Abstract
Background:
There is growing evidence that excess generation of highly reactive free radicals, largely due to hyperglycaemia causes oxidative stress, which further exacerbates the development and progression of type 2 diabetes and its complications.
Objectives:
In this study, the level of oxidative stress was compared with glycaemic control in type 2 diabetic patients.
Method:
Fifty confirmed type 2 diabetic patients, aged between 25 and 70 years were used for the study. 20 patients with good glycaemic control served as positive control while 20 apparently healthy non-diabetic age-matched individuals served as negative control. The FBS, MDA and HbA1 c were determined in fasting blood samples using standard methods.
Results:
Patients with poor glycaemic control had significantly raised MDA and HbA1c (P =0.0001) when compared with non diabetics However, those with good glycaemic control only had a significant increase in the MDA when compared to non diabetic. (P =0.0001).).The MDA level when compared to fasting blood sugar (FBS) and glycated haemoglobin (HbA1c) showed a positive correlation.( r= 0.77; P = 0.0001 and r=0.69; P = 0.0001 respectively)
Conclusion:
This study showed that both glycaemic control and lipid peroxidation are factors to be monitored or evaluated in the management of type2 diabetics to avoid the development of diabetic complications.
Introduction
Diabetes mellitus is defined as a group of metabolic diseases characterised by high blood glucose levels that result from defects in insulin secretion or action or both.1 It is associated with a significant long-term risk of early mortality and morbidity.2 Diabetes is largely responsible for blindness, amputations, nerve damage, and end stage renal failure, with increased coronary artery, cerebrovascular and peripheral vascular disease with up to 80% of deaths in people with diabetes caused by cardiovascular disease. Considering these facts, it is important to recognise and treat this devastating disease early, to ameliorate or prevent the serious complications associated with it.3 Insufficient production of insulin (either absolutely or relatively to the body's needs), production of defective insulin or inability of cells to properly and efficiently utilise insulin leads to hyperglycaemia and diabetes. This later condition affects mostly the cells of muscle and fat tissues and results in a condition known as “insulin resistance”.1 Basically, three types of diabetes mellitus exist; type 1, type 2 and pregnancy induced diabetes mellitus. In type 1 diabetes mellitus, there is a progressive inability of the pancreas to secret insulin because of autoimmune destruction of the beta cells.4
Type 2 diabetes mellitus is referred to as non-insulin dependent diabetes mellitus (NIDDM) or adult onset diabetes mellitus (AODM). It is characterised by insulin resistance which may be combined with relatively reduced insulin secretion. The defective responsiveness of body tissues to insulin is believed to involve the insulin receptors.2 Type 2 diabetes mellitus is the most common form of diabetes and most individuals with the disease are adults. However, children and adolescents can develop type 2 diabetes, too, particularly if they are overweight and have a history of type 2 diabetes in their family.5
Glycated haemoglobin (haemoglobin Hb1C also HbA1c) is a form of haemoglobin used primarily to identify the average plasma glucose concentration over prolonged period of time. It is formed in a non-enzymatic pathway by haemoglobin's normal exposure to high plasma levels of glucose. Glycation of haemoglobin had been associated with cardiovascular disease, nephropathy, and retinopathy in diabetes mellitus.6 Glycated haemoglobin is recommended for checking blood sugar control in people who might be pre-diabetic and monitoring blood sugar control in patients with more elevated levels.7
Currently, type 2 diabetes mellitus has been linked to oxidative stress through a single unifying mechanism of superoxide radical production. These radicals cause peroxidative damage to cell membrane and DNA, leading to the increase in the plasma levels of some of the end products like Malondialdehyde (MDA). This is the common pathogenic factor leading to insulin resistance, B-cell dysfunction, impaired glucose tolerance and ultimately type 2 diabetes mellitus.8 Furthermore, this mechanism has been implicated as underlying cause of both the macrovascular and microvascular complications associated with type 2 diabetes mellitus.
Excess nourishment and sedentary lifestyle lead to glucose and fatty acid accumulation within muscle, adipose tissue and pancreatic cells resulting in production of excess reactive oxygen species (ROS) particularly superoxide anion, through the mitochondrial electron transport chain.9 The manifestation of type 2 diabetes mellitus is an elevated fasting blood sugar secondary to insufficient insulin action. This is of two folds: the presence of insulin resistance, and the reduction in endogenous insulin. The United Kingdom Prospective Diabetes Study (UKPDS) demonstrated a progressive decline of endogenous insulin release and demonstrated β-cell function of less than 60% at baseline for patients with type 2 Diabetes mellitus.10
It has been shown that elevated HbA1c is associated with an increased risk of complications in patients with type 2 diabetes mellitus and that lowering HbA1c reduces such risk. Thus, HbA1c serves as a surrogate for the risk of microvascular and macrovascular complications, and these results firmly establish HbA1c as a useful measure of long-term glycaemic control.11 The relationship between glycaemic control, oxidative stress and antioxidants has received limited attention in our environment. Consequently, this study was undertaken to obtain baseline information on this topic.
Subjects and Methods
This is a crossectional study consisting of 50 poorly controlled type 2 diabetic patients. Twenty (20) subjects with good glycaemic control (normal HbA1c) served as positive control, while 20 non diabetic apparently healthy subjects served as negative control. They were randomly selected by a simple lucky dip of “YES” or “NO” and were of the same age range (35-70years). Pregnant subjects and indeed subjects with any other diagnosed medical condition were excluded from the study. Test Subjects were drawn from diabetic clinic of University of Nigeria Teaching Hospital Ituku-Ozalla, Enugu State. Ethical clearance and informed oral consent was obtained.
Collection of Blood Samples
Blood samples from the patients and the controls were drawn after an overnight fast by a clean venepuncture from the antecubital vein using a 10ml disposal syringe and needle under aseptic conditions. The samples were collected while the subjects were in a sitting position with minimum stasis. Two (2) mls of blood were collected into fluoride oxalate bottles and centrifuged at 3,500 rpm for 15 minutes. The plasma was separated into sterile tubes for FBS. 3mls of blood were collected into EDTA tubes for Glycated haemoglobin (HbA1c) and malondialdehyde (MDA).
Analytical methods: Bio-Rad IN2IT (1) Analyser kit produced by Bio-Rad Laboratories Inc. UK. was used for estimation of glycated haemoglobin,12 while spectrophotometric method for lipid peroxidation was used for MDA (measured as thiobarbituric acid reacting substances-TBARS)13 Enzymatic- colorimetric estimation of plasma fasting sugar was done using the glucose oxidase- perioxidase method.14
Statistical Analysis
Values were recorded as mean and standard deviation using SPSS statistical software version17. Statistical significance was determined using student t-test. Correlation of the FBS with HbA1c and MDA was also done.
Results
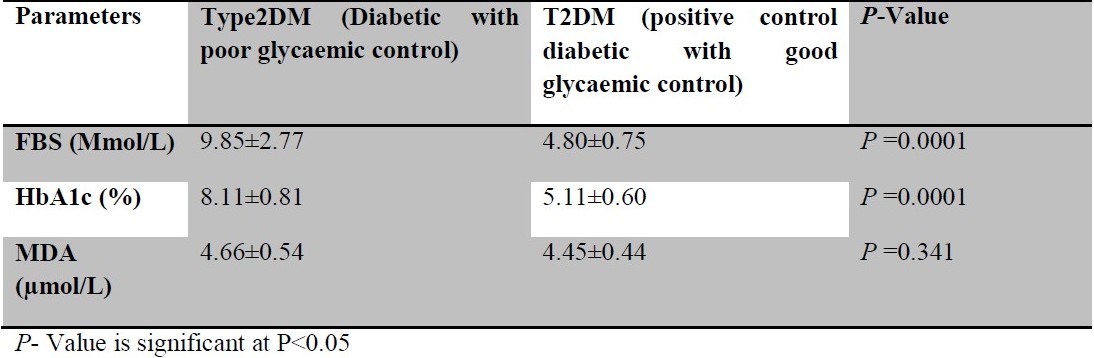
Patients with poor glycaemic control had significantly raised MDA (μmol) and HbA1c (%) (P =0.0001) when compared with non diabetics. [Table 1]. However, those with good glycaemic control only had a significant (P =0.0001) increase in the MDA (Mmol/L) when compared to non diabetic. (Table 2). FBS and HbA1c of diabetic patients with poor glycaemic control were significantly increased compared to those with good glycaemic control (P =0.0001), while there was no significant difference between their MDA values. (P =0.341). (Table 3). The MDA level correlated positively with fasting blood sugar (FBS) and glycated haemoglobin (HbA1c) between the poorly controlled and non diabetics (r= 0.77; P = 0.0001 and r = 0.69; P = 0.0001 respectively). FBS also correlated positively with HbA1c (r=0.96; P = 0.0001).
Table 1.
The mean standard deviation of fasting blood sugar FBS (Mmol/L) HbA1c and MDA in type 2 diabetic patients with poor glycaemic control and non-diabetic patients who served as negative control.
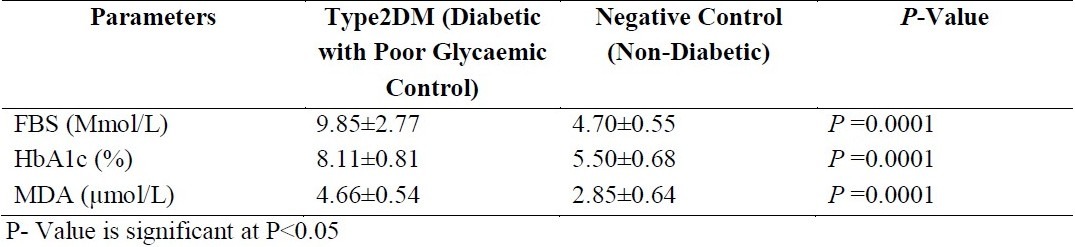
Table 2.
The mean and standard deviation of fasting blood sugar (FBS), glycated haemoglobin (HbA1c) and Malondialdehyde (MDA) in type 2 diabetic patients with good glycaemic control who served as positive control and non-diabetic patients who served as negative control
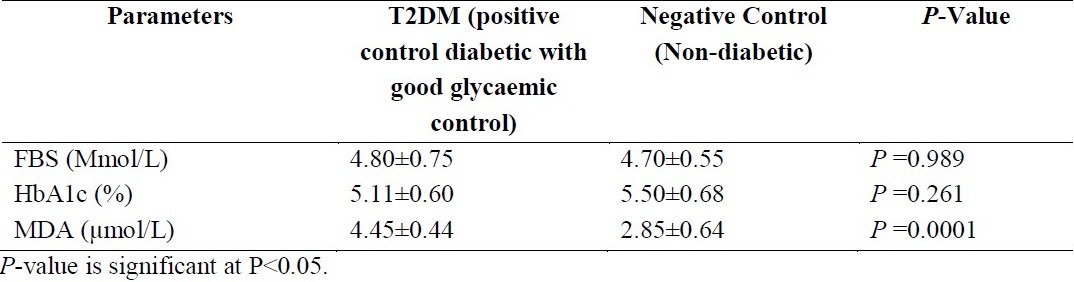
Table 3.
the mean and standard deviation of fasting blood sugar (FBS), glycated haemoglobin (HbA1c) and Malondialdehyde (MDA) in type 2 diabetic patients with poor glycaemic control and type 2 diabetic patients with good glycaemic control
Discussion
Diabetic mellitus has been known to be a state of excess generation of free radicals contributed by several mechanisms including hyperglycaemia which causes oxidative stress. This oxidative stress exacerbates the development and progress of diabetes and its complications.15 The results obtained showed that subjects with uncontrolled type 2 diabetics had significantly higher levels of FBS, HbA1c and MDA when compared with the negative control; The increased HbA1c levels (> 6.5%) reflect the poor metabolic control of diabetic patients.
The results obtained from this study also show that the type 2 diabetic patients with good glycaemic control had both their FBS within the normal range and their MDA levels were significantly increased when compared with nondiabetics but reduced when compared to diabetics without good glycaemic control. This indicates that oxidative stress measured as MDA influenced glycaemic control in type 2 diabetics.
In type 2 diabetics, if glycaemic control improves, the oxidative stress indicators such as MDA will partially decrease.16 The result as well showed that MDA when compared to FBS and HbA1c correlated positively, thus, as fasting blood sugar increases, MDA and HbA1c increase as well. There is growing evidence that excess generation of highly reactive free radicals, largely due to hyperglycaemia causes oxidative stress, which further exacerbates the development and progression of type 2 diabetes and its complications.16 In this study, as the FBS increases, the HbA1c also increases. Higher levels of HbA1c have been found in the people with elevated blood sugar (FBS) as seen in type 2 diabetes mellitus.6
This study supports the fact that in type 2 diabetes mellitus patients, there are increased levels of FBS and MDA than in non-diabetic patients and that oxidative stress measured as MDA is strictly influenced by glycaemic control. Type 2 diabetes is associated with free-radical production and this increase together with insulin resistance can lead to the development of cardiovascular complications. However, the relationship between the parameters evaluated and insulin resistance was not established in this study.
It is thus suggested that the inclusion of adjuvant antioxidant micronutrients to hypoglycaemic agents in the management of diabetes, might go a long way in the reduction of associated complications. Large scale randomised controlled trails are needed to validate this suggestion.
References
- 1.William H, Herman DW. Division of Endocrinology and metabolism University of Michigan Medical Centre USA. BMJ. 1999;319:104–106. [Google Scholar]
- 2.Department of Non communicable Disease Surveillance Definition, Diagnosis and classification of Diabetes Mellitus and Complications. World Health Organization (WHO) 1999 [Google Scholar]
- 3.Wright EJ, Scism-Bacon JL, Glass LC. Oxidative Stress in Type 2 Diabetes: The Role of Fasting and Post Prandialglycaemia. Int J Clin Prac. 2006;60(3):308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daneman D. Type I diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 5.Arlan R, Janet HS. Type 2 Diabetes in Children and Adolescents. A Clinician's Guide to diagnosis, Epidemiology, pathogenesis, prevention, and treatment. American Diabetes Association. 2003 [Google Scholar]
- 6.Reynolds TM, Smellie WS, Twomey PJ. Glycated haemoglobin (HbA1c) monitoring. BMJ. 2006;333:586–588. doi: 10.1136/bmj.38947.627847.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehman R, Krumholz HM. Tight control of Blood Glucose in long Standing type 2 Diabetes. BMJ. 2009;338:6800. doi: 10.1136/bmj.b800. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Quagliaro L, Catone B. Role of Hyperglycemia in Nitotyrosine postprandial generation. Diabetes Care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 9.Lisa LW, Eric SA. Achieving Glucose control in type 2 diabetes: A practical Guide for Clinicians on Oral Hypoglycemics. The Southern Medical Journal. 2004;97(11):1088–1092. doi: 10.1097/01.SMJ.0000140831.28281.7E. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes control and Complications trial DCCT, Research Group. The relationship of Glycemic exposure (HbA1c) to the risk of development and progress of retinopathy in the diabetes control and complications Trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 11.United Kingdom prospective diabetes study group. Lancet. 1998;352:837–853. doi: 10.1016/s0140-6736(98)22937-0. [DOI] [PubMed] [Google Scholar]
- 12.Beuge JA, Aust CD. Spectrometric estimation and lipid peroxidation. Enzymol. 1978;52:30. [Google Scholar]
- 13.Ochie J, Kolhatkar A. Medical Laboratory Science, Theory and Practice. 6th ed. Indian: Tata McGraw-Hill New Publishing Company Limited Delhi; 2008. Estimation of Blood Glucose; pp. 102–103. [Google Scholar]
- 14.Kalaivanam KN, Dharmalingam M, Marcus SR. Lipid Peroxidation in type 2 Diabetes Mellitus. Int J Diab Dev Ctries. 2006;26(1):30–32. [Google Scholar]
- 15.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and use of antioxidants in Diabetes. Cardiovascular Diabetol. 2005;4(1):5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


