Abstract
Although the control of carbon fixation and nitrogen assimilation has been studied in detail, relatively little is known about the regulation of carbon and nitrogen flow into amino acids. In this paper we report our study of the metabolic regulation of expression of an Arabidopsis aspartate kinase/homoserine dehydrogenase (AK/HSD) gene, which encodes two linked key enzymes in the biosynthetic pathway of aspartate family amino acids. Northern blot analyses, as well as expression of chimeric AK/HSD-β-glucuronidase constructs, have shown that the expression of this gene is regulated by the photosynthesis-related metabolites sucrose and phosphate but not by nitrogenous compounds. In addition, analysis of AK/HSD promoter deletions suggested that a CTTGACTCTA sequence, resembling the binding site for the yeast GCN4 transcription factor, is likely to play a functional role in the expression of this gene. Nevertheless, longer promoter fragments, lacking the GCN4-like element, were still able to confer sugar inducibility, implying that the metabolic regulation of this gene is apparently obtained by multiple and redundant promoter sequences. The present and previous studies suggest that the conversion of aspartate into either the storage amino acid asparagine or aspartate family amino acids is subject to a coordinated, reciprocal metabolic control, and this biochemical branch point is a part of a larger, coordinated regulatory mechanism of nitrogen and carbon storage and utilization.
The storage of photosynthesis-fixed carbon in vegetative tissues partitions between carbohydrate molecules such as Suc or starch and amino acids that accumulate either in their free forms or are incorporated into proteins. The regulation of carbon partition between carbohydrates and amino acids is subject to a complex metabolic regulation by light, photosynthesis-related metabolites, and nitrogenous compounds (Champigny and Foyer, 1992; Staswick, 1994; Lam et al., 1995). Under conditions of high sugar but limiting nitrogen levels, carbohydrates accumulate preferentially, whereas under conditions of high carbon and high nitrogen levels, a larger proportion of the carbon is shifted toward production of amino acids and proteins (Champigny and Foyer, 1992; Sadka et al., 1994; Staswick, 1994).
Nitrogen is first assimilated into Gln and glutamate by Gln synthase and GOGAT (Champigny and Foyer, 1992; Staswick, 1994; Lam et al., 1995). Glutamate is then transaminated into aspartate, which is then either converted into Asn by Asn synthetase or used for the production of Lys, Thr, Met, and Ile through the aspartate family pathway. This pathway is initiated by the activity of AK (Galili, 1995; Lam et al., 1995), which competes with Asn synthase for their common substrate, aspartate (Galili, 1995; Lam et al., 1995). The metabolism of Gln, glutamate, aspartate, and Asn is subject to a coordinated metabolic regulation. Whereas the expression of an Arabidopsis and pea Gln synthetase was shown to be stimulated by light (Lam et al., 1994, 1995), the expression of an Arabidopsis Asn synthetase was repressed by light and Suc, and stimulated by dark and nitrogen (Tsai and Coruzzi, 1990, 1991; Lam et al., 1995; Chevalier et al., 1996). It has been suggested that during the day plants accumulate Gln, glutamate, and aspartate, which are apparently used for the synthesis of other amino acids, whereas during the night, aspartate is converted into Asn for storage (Galili, 1995; Lam et al., 1995).
To unravel the regulation of the biochemical branch point through which aspartate can be converted into either Asn or the aspartate family amino acids, in the present paper we report the results of our study of the metabolic regulation of expression of an Arabidopsis AK/HSD gene. We found that the expression of this gene, which encodes two linked enzymes of the aspartate family amino acid synthesis, is mediated by light and photosynthesis-related metabolites in a coordinated, reciprocal manner to the expression of the Asn synthetase gene. In addition, analysis of chimeric AK/HSD-GUS constructs in transgenic plants suggested that metabolic regulation of the AK/HSD gene operates at the transcriptional level.
MATERIALS AND METHODS
Plant Growth and Treatments
Arabidopsis thaliana seeds were germinated in a growth chamber with a 16-h light/8-h dark cycle in flasks with liquid MS media (Murashige and Skoog, 1962) in which Suc, nitrogenous compounds, or phosphate was depleted. On the 10th d after culturing, various compounds were added at the following concentrations: Suc, 200 mm; mannitol, 200 mm; Gln, 100 mm; Asn, 100 mm; NH4NO3, 20 mm; and phosphate, 1.25 or 6.25 mm KH2PO4, as stated in Figure 3. The germinating seedlings were then incubated for an additional 24 h, harvested, and immediately frozen in liquid nitrogen.
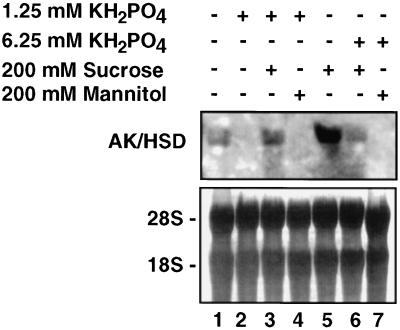
Figure 3.
Effect of phosphate on the expression of the Arabidopsis AK/HSD gene. Arabidopsis seedlings were incubated in the absence (−) or in the presence (+) of various combinations of phosphate, Suc, and mannitol, as described at the top. Northern-blot analysis using AK/HSD as a probe (top) and methylene blue staining of the 18S and 28S rRNA bands (bottom) were performed as described in Figure 2.
Transgenic tobacco (Nicotiana tabacum L.) seeds were germinated on Nitsch plates (Nitsch, 1970) with kanamycin (100 μg mL−1) as a selection. The seedlings were then transferred to the greenhouse under controlled conditions. Leaves of 1-month-old transgenic tobacco plants 5 to 10 cm tall were collected, surface sterilized with 10% bleach and 0.05% Tween 20, and thoroughly rinsed with double-distilled water. Leaf pieces (1 cm2) were excised from the leaves and incubated in 2 mL of buffer (50 mm Mes, pH 6.0, 40 mm KCl, and 10 mm CaCl2) in 24-well plastic plates. Different treatments were applied by adding various compounds at the following concentrations: Suc, 200 mm; mannitol, 200 mm; Gln, 100 mm; Asn, 100 mm; and NH4NO3, 20 mm. The leaf pieces were incubated for an additional 24 h before they were collected and immediately frozen in liquid nitrogen for further protein extraction.
Construction of Arabidopsis AK/HSD Promoter-GUS Constructs and Plant Transformation
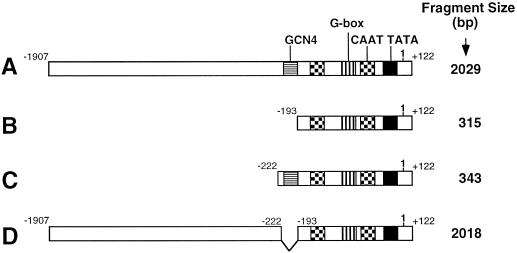
DNA fragments of 2029 and 315 bp of the Arabidopsis AK/HSD promoter region (Fig. 1, A and B, DNA fragments starting at +122 and ending at −1907 and −193, respectively, upstream to the transcription initiation site) were previously produced by PCR (Zhu-Shimoni et al., 1997) and designated “A” and “B,” respectively. Construct A (Fig. 1A) was digested by restriction enzyme HindIII, which has a site just upstream of the 5′-CTTGACTCTA-3′ GCN4-like box (positioned at −198 to −208) and another site on the vector at the 5′ termini of the promoter fragment. By doing so, a 5′ upstream fragment with a size of approximately 1.7 kb was excised, and then the plasmid was re-ligated at HindIII sites. The new clone, containing a 343-bp promoter fragment (including the GCN4-like box), was designated “C” (Fig. 1C). The approximately 1.7-kb 5′ upstream promoter fragment released by HindIII, as described above, was ligated into construct B by its HindIII site on its vector. This step resulted in a new clone designated “D,” containing a 2018-bp promoter fragment lacking the 10 nucleotides of the GCN4-like box (Fig. 1D).
Figure 1.
Schematic representation of deletion constructs of the Arabidopsis AK/HSD promoter. DNA fragment sizes of the different promoter fragments (A–D) are shown on the right. The − and + positions of the truncation sites relative to the putative transcription start site (labeled as 1 on top of the bars) are given to the left and right ends of the construct bars. Different putative regulatory elements are indicated by different patterned boxes and are marked on top of the upper bar. Truncations are aligned and internal deletions are indicated by single lines.
All constructs were subcloned into the binary vector pBI101.3 (Clontech, Palo Alto, CA) upstream of the coding region of the GUS reporter gene, which included a 5′ Ω translation enhancer from the coat protein of tobacco mosaic virus (Gallie et al., 1987, 1989), followed by the 3′ transcription termination sequence of the nopaline synthetase gene. The plasmids were transformed into the Agrobacterium tumefaciens strain LBA4404 and were used to transform N. tabacum cv Samsun NN by the leaf-disc protocol (Horsch et al., 1985). Transgenic plants were selected in MS medium supplemented with 100 mg L−1 kanamycin and grown at 25°C under a 16-h light/8-h dark cycle. Transgenic T0 plants were grown in the greenhouse and selfed. T1 seeds were germinated on MS medium supplemented with 100 mg L−1 kanamycin.
RNA Extraction and Northern Analysis
Total RNA was extracted from various tissues using the Tri-Reagent (MRC, Inc., Cincinnati, OH), according to the protocol provided by the manufacturer. RNA samples (10 μg) were electrophoresed in 1% agarose gels containing 2.2 m formaldehyde and 50 mm Mops, pH 7.0, and transferred to nylon (Hybond-N, Amersham). The blots were hybridized for 12 to 16 h at 65°C with 32P-labeled probes that contained a PCR-generated AK domain of Arabidopsis AK/HSD cDNA (J.X. Zhu-Shimoni and G. Galili, unpublished results). Hybridization was performed in 5× SSPE, 5× Denhardt's solution, and 1% SDS. Blots were washed twice for 10 min at 65°C in 1× SSPE and 0.1% SDS, followed by 15 min at 65°C in 0.1× SSPE and 0.1% SDS. Equal loading of RNA samples, as well as the efficiency of transfer, was determined according to the quantity of 28S and 18S rRNA observed upon methylene blue staining of blots prior to hybridization.
Fluorometric Analysis of GUS Activity
Fluorometric analysis of GUS activity was carried out according to a previous procedure (Jefferson et al., 1987). GUS activity was expressed as picomoles of 4-methyl umbelliferone per milligram of protein per minute. Protein concentration in the extracts was determined by the method of Bradford (1976).
RESULTS
Metabolic Regulation of AK/HSD Gene Expression by Suc and Nitrogenous Compounds
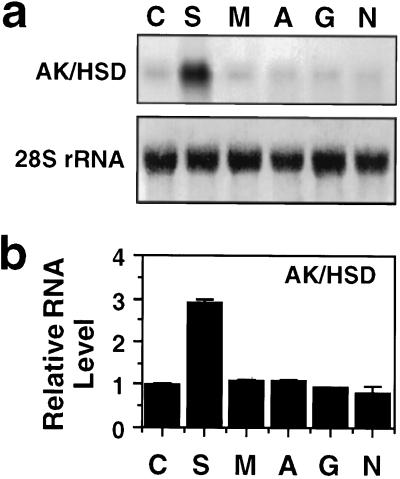
Production of amino acids requires carbon and nitrogen. Therefore, we have studied whether the expression of the Arabidopsis AK/HSD gene in vegetative tissues is regulated by the availability of Suc (a carbon source and a product of photosynthesis) and by compounds related to nitrogen assimilation. To address this, germinating seedlings of Arabidopsis in culture were treated with Suc, mannitol, Asn, Gln, or NH4NO3, and then total RNA was extracted and subjected to northern-blot analysis using the AK/HSD cDNA as a probe. As shown in Figure 2, Suc significantly increased the AK/HSD mRNA level by nearly 3-fold, whereas treatments with Asn, Gln, or NH4NO3 had no effect. In addition, the control treatment with mannitol showed no effect on the level of AK/HSD mRNA, ruling out the possibility that the Suc effect was due to increased osmoticum.
Figure 2.
Effect of Suc and nitrogen on the expression of the Arabidopsis AK/HSD gene. Arabidopsis seedlings were incubated in the absence (C) or in the presence of Suc (S), mannitol (M), Asn (A), Gln (G), or NH4NO3 (N) as described in Methods. a, AK/HSD mRNA level was assayed in northern blots and hybridized with an AK/HSD cDNA probe (top). The intensity of the 28S rRNA bands upon methylene blue staining of the same blot prior to hybridization is given at the bottom. b, The average quantitative hybridization signals from blots of three separate experiments similar to the one shown in a were obtained by phosphor-imager scanning using the MacBAS program. Bars on top of the histograms represent the ses.
Previous studies suggested that the Suc-responsive regulatory effect on plant gene expression may be related to photosynthesis, and that this regulation may be exerted indirectly by a Suc-dependent fluctuation of phosphates (Preiss, 1984; Walker and Sivak, 1986; Sadka et al., 1994). Suc is apparently converted into hexose phosphates, reducing the free phosphate levels in the cell (Thorne and Giaquinata, 1984; Krapp et al., 1993). To test whether the stimulation of AK/HSD gene expression by Suc was related to photosynthesis, we tested the effect of phosphate on the expression of this gene. Arabidopsis seedlings were incubated in medium containing phosphate or phosphate plus Suc, and then the AK/HSD mRNA level was analyzed in northern blots. As shown in Figure 3, lanes 1 and 2, treatment with 1.25 mm KH2PO4 significantly reduced the expression of AK/HSD compared with an untreated control. Addition of 200 mm Suc, together with 1.25 mm KH2PO4, restored AK/HSD expression to slightly over the basal level, whereas Suc alone induced the AK/HSD expression (compare lanes 1, 3, and 5). Treatment with 6.25 mm KH2PO4 reduced the mRNA levels of AK/HSD, and this could not be restored effectively by the addition of 200 mm Suc, compared with 1.25 mm KH2PO4 and 200 mm Suc (compare lanes 3 and 6). This suggested that Suc and phosphate had an antagonistic effect on the expression of the AK/HSD gene. Mannitol had no combined effect with phosphate, confirming again that the effect of Suc was not due to a change in osmoticum (compare lanes 2 to 4 and 7).
The Metabolic Regulation of AK/HSD Occurs at the Transcriptional Level
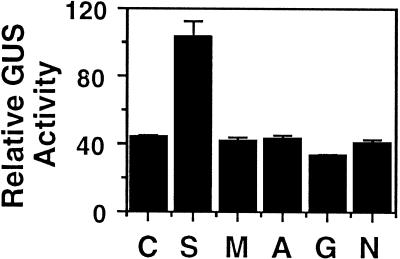
To test whether the stimulatory effect of Suc, but not nitrogenous compounds, on the expression of the AK/HSD gene was due to a transcriptional control, we used transgenic tobacco plants expressing a chimeric GUS construct fused to a 2029-bp DNA fragment containing the promoter of the Arabidopsis AK/HSD gene (Fig. 1; see also Methods). Leaf discs from these transgenic plants were treated with Suc, mannitol, Asn, Gln, or NH4NO3 and then analyzed for GUS levels. As shown in Figure 4, Suc, but not mannitol, treatment significantly increased the GUS level by about 3-fold, whereas nitrogenous compounds had no effect, which is similar to the results shown in the northern blot of Figure 2. The similarity between the effects of Suc and nitrogen on the expression of the endogenous Arabidopsis AK/HSD gene and the AK/HSD promoter-GUS constructs suggested that these regulatory effects occur at the transcriptional level. Moreover, these similarities ruled out possible artificial effects of GUS in this specific case and, therefore, enabled us to study the metabolic regulation of AK/HSD on the transcriptional level using promoter-GUS constructs.
Figure 4.
Effect of Suc and nitrogen on GUS activity in transgenic tobacco plants expressing the 2029-bp Arabidopsis AK/HSD-GUS construct. Leaf discs were treated in the absence (C) or in the presence of Suc (S), mannitol (M), Asn (A), Gln (G), or NH4NO3 (N), as described in Methods. GUS activity was measured by the MUG assay. Bars on top of the histogram represent the ses, which were calculated from at least 10 repeats obtained from three independent transformants per each treatment.
Expression of Chimeric GUS Constructs Containing Truncated AK/HSD Promoter Fragments
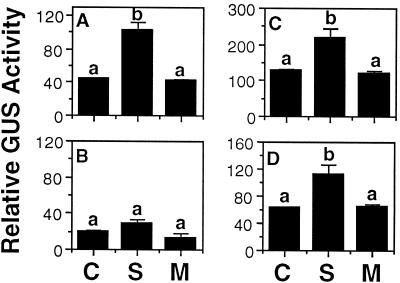
We have previously shown that a chimeric GUS construct containing a 315-bp region upstream of the Arabidopsis AK/HSD gene was expressed at a very low level (Zhu-Shimoni et al., 1997). Notably, this promoter fragment was truncated immediately downstream to a sequence resembling a GCN4 element (Fig. 1B). This element is present in a number of plant gene promoters (Muller and Knudsen, 1993) and was previously shown to regulate the seed-specific and nitrogen-dependent expression of storage protein genes (Muller and Knudsen, 1993; Albani et al., 1997). It was thus interesting to test whether this truncated promoter could still respond to Suc. As shown in Figure 5B, this truncated promoter region produced relatively low GUS levels, and no significant effect of either Suc or mannitol was observed (Fig. 5, compare A and B). To test further whether the GCN4-like element may play a functional role in the expression of the AK/HSD gene, we expressed in transgenic tobacco plants a chimeric GUS construct containing a 343-bp upstream region of the AK/HSD gene, which includes the GCN4-like element but not any upstream promoter sequence (Fig. 1C). As shown in Figure 5C, GUS production in these plants was stimulated by Suc but not by mannitol in a manner similar to the chimeric GUS construct containing the 2029-bp upstream sequence (Fig. 5, compare A and C). Notably, transgenic plants containing the 343-bp upstream region of the AK/HSD gene (Fig. 5C) exhibited an average GUS level that was about twice as much as those expressing the longest promoter region (Fig. 5A). Although this result suggests that the AK/HSD promoter may contain negative elements located upstream of the GCN4-like sequence, this has yet to be confirmed by more extensive analysis using a larger number of independent transformants.
Figure 5.
Effect of Suc and mannitol on GUS activity in transgenic tobacco plants expressing various AK/HSD-GUS deletion constructs. Leaf discs were treated in the absence (C) or in the presence of Suc (S) or mannitol (M) as described in Methods. GUS activity was measured by the MUG assay using the protein extracts of treated leaves. A to D represent transgenic plants expressing the AK/HSD promoter fragments shown in Figure 1, A to D, respectively. Bars on top of the histogram represent the ses, which were calculated from at least 10 repeats obtained from three independent transformants per each treatment. Different letters above the bars represent significant differences at the level of 0.01, as determined by the analysis of variance test.
The above results suggested that the GCN4-like element was functional in regulating the expression of the AK/HSD gene. To test further whether this element was absolutely essential, we also expressed in transgenic tobacco plants a chimeric GUS construct containing the 2029-bp upstream sequence of the AK/HSD gene, from which a small region containing only the GCN4-like element was deleted (Fig. 1D). As shown in Figure 5D, the level of GUS expression in these plants was comparable to that in plants expressing the 2029-bp AK/HSD-GUS construct (Fig. 5, compare A and D), and was also significantly induced by Suc but not by mannitol.
DISCUSSION
Transcription of the Arabidopsis AK/HSD Gene Is Stimulated by Photosynthesis-Related Signals but Not by Nitrogen
We previously showed that the expression of the Arabidopsis AK/HSD gene is subject to a spatial and temporal transcriptional control in vegetative tissues, flowers, and developing seeds (Zhu-Shimoni et al., 1997). In addition, upon exposure of young etiolated seedlings to light, expression of this gene was significantly up-regulated in cotyledons (Zhu-Shimoni et al., 1997). In the present study, northern analysis demonstrated further that expression of this AK/HSD gene in vegetative tissues is regulated by the photosynthesis-related metabolites Suc and phosphate but not by nitrogen. In addition, analysis of transgenic tobacco plants expressing chimeric GUS constructs containing the promoter of this gene showed that this regulation occurs at the transcriptional level. The antagonistic effects of Suc and phosphate on the expression of the Arabidopsis AK/HSD gene, as observed in this study, indicate that the Suc-induced expression of AK/HSD might be manipulated at least in part through the fluctuation of cellular phosphate levels affected by Suc (Thorne and Giaquinata, 1984; Krapp et al., 1993; Sadka et al., 1994).
The GCN4-Like Element Is Functional but Not Essential
Analysis of the chimeric AK/HSD-GUS constructs truncated immediately downstream and upstream of the GCN4-like element showed that this element does play a functional role in the expression of the AK/HSD gene. This result is of special interest, since expression of the AK/HSD gene was not affected by nitrogenous compounds, whereas previous studies showed that a similar element was responsible for a nitrogen-regulated expression of storage protein genes (Muller and Knudsen, 1993; Albani et al., 1997). A possible explanation for this result is that plants may possess a gene family of GCN4-related transcription factors, which are differentially expressed in various tissues and under various physiological conditions, and which regulate a wide range of genes by binding to common GCN4 elements in their promoters.
Despite the functional role of the GCN4-like element, our results showed that this element is not absolutely essential for the general expression level and metabolic regulation of the AK/HSD gene. This was concluded from the observation that the 2029-bp promoter fragment of the AK/HSD gene, which lacked the GCN4-like element, demonstrated an expression level and Suc inducibility similar to that of the 2029-bp promoter fragment containing the GCN4-like element. These results imply that the promoter of the AK/HSD gene apparently contains additional regulatory sequences that may function in a similar manner to the GCN4-like element and cause some regulatory redundancy.
The exact functional role of the GCN4-like element in the expression of the AK/HSD gene is still unknown. Although transgenic plants expressing the chimeric 343-bp AK/HSD-GUS constructs exhibited similar Suc-stimulated expression as the plants expressing the 2029-bp AK/HSD-GUS construct, we cannot conclude at present whether this element functions in the developmental and metabolic regulation of the AK/HSD gene. It is still possible that the GCN4-like element functions only in the general expression level of the AK/HSD gene, whereas its metabolic regulation is conferred by other promoter elements that operate in concert with the GCN4-like element. Analysis of this issue is complicated by the fact that these regulatory properties are apparently controlled by multiple redundant elements on the promoter of the AK/HSD gene.
Coordinated Metabolic Regulation of AK/HSD Gene Expression and Assimilation of Nitrogen into Amide Amino Acids
Aspartate, the substrate of AK, serves not only as the precursor for the aspartate family amino acids but is also among the four major amino acids (Asn, Gln, glutamate, and aspartate) that function in nitrogen transport and storage (Lam et al., 1995). In plants nitrogen is first assimilated into Gln and glutamate by Gln synthetase and GOGAT, whereas glutamate is further transformed into aspartate by aspartate amino transferases. Aspartate may then either be converted by AK into aspartate family amino acids or be converted into Asn (for storage and transport) through the action of Asn synthetase. The accumulation of Asn, Gln, glutamate, and aspartate appears to be subject to a coordinated metabolic regulation. During the day, light and sugars produced by photosynthesis stimulate the expression of genes encoding the chloroplastic Gln synthetase (GS2), as well as GOGAT. This results in efficient biosynthesis of Gln, which is further transaminated into aspartate. However, these metabolic signals also repress the expression of the gene encoding Asn synthetase, blocking further conversion of aspartate into Asn ( Tsai and Coruzzi, 1990, 1991; Lam et al., 1994, 1995; Chevalier et al., 1996) In contrast, at night, when the carbon-to-nitrogen ratio is relatively low, dark and relatively high nitrogen levels stimulate the expression of Asn synthetase, resulting in efficient conversion of aspartate into Asn (Lam et al., 1995). Our previous (Zhu-Shimoni et al., 1997) and present reports showed further that the conversion of aspartate into Asn or into aspartate family amino acids is also subject to a coordinated, reciprocal metabolic regulation of the expression of AK/HSD and Asn synthetase genes. Light and photosynthesis-related metabolites repress the expression of Asn synthetase, whereas they stimulate the expression of the AK/HSD gene, apparently resulting in efficient conversion of aspartate into aspartate family amino acids during the day. In contrast, dark stimulates the expression of Asn synthetase genes, whereas it apparently represses the expression of the AK/HSD gene, resulting in the conversion of aspartate into Asn during the night. Similarly, nitrogen, which stimulates the expression of Asn synthetase genes (Lam et al., 1995; Chevalier et al., 1996), had no effect on the expression of the AK/HSD gene. A similar reciprocal metabolic regulation was also suggested to operate between the expression of the genes encoding GS2 and Asn synthetase (Lam et al., 1995).
ACKNOWLEDGMENTS
We are grateful to Drs. Gideon Grafi and Simcha Lev-Yadun for critical reading of the manuscript.
Abbreviations:
- AK
aspartate kinase
- GOGAT
glutamate synthase
- HSD
homoserine dehydrogenase
- MS
Murashige-Skoog
Footnotes
This research was supported by the United States-Israel Binational Science Foundation (grant no. 91–00277/1) and by the Leo and Julia Forchheimer Center for Molecular Genetics. G.G. is an incumbent of the Bronfman Chair of Plant Sciences.
LITERATURE CITED
- Albani D, Hammond-Kosack MCU, Smith C, Conlan S, Colot V, Holdsworth M, Bevan M. The wheat transcriptional activator SPA: a seed-specific bZIP protein that recognizes the GCN4-like motif in the bifactorial endosperm box of prolamin genes. Plant Cell. 1997;9:171–184. doi: 10.1105/tpc.9.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Champigny M-L, Foyer CH. Nitrate activation of cytosolic protein kinase diverts photosynthetic carbon flow from sucrose to amino acid biosynthesis. Basis for a new concept. Plant Physiol. 1992;100:7–12. doi: 10.1104/pp.100.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C, Bourgeois E, Just D, Raymond P. Metabolic regulation of asparagine synthetase gene expression in maize (Zea mays L.) root tips. Plant J. 1996;9:1–11. doi: 10.1046/j.1365-313x.1996.09010001.x. [DOI] [PubMed] [Google Scholar]
- Galili G. Regulation of lysine and threonine synthesis. Plant Cell. 1995;7:899–906. doi: 10.1105/tpc.7.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Lucas WJ, Walbot V. Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell. 1989;1:301–311. doi: 10.1105/tpc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TMA. The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987;15:3257–3273. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucoronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Hofmann B, Schafer C, Stitt M. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates. A mechanism for the “sink regulation” of photosynthesis? Plant Physiol. 1993;99:627–631. [Google Scholar]
- Lam H-M, Coschigano K, Schultz C, Melo-Oliveira R, Tjaden G, Oliveira I, Ngai N, Hsieh M-H, Coruzzi G. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell. 1995;7:887–898. doi: 10.1105/tpc.7.7.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H-M, Peng SS, Coruzzi GM. Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol. 1994;106:1347–1357. doi: 10.1104/pp.106.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Knudsen S. The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and endosperm box. Plant J. 1993;4:343–355. doi: 10.1046/j.1365-313x.1993.04020343.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nitsch JP. Experimental androgenesis in Nicotiana. Phytomorphology. 1970;19:389–404. [Google Scholar]
- Preiss J. Starch, sucrose biosynthesis and partition of carbon in plants are regulated by orthophosphate and triose-phos- phates. Trends Biochem Sci. 1984;9:24–27. [Google Scholar]
- Sadka A, DeWald DB, May GD, Park WD, Mullet JE. Phosphate modulates transcription of soybean VspB and other sugar-inducible genes. Plant Cell. 1994;6:737–749. doi: 10.1105/tpc.6.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE. Storage proteins of vegetative plant tissues. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:303–322. [Google Scholar]
- Thorne JH, Giaquinata RT. Pathways and mechanisms associated with carbohydrate translocation in plants. In: Lewis DH, editor. Storage Carbohydrates in Vascular Plants: Distribution, Physiology and Metabolism. Cambridge, UK: Cambridge University Press; 1984. pp. 75–96. [Google Scholar]
- Tsai F-Y, Coruzzi GM. Dark-induced and organ-specific expression of two asparagine synthetase genes in Pisum sativum. EMBO J. 1990;9:323–332. doi: 10.1002/j.1460-2075.1990.tb08114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F-Y, Coruzzi GM. Light represses the transcription of asparagine synthetase genes in photosynthetic and non-photo- synthetic organs of plants. Mol Cell Biol. 1991;11:4966–4972. doi: 10.1128/mcb.11.10.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DA, Sivak MN. Photosynthesis and phosphate: a cellular affair? Trends Biochem Sci. 1986;11:176–179. [Google Scholar]
- Zhu-Shimoni JX, Lev-Yadun S, Matthews B, Galili G. Expression of an aspartate kinase homoserine dehydrogenase gene is subject to specific spatial and temporal regulation in vegetative tissues, flowers, and developing seeds. Plant Physiol. 1997;113:695–706. doi: 10.1104/pp.113.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]