Abstract
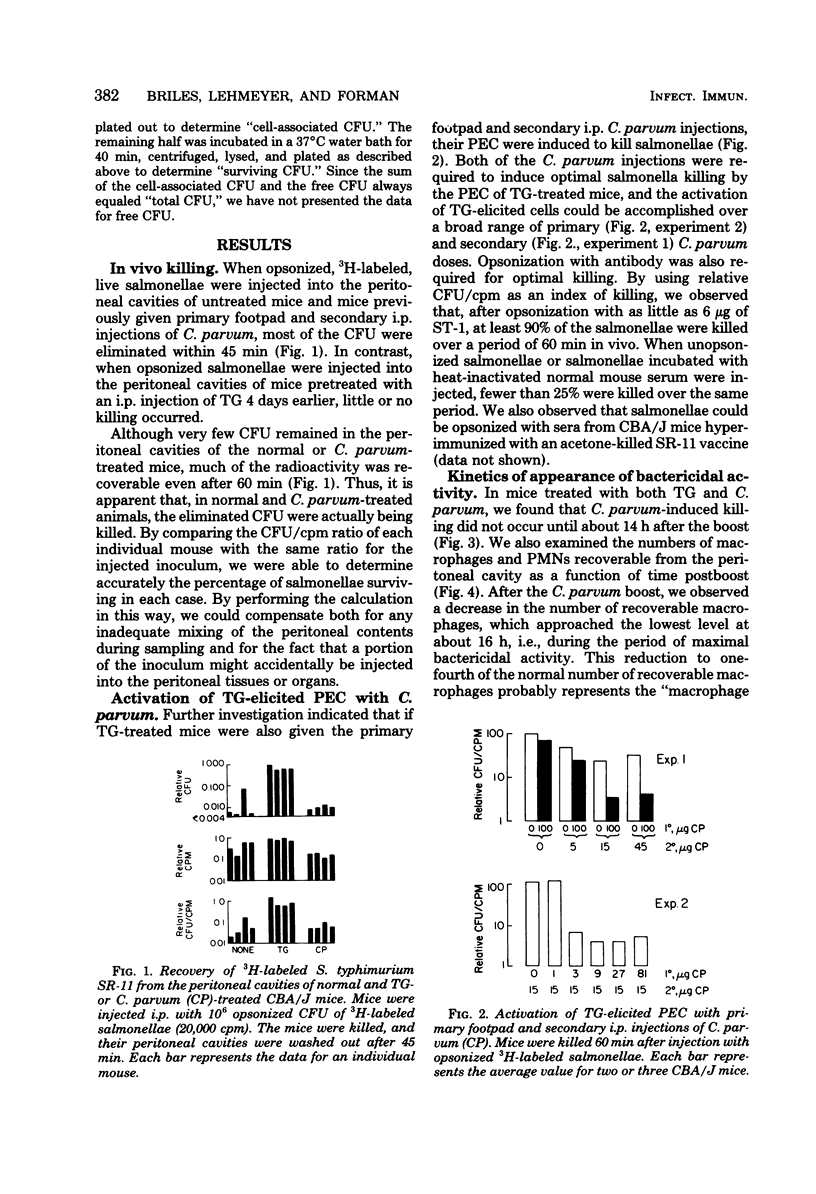
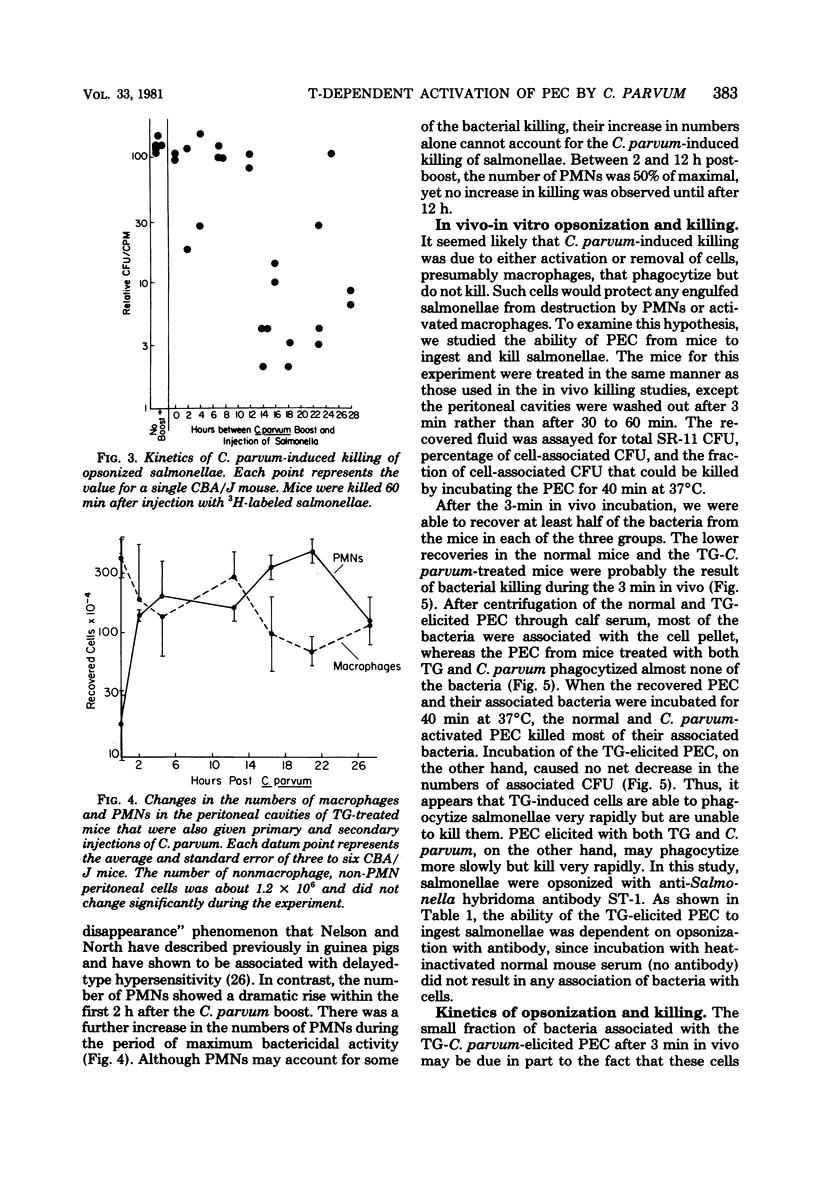
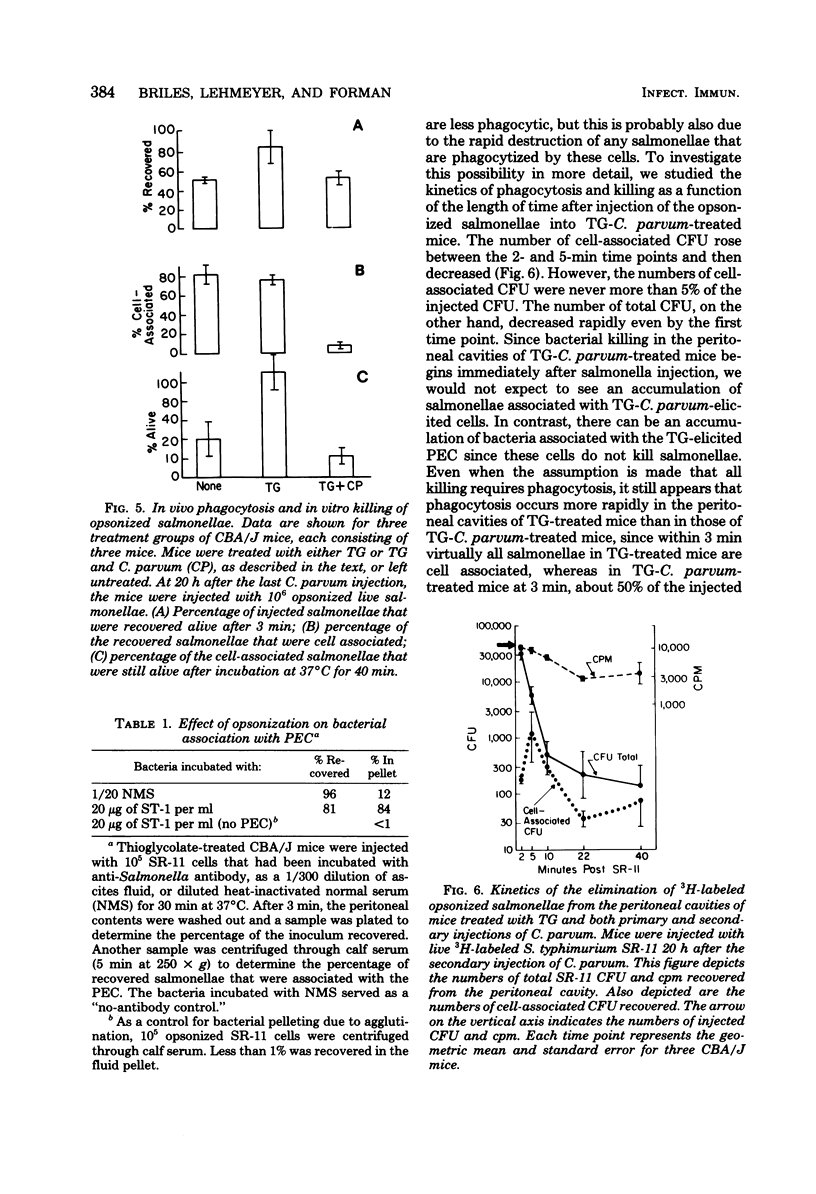
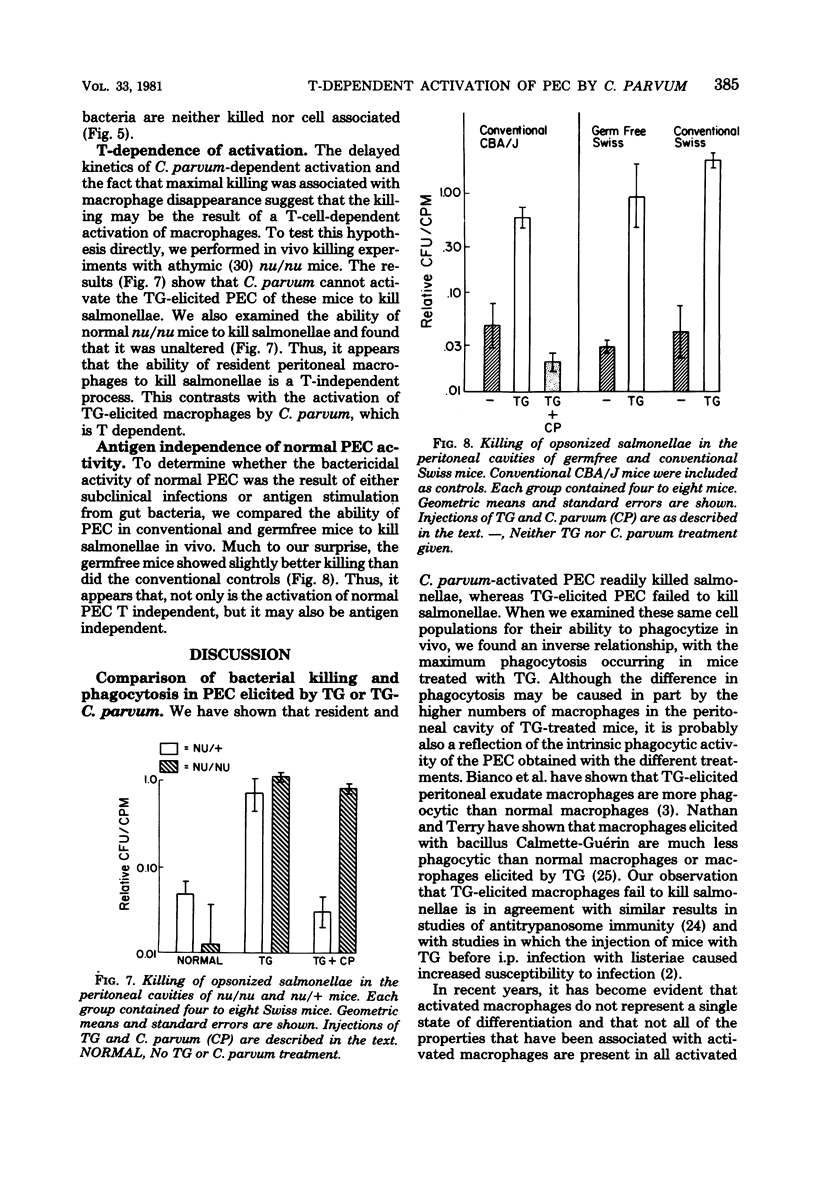
Normal peritoneal cells from conventional, germfree, or nu/nu mice readily killed opsonized salmonellae, an observation that suggests that this activity in the normal peritoneal cavity may not be dependent on either environmental antigenic stimulation or T-cell mediation. In contrast, peritoneal cells elicited 4 days after injection with thioglycolate medium failed to kill opsonized salmonellae but appeared to be highly phagocytic. Peritoneal cells from thioglycolate-treated mice could be induced to kill opsonized salmonellae by giving the mice a primary footpad injection and a secondary intraperitoneal injection of Corynebacterium parvum. This activation by C. parvum appeared to be thymus dependent, since it did not occur in nu/nu mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L. A., Campbell P. A. Thioglycolate medium decreases resistance to bacterial infection in mice. Infect Immun. 1980 Feb;27(2):455–460. doi: 10.1128/iai.27.2.455-460.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Hochadel J. F., Keller K. F. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J Infect Dis. 1977 May;135(5):813–823. doi: 10.1093/infdis/135.5.813. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. PARTIAL PURIFICATION OF THE "PROTECTIVE" ANTIGEN OF SALMONELLA TYPHIMURIUM AND ITS DISTRIBUTION AMONGST VARIOUS STRAINS OF BACTERIA. Aust J Exp Biol Med Sci. 1965 Feb;43:65–78. doi: 10.1038/icb.1965.5. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Cooper M. D., Lawton A. R. B cell differentiation induced by lipopolysaccharide. IV. Development of immunoglobulin class restriction in precursors of IgG-synthesizing cells. J Immunol. 1976 Nov;117(5 Pt 1):1567–1572. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kong Y. C., Savage D. C., Kong L. N. Delayed dermal hypersensitivity in mice to spherule and mycelial extracts of Coccidioides immitis. J Bacteriol. 1966 Feb;91(2):876–883. doi: 10.1128/jb.91.2.876-883.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hammerling G. J., Hohmann C., Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse. Nature. 1978 Jan 19;271(5642):249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Cellular immunity to toxoplasma and besnoitia in hamsters: specificity and the effects of cortisol. Infect Immun. 1977 Mar;15(3):855–862. doi: 10.1128/iai.15.3.855-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Aber V. R., Carrol M. E. Division and death rates of Salmonella typhimurium inside macrophages: use of penicillin as a probe. J Gen Microbiol. 1979 Feb;110(2):409–419. doi: 10.1099/00221287-110-2-409. [DOI] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Buchmüller Y., Behin R. Studies on the mechanisms of macrophage activation. I. Destruction of intracellular Leishmania enriettii in macrophages activated by cocultivation with stimulated lymphocytes. J Exp Med. 1978 Aug 1;148(2):393–407. doi: 10.1084/jem.148.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntrye J., Rowley D., Jenkin C. R. The functional heterogeneity of macrophages at the single cell level. Aust J Exp Biol Med Sci. 1967 Dec;45(6):675–680. doi: 10.1038/icb.1967.67. [DOI] [PubMed] [Google Scholar]
- NELSON D. S., NORTH R. J. THE FATE OF PERITONEAL MACROPHAGES AFTER THE INJECTION OF ANTIGEN INTO GUINEA PIGS WITH DELAYED-TYPE HYPERSENSITIVITY. Lab Invest. 1965 Jan;14:89–101. [PubMed] [Google Scholar]
- Nathan C. F., Terry W. D. Decreased phagocytosis by peritoneal macrophages from BCG-treated mice: induction of the phagocytic defect in normal macrophages with BCG in vitro. Cell Immunol. 1977 Mar 15;29(2):295–311. doi: 10.1016/0008-8749(77)90324-0. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Auzins I., Jenkin C. R. Further studies regarding the question of cellular immunity in mouse typhoid. Aust J Exp Biol Med Sci. 1968 Aug;46(4):447–463. doi: 10.1038/icb.1968.38. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Glode L. M., Rosenstreich D. L. Lack of responsiveness of C3H/HeJ macrophages to lipopolysaccharide: the cellular basis of LPS-stimulated metabolism. J Immunol. 1979 Mar;122(3):932–935. [PubMed] [Google Scholar]
- SCHNEIDER H. A., ZINDER N. D. Nutrition of the host and natural resistance to infection. V. An improved assay employing genetic markers in the double strain inoculation test. J Exp Med. 1956 Feb 1;103(2):207–223. doi: 10.1084/jem.103.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle R. L., North R. J. Mechanisms of antitumor action of Corynebacterium parvum: replicating short-lived T cells as the mediators of potentiated tumor-specific immunity. J Reticuloendothel Soc. 1976 Sep;20(3):209–216. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- de Sousa M. A., Parrott D. M. Induction and recall in contact sensivitity. Changes in skin and draining lymph nodes of intact and thymectomized mice. J Exp Med. 1969 Oct 1;130(4):671–690. doi: 10.1084/jem.130.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]



