopnea is defined as a reduction in airflow resulting short period of awakening (arousal) or decreased oxygenation (destauration of 3–4%).
Table 3.
Clinical presentation
When to refer patients for evaluation?
Patients who report excessive daytime sleepiness should be asked specific questions to assess the severity of this sleepiness and whether it occurs in situations that require alertness such as during driving or at work. Epworth sleepiness scale is the most common questionnaire used in the clinical settings to assess the level of sleepiness and to follow it objectively. In specific patient phenotypes such as stroke, spinal cord injury, or heart failure, there is dissociation between OSAHS from hypersomnia and obesity. Therefore, in such patients a high index of suspension coupled with meticulous evaluation and clinical history followed by screening study may be necessary as early as possible.
In general, OSAHS should be suspected by the health care provider in the following cases:
Loud snoring
Obesity: BMI >40 kg/m2
Excessive daytime sleepiness
Stroke patients
Neuromuscular disorders (such as spinal cord injury or Parkinson's disease).
How is the diagnosis made?
The diagnosis of sleep disordered breathing is confirmed by an overnight sleep study or polysomnography that is usually done in the sleep laboratory or the patient home. During laboratory-based polysomnography, multiple physiological parameters are measured and recorded simultaneously with the sleep stages measured by a standard electroencephalogram. The respiratory parameters include a flow channel (usually a thermistor placed on the nose and mouth), effort channels (such as respiratory impedance plethysmography), and pulse oximetry. The analysis of the thermistor signal alone cannot accurately score hypopneic events and that nasal pressure analysis measured via nasal prongs connected to a pressure transducer is a simple and useful way to identify nocturnal breathing abnormalities. Other signals that are measured also include electromyogram from the legs and chin, electrocardiogram, and snoring channel. Sleep disordered breathing can be diagnosed also by in-house polysomnography portable monitors (PM). PM are usually divided into three categories: Level II, full polysomnography; level III, at least four channels (flow, effort, oximetry, and ECG); level IV, less than four channels (including oximetry). There is now strong evidence that level III portable monitoring can confirm or exclude the diagnosis of OSAHS with absolute differences between the home and laboratory respiratory disturbance index less than 10 for 65% of patients.[32]
The 2007 Clinical Guidelines advised that PM should be used in patients with a “high pretest probability” for OSA.[27] PM is not appropriate for the diagnosis of OSA in patients with significant comorbid medical conditions, for the diagnosis of comorbid sleep disorders, or for general screening of asymptomatic populations. The guidelines also stated that PM may be indicated for the diagnosis of OSA in patients for whom in-laboratory PSG is not possible. It is required that PM must record airflow, respiratory effort, and blood oxygenation. The airflow, effort, and oximetric biosensors conventionally used for in-laboratory PSG should be used in PM. The AASM task force recommendations for PM use are summarized in Table 4. In highly suspected cases, the utility of a diagnostic algorithm in conjunction with autoadjusting positive airway pressure titration in the initial management of obstructive sleep apnea has been reported in one study to be equivalent to conventional polysomnography.[33]
Table 4.
The AASM task force recommendations for portable monitors testing in the diagnosis of OSA[34]

This approach can be used alternatively in areas with limited resources such the Middle East. It should be noted, however, that when the test is negative with high clinical suspension, a full polysomnography should be performed to rule out the disease.
What is the treatment for OSAHS?
The treatment of OSAHS is beyond the scope of this review. Nevertheless the following management strategies are available: Lifestyle changes, mouthpieces, breathing devices, and surgery are used to treat OSAHS. The goals of treating sleep apnea are to relieve symptoms such as loud snoring and daytime sleepiness and to restore normal breathing during sleep. Lifestyle changes (weight loss and exercise), positional therapy, and mouthpieces can be used to treat mild sleep apnea. People who have moderate or severe sleep apnea will need breathing devices, such as nasal continuous positive airway pressure or surgery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–14. [PubMed] [Google Scholar]
- 4.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi N, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: An outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–5. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badr MS. Effect of ventilatory drive on upper airway patency in humans during NREM sleep. Respir Physiol. 1996;103:1–10. doi: 10.1016/0034-5687(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 7.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 8.Rowley JA, Sanders CS, Zahn BR, Badr MS. Gender differences in upper airway compliance during NREM sleep: Role of neck circumference. J Appl Physiol. 2002;92:2535–41. doi: 10.1152/japplphysiol.00553.2001. [DOI] [PubMed] [Google Scholar]
- 9.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 10.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: Comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–8. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight J, Arzt M, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–72. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–80. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 13.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–14. [PubMed] [Google Scholar]
- 14.Sanders MH, Moore SE. Inspiratory and expiratory partitioning of airway resistance during sleep in patients with sleep apnea. Am Rev Respir Dis. 1983;127:554–8. doi: 10.1164/arrd.1983.127.5.554. [DOI] [PubMed] [Google Scholar]
- 15.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol. 1986;61:1438–43. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- 16.Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am J Respir Crit Care Med. 2009;179:313–9. doi: 10.1164/rccm.200805-741OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider H, Boudewyns A, Smith PL, O’Donnell CP, Canisius S, Stammnitz A, et al. Modulation of upper airway collapsibility during sleep: Influence of respiratory phase and flow regimen. J Appl Physiol. 2002;93:1365–76. doi: 10.1152/japplphysiol.00942.2001. [DOI] [PubMed] [Google Scholar]
- 18.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 19.Morrell MJ, Arabi Y, Zahn B, BaDr MS. Progressive retro palatal n arrowing preceding obstructive apnea. Am J Respir Crit Care Med. 1998;158:1974–81. doi: 10.1164/ajrccm.158.6.9712107. [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke, the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portier F, Portmann A, Czernichow P, Vascaut L, Devin E, Benhamou D, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162:814–8. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 22.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J Cooperative Group Burgos-Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 23.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: A randomized validation study. Ann Intern Med. 2007;146:157–66. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 24.Javaheri S, Wexler L. Prevalence and treatment of breathing disorders during sleep in patients with heart failure. Curr Treat Options Cardiovasc Med. 2005;7:295–306. doi: 10.1007/s11936-005-0040-0. [DOI] [PubMed] [Google Scholar]
- 25.Leduc BE, Dagher JH, Mayer P, Bellemare F, Lepage Y. Estimated prevalence of obstructive sleep apnea-hypopnea syndrome after cervical cord injury. Arch Phys Med Rehabil. 2007;88:333–7. doi: 10.1016/j.apmr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–7. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 27.Bassetti C, Aldrich MS, Chervin RD, Quint D. Sleep apnea in patients with transient ischemic attack and stroke: A prospective study of 59 patients. Neurology. 1996;47:1167–73. doi: 10.1212/wnl.47.5.1167. [DOI] [PubMed] [Google Scholar]
- 28.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 29.Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 30.Sankri-Tarbichi AG, Rowley JA, Badr MS. Inhibition of ventilatory motor output increases expiratory retro palatal compliance during sleep. Respir Physiol Neurobiol. 2011;176:136–43. doi: 10.1016/j.resp.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184:1183–91. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 33.Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 34.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]


