Abstract
Background:
In developing countries, under nutrition is common, and this plays a crucial role in the pathogenesis of malaria and anemia. Indeed it has been associated with adverse pregnancy outcomes. Unfortunately, published evidence concerning the situation is lacking.
Objectives:
To evaluate some nutritional function indices of pregnant women in a rural Nigerian community.
Subjects and Methods:
This is a cross sectional study involving of 171 pregnant women from a rural area in South-eastern Nigeria. They included 72 and 99 women in their second and third trimesters respectively. The control group was of 60 women, matched in age, parity and socioeconomic conditions, non-pregnant, non-menstruating and non lactating apparently healthy women. The parameters measured by standard methods included serum iron, total proteins, albumin, globulin, packed cell volume and hemoglobin.
Results:
The results obtained from control group, second and third trimesters, recorded as Mean (SEM) were 134.60(3.12) μg/L, 101.20(4.48) μg/L and 91.87(3.42) μg/L respectively for iron; 69.12(0.80) g/L, 63.60(0.71) g/L and 57.74(0.75) g/L for total proteins; 42.95(0.92) g/L, 35.74(1.00) g/L and 35.26(0.64) g/L for albumin; 26.77(1.00) g/L, 27.78(1.07) g/L and 22.93(0.88) g/L for globulin; 32.80(0.36%), 27.92(0.37%) and 27.73(0.34%) for packed cell volume, and 11.25(0.11) g/L, 9.59(0.13) g/L and 9.57(0.14) g/L for hemoglobin respectively. These results showed that all the parameters decreased significantly in pregnancy (P<0.001) except globulin that did not show immediate, significant decrease.
Conclusion:
There is a general decrease in the nutrition parameters studied among the pregnant women from our study area, indicating under-nutrition. Urgent measures should be taken to improve the nutritional status of rural dwellers especially the antioxidant micronutrients.
Keywords: Nutritional status, Pregnancy outcome, Rural area
Introduction
Good nutritional status during pregnancy is one of the best predictors of optimal pregnancy outcome. Nutritional status during pregnancy is determined by the nutrient intake and dietary planning during pregnancy, including the macro and micro nutrients. Conception period and some weeks thereafter are the days when most organs and systems of the fetus are formed. The energy needed to develop these systems comes from the energy and nutrients in the mother's circulation and metabolism; hence adequate nutrient intake during pregnancy is particularly crucial. Undoubtedly, the foods a woman eats are the main sources of nutrients for the body and as the baby grows the requirements for these nutrients increase.[1] The increased nutrient intake will not only improve the growth and development of the baby but will also help the woman to adapt to changes to promote healthy childbirth. Hence, it has been noted that women who eat well and avoid known risks tend to have fewer complications during pregnancy and delivery, and are more likely to make larger, healthier babies.[2]
Moreover, under nutrition or protein energy malnutrition and the consequent micro and macro-nutrient deficiencies have been implicated as significant causes of various medical disorders in developing countries. Indeed, dietary impropriety has been recognized as a critical factor in the pathogenesis of some chronic diseases like atherosclerosis, diabetes mellitus, cancers and hypertension.[3] Furthermore, poor nutrition aggravates malaria anemia,[4] ensuring that people from rural areas with heavy socio-economic burden are constantly bombarded with persistent malaria parasitization and helminthic infestations. Pregnancy in such circumstances grossly exacerbates the situation and exposes the women and their unborn children to avoidable morbidity and mortality.
There is no doubt that the nutritional status of pregnant women has been recognized as an essential determinant of fetal growth and survival.[5] Incidentally, though this situation may be well known to many urban women and their pregnancy caregivers, relatively little attention has been given to it in the rural areas.[6] Moreover, in these rural areas, the concept of well balanced nutrient intake for pregnant women in particular and the inhabitants in general is not given the deserved attention. As a result, the situation has degenerated into complicated pregnancies: Spontaneous abortion, still birth, preterm birth and low birth weight.[7]
The exact nutrient needs of our pregnant women have not been adequately evaluated. Thus, replacements have been arbitrarily given with various fortified foods and nutrients. This practice has not improved the maternal and perinatal mortality indices, as they still remain embarrassingly high,[8] and prejudice the achievement of the millennium developmental goals (MDG). This calls for urgent evaluation and documentation of the nutritional status of pregnant women from rural areas. This will help obstetricians and midwives and indeed other traditional caregivers in educating such women for optimal pregnancy outcome. Furthermore, policy makers can benefit tremendously in formulating and articulating healthy nutritional policies that are optimal for feto-maternal survival. Developing countries are confronted with adverse socioeconomic environment and thus, pregnant women hardly enroll for antenatal care within their first trimester, except few primigravidae.[9,10] Yet, there is a definite need for early assessment of some nutritional parameters, particularly serum iron, as it has been found that iron supplementation started after mid pregnancy may not be able to prevent the consequences of iron-deficiency anemia.[11,12] In this study, some vital nutrient status of pregnant women from a rural area of Eastern Nigeria is evaluated.
Subjects and Methods
Study area
The study area was the rural Ngbo Community of Ohaukwu Local Government Area of Ebonyi State – an educationally disadvantaged state in the eastern part of Nigeria. According to an earlier study,[10] the area has an estimated population of about one hundred and ninety-six thousand (196,000). The inhabitants are mainly subsistent farmers with a few petty traders and primary school teachers. Their staple foods include yam, foo-foo and tapioca (from cassava), rice and corn. The main religions are Christianity and African Traditional Religion. Each of the two health centers in the community was manned by one trained Nurse/Midwife and two trained community health extension workers, while the remaining were inexperienced hands. There were no facilities for blood banking and other laboratory diagnostic procedures except the use of Sahli's tube for the estimation of hemoglobin levels.
Subjects
The subjects included apparently healthy 171 pregnant women aged between 18 and 40 years, who were on their first antenatal visits to Ngbo health center. 72 women were in their second trimester and 99 in their third trimester. The controls were 60 age-matched non-pregnant, non-menstruating women volunteers who were either workers in the health centers, school teachers or mobilized petty traders. After informed verbal consent and, ethical clearance from the relevant authorities, subjects who met the inclusion criteria were recruited.
Exclusion criteria
All subjects who presented with signs indicating malaria parasitemia including fever, headache, anorexia, weakness and general body pain were excluded from the study. Also, excluded were patients known to have chronic diseases like hypertension, diabetes mellitus and HIV infection.
Dietary recall
The dietary indices of the study area were calculated from the preliminary investigations using a 24-h dietary recall and estimated food records.[13] The results and information obtained during counseling showed that both patients and controls have similar dietary indices.
Samples
A total of 5 ml of venous blood was collected from each subject. Out of this, 2 ml was dispensed into EDTA-anticoagulated tube for hemoglobin and packed cell volume estimation while the remaining was dispensed into a chemically cleaned glass test tube. This was allowed to clot and retract and then centrifuged at 3,000 (rpm) for 5 min. The serum obtained was used for determination of serum iron and protein concentrations. Hemoglobin (Hb) and packed cell volume (PCV) estimations were usually done on the day of sample collection while the serum samples were stored frozen to gather to a reasonable number before analysis. For the control group, samples were taken on the 5th day of the last menstrual period after an early morning negative pregnancy test.
Ethical clearance
Ethical approval was obtained from the Ethics and Research Committee of Ebonyi State University Teaching Hospital, Abakaliki, while consent was sought and obtained from the subjects.
Laboratory Methods
Serum iron was determined using bathophenanthroline method.[14] Total protein was estimated by Biuret method,[15] while albumin was estimated by dye-binding method using bromocresol green – BCG.[16] Globulin was calculated as the difference between the total protein and albumin concentrations. PCV was determined by micro-hematocrit centrifugation and Hb by cyanmethemoglobin method.[17]
Analyses
Statistical analyses were done using Graph pad prism version 5.0 series. Tests of significance were determined using student t-test and ANOVA wherever appropriate. Results were given as means±standard error means (SEM) and level of significance was considered to be P<0.05. Pearson correlation was used to determine the relationship between the nutritional parameters.
Results
Figures 1a–1f are the bar charts showing the means (SEMs) of all the parameters – iron, total protein, albumin, globulin, PCV and Hb, in the controls, second and third trimesters. The values were 134.60(3.12) μg/L, 101.20(4.48) μg/L and 91.87(3.42) μg/L for iron; 69.12(0.80) g/L, 63.60(0.71) g/L and 57.74(0.75) g/L for total proteins; 42.95(0.92) g/L, 35.74(1.00) g/L and 35.26(0.64) g/L for albumin; 26.77(1.00) g/L, 27.78(1.07) g/L and 22.93(0.88) g/L for globulin; 32.80(0.36%), 27.92(0.37%) and 27.73(0.34%) for packed cell volume, and 11.25(0.11) g/L, 9.59(0.13) g/L and 9.57(0.14) g/L for hemoglobin respectively. There was no significant difference (P=0.093) between the serum iron concentration in the second and third trimesters. But the difference between each trimester and control was significant (P<0.001 each). Similar results were obtained with serum albumin, PCV and Hb. On the other hand, there was a significant decrease in the concentration of the total protein between each trimester and control and between the second and third trimesters (P<0.001 each). Likewise, the concentration of serum globulin showed a significant difference between second and third trimesters (P=0.001), and between third trimester and control (P=005), while the difference between second trimester and controls was not significant (P=0.494).
Figure 1a.
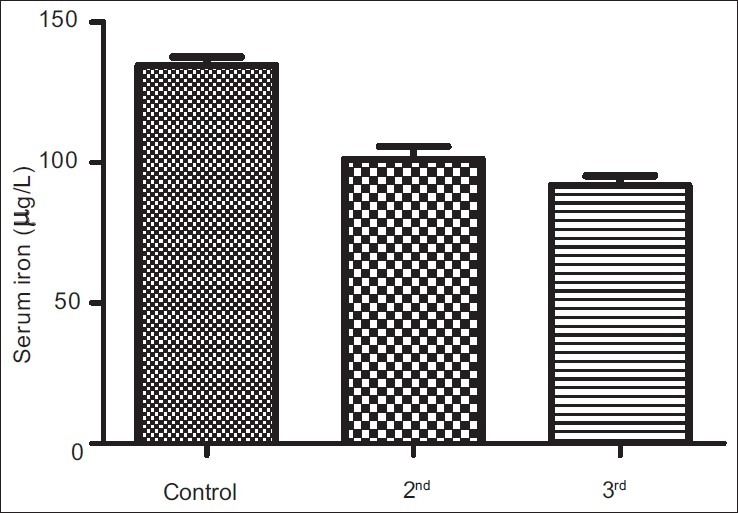
Bar chart showing means (SEMs) of serum iron concentrations in controls, second and third trimesters
Figure 1f.
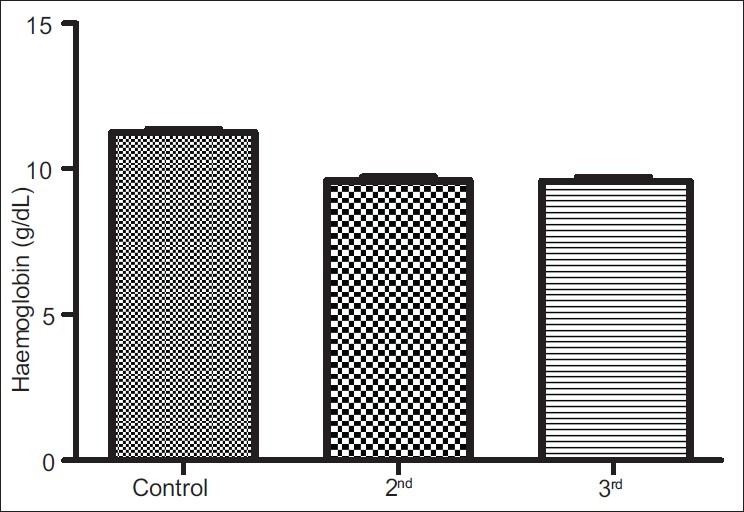
Bar chart showing means (SEMs) of hemoglobin concentrations in controls, second and third trimesters
Figure 1b.
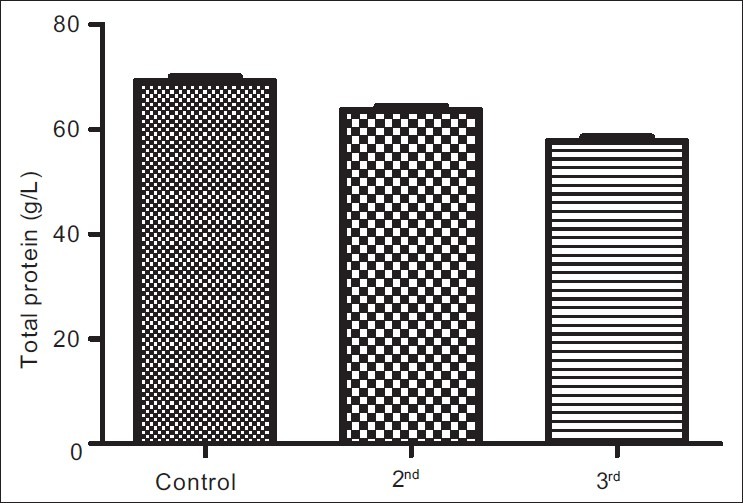
Bar chart showing means (SEMs) of total protein concentrations in controls, second and third trimesters
Figure 1c.
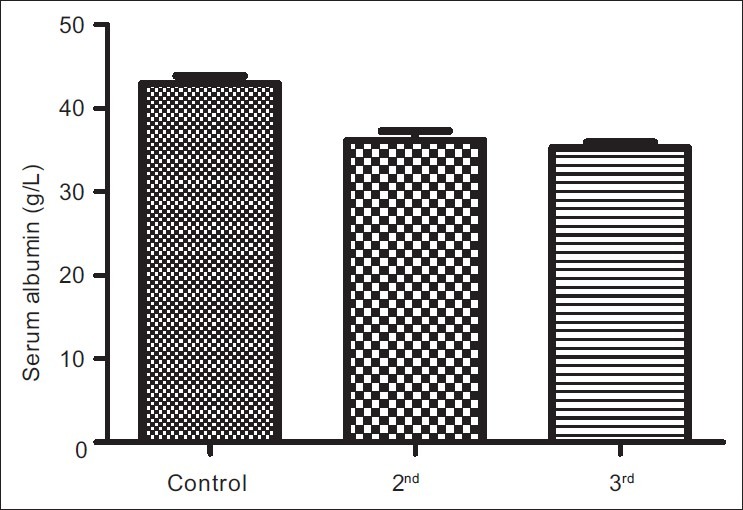
Bar chart showing means (SEMs) of serum albumin concentrations in controls, second and third trimesters
Figure 1d.
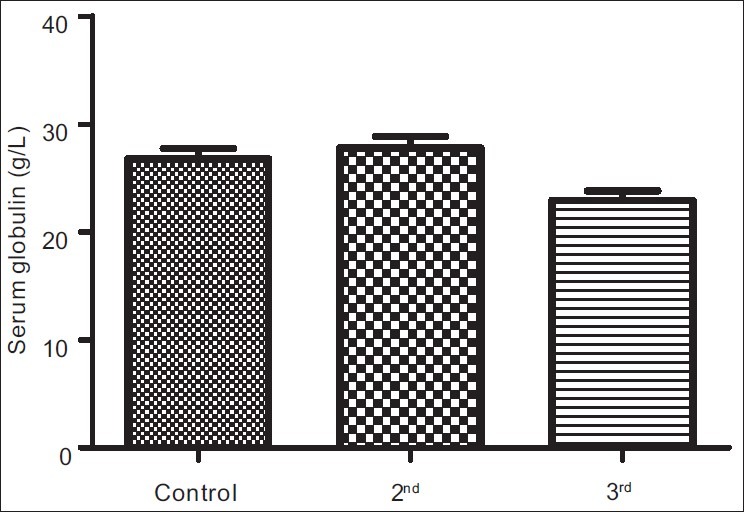
Bar chart showing means (SEMs) of serum globulin concentrations in controls, second and third trimesters
Figure 1e.
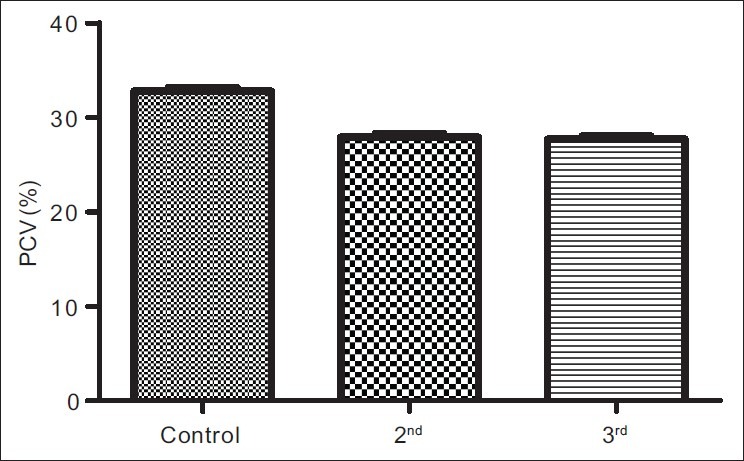
Bar chart showing means (SEMs) of packed cell volumes in controls, second and third trimesters
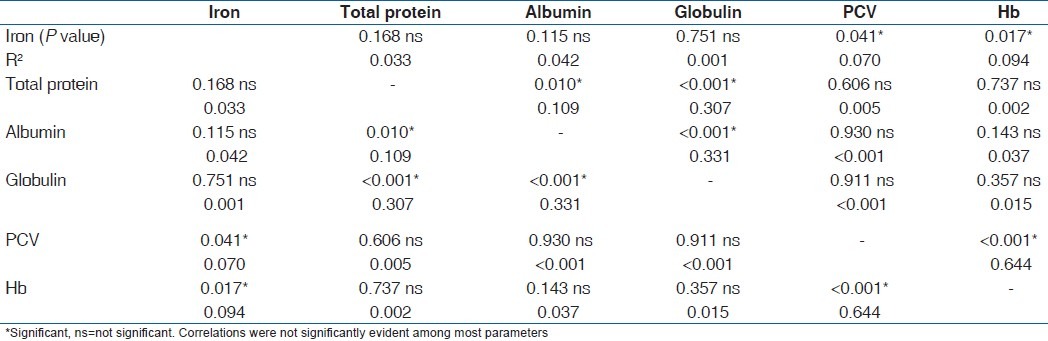
Table 1 is the result of a correlation study of the parameters in the second trimester. It showed that there was a strong correlation between serum iron and total proteins and albumin (P<0.001) but not serum globulin, PCV or Hb (P=0.216; 0.714 and 0.968 respectively). Total protein correlated significantly with all other parameters (P=0.041) except PCV and Hb (P=0.341 and 0.178 respectively). On the other hand, serum albumin correlated with all other parameters (P=0.043), while globulin correlated with all (P<0.001) except serum iron (P=0.216). PCV and Hb correlated with each other, and with serum albumin and globulin (P=0.002). Table 2 is the result of correlation study of the parameters in the third trimester. Serum iron correlated with albumin only (P=0.004), total protein with all (P<0.001) except serum iron (P=0.456) and serum albumin with all other parameters (P=0.005), while others correlated with two or three other parameters. These correlations were not seen among these parameters in the control subjects [Table 3] as one parameter correlated with only one or two other parameters.
Table 1.
Correlation among the parameters in the second trimester
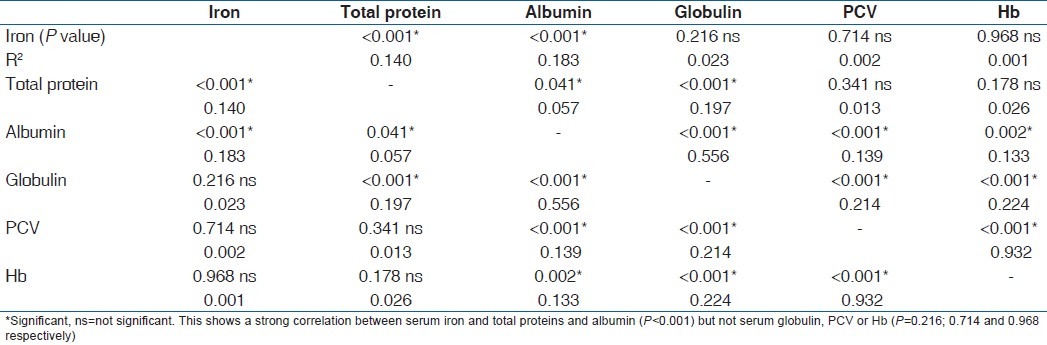
Table 2.
Correlation among the parameters in the third trimester
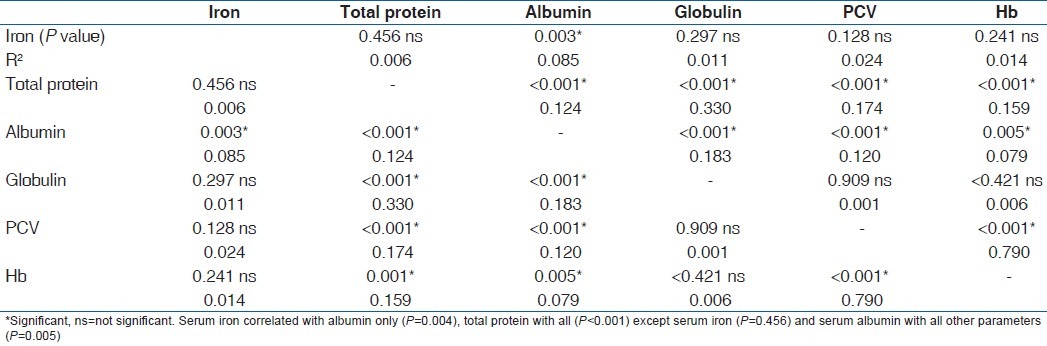
Table 3.
Correlation among the parameters in control subjects
Discussion
Serum iron decreased expectedly as pregnancy progressed, due to increased iron required necessary to optimize the red cell mass, expand the plasma volume and allow for the growth of the fetal-placental unit.[12] Early iron supplementation is generally recommended during pregnancy in order to meet the needs of both mother and fetus and prevent the consequences of iron deficiency anemia (IDA) – preterm delivery (resulting from hypoxia), oxidative stress and infection.[11,12] Moreover, only anemia diagnosed before mid pregnancy has been found to be associated with small for gestational age,[18] while maternal anemia that ensued during the third trimester has not been associated with overt risk of adverse pregnancy outcome.[12] This therefore calls for early assessment of iron stores at each stage and condition of pregnancy. This is necessary because some disease conditions, especially malaria parasitemia and pregnancy-induced hypertension,[19,20] are known to cause an increase in serum iron, which may induce oxidative stress, with associated adverse pregnancy outcome.[21,22]
Other parameters, total protein, albumin, PCV and hemoglobin, showed a similar trend as serum iron except serum globulin. The situation was different with serum globulin, where there was no significant difference between its values in controls and second trimester. However, the difference between its values in controls and third trimester, and second and third trimesters were statistically significant showing a steady decline as pregnancy progressed. Total serum protein represents the sum of albumin and globulin in broad term; therefore, it is also applicable to consider which of the fractions was affected at any change in the value of total protein. In this case, the decrease in the second trimester is most likely due to decrease in albumin fraction, and probably hypervolemia (which is consistent with pregnancy) that causes dilution effects. Moreover, because of its molecular weight, albumin is the fraction that is usually lost during proteinuria in pregnancy. Thus, hypoalbuminemia in pregnancy may not necessarily be only due to decrease in production but also dilution (due to increased volume) and increased loss in urine (proteinuria). For the serum globulin, pervious study[23] has shown that pregnant women, especially primigravidae and secundigravidae, have depressed immune functions, exposing them to malaria infections and other stress-causing factors. During pregnancy onset, there appears to be down-regulation or suppression of the T-cell-mediated or adaptive immune response. According to studies,[24,25] this is probably due to catabolism of tryptophan by maternal dendritic cells, as well as suppression of production of pro-inflammatory cytokines such as interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α) and interferon–γ (IFN-γ). These alterations to the immune status of pregnant women are required to enable mothers tolerate genetically different fetal tissues during pregnancy, but may also increase susceptibility of women to infections.[26] These alterations may have accounted for the initial non-significant reduction of serum globulin during the second trimesters and as the pregnancy progressed.
Decreases in packed cell volume and hemoglobin are consequences of decreases in serum iron and albumin. While iron store determines, to a large extent, the level of hemoglobin, protein level is one of the determinants of the value of packed cell volume. Thus, while serum iron and albumin need to be improved to increase packed cell volume and hemoglobin – to prevent anemia, globulin is particularly significant in its role as immune protein – to prevent immune-related complications including infections and infestations. Though the development of immunity, especially against malaria, is said to increase with parity,[27–29] low serum globulin and therefore, low immunity is a threat to primigravidae and secundigravidae.
This study has re-awakened our consciousness on the magnitude of maternal malnutrition in our rural areas. However, identifying the women before pregnancy and longitudinally following them throughout pregnancy would have been more appropriate. Furthermore, a 24 h dietary recall may not yield an accurate representation of the nutrient status and preferably, dietary recall for a month is more valuable. This is, however, difficult to achieve as most of the women may not be able to recall the exact food they took during the past one month. These short comings will be taken care of in future studies.
It is less than half a decade to the MDGs and maternal nutritional indices are not encouraging. Maternal/perinatal morbidity and mortality has remained unacceptably high, and it is patently obvious that the attainability of the MDGs is questionable. Regrettably, no concerted effort seems to be in place either by the government or the nongovernmental agencies. All energies and resources have been channeled into HIV/AIDs prevention and treatment with minimal input on the prevention of maternal malnutrition. This calls for desperate measures to improve the appalling situation, especially in the rural areas. This will include not only improving the macronutrients but also the micronutrients, particularly antioxidant micronutrients known to ameliorate the effects of oxidative stress. There is also absolute need for periodic public health education in these areas to notify women on the need for early antenatal care and nutritional blueprint for intending and expectant mothers.
Acknowledgement
We are grateful to the Health Officer in Ohaukwu LGA for permission and provision of logistic support for the study. We are also grateful to the nurses and other employees of the health centers at Ngbo for their co-operation during the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.ACOG. Nutrition during pregnancy. The American Congress of Obstetricians and Gynecologists. (ACOG Education Pamphlet AP001, 2010) [Last accessed on 2011 Jan 14]. Available from: http://www.acog.org/publications/patient_education/bp001.cfm .
- 2.Isenberg MH. Nutrition during pregnancy. 1999. Jan 1, [Last accessed on 2011 Jan 14]. Available from: http://www.ivillage.com/nutrition-duringpregnancy/6-a-144752 .
- 3.Human Pathology. Nutritional deficiencies. (HP 14724, 2010) [Last accessed on 2011 Jan 14]. Available from: http://www.humpath.com/spip.php?article14724 .
- 4.Crawley J. Reducing the burden of anemia in infants and young children in malaria-endemic countries of africa: From evidence to action. Am J Trop Med Hyg. 2004;71(2 Suppl):25–34. [PubMed] [Google Scholar]
- 5.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, et al. Vitamins and perinatal outcomes among HIV-Negative women in Tanzania. N Engl J Med. 2007;356:1423–31. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 6.Saxena V, Srivastava VK, Idris MZ, Mohan U, Bhushan V. Nutritional Status of Rural Pregnant Women. Indian J Community Med. 2000;25:104–7. [Google Scholar]
- 7.Sharma RK, Cooner PP, Sekhon AS, Dhaliwal DS, Singh K. A study of effect of maternal nutrition on incidence of low birth weight. Indian J Community Med. 1999;24:39–43. [Google Scholar]
- 8.Nwagha UI, Nwachukwu D, Dim C, Ibekwe PC, Onyebuchi A. Maternal mortality trend in South East Nigeria; less than a decade to the millennium developmental goals. J Womens Health (Larchmt) 2010;19:323–7. doi: 10.1089/jwh.2008.1028. [DOI] [PubMed] [Google Scholar]
- 9.Nwagha UI, Ugwu OV, Nwagha TU, Anyaehie US. The influence of parity on the gestational age at booking among pregnant women in Enugu, South East Nigeria. Niger J Physiol Sci. 2008;23:67–70. doi: 10.4314/njps.v23i1-2.54928. [DOI] [PubMed] [Google Scholar]
- 10.Ogbodo SO, Nwagha UI, Okaka AN, Ogenyi SC, Okoko RO, Nwagha TU. Malaria parasitaemia among pregnant women in a rural community of eastern Nigeria: Need for combined measures. Niger J Physiol Sci. 2009;24:95–100. doi: 10.4314/njps.v24i2.52923. [DOI] [PubMed] [Google Scholar]
- 11.Allen LH. Biological mechanisms that might underlie iron's effects on fetal growth and preterm birth. J Nutr. 2001;131:581S–9S. doi: 10.1093/jn/131.2.581S. [DOI] [PubMed] [Google Scholar]
- 12.Scholl TO. Iron status during pregnancy: Setting the stage for mother and infant. Am J Clin Nutr. 2005;81:1218S–22S. doi: 10.1093/ajcn/81.5.1218. [DOI] [PubMed] [Google Scholar]
- 13.Oguntawo EB, Akinyele IO. Nutritional composition of commonly eaten foods in Nigeria; raw, processed and prepared. Ibadan Nigeria: Food Basket Foundation Publication Series; 1995. Food consumption of individuals; pp. 37–53. [Google Scholar]
- 14.Nuttal KL, Klee GG. Analyses of haemoglobin metabolism – porphyrins, iron and bilirubin. In: Burtis CA, Ashwood ER, editors. Tietz Fundamentals of Clinical Chemistry. 5th ed. India: Elsevier; 2006. pp. 584–606. [Google Scholar]
- 15.Reinhold JG. Total protein, albumin and globulin. In: Reiner M, editor. Standard methods in Clinical Chemistry. New York: Academic Press Inc; 1953. pp. 88–97. [Google Scholar]
- 16.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 17.Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. UK: Cambridge University Press; 2005. pp. 299–314. [Google Scholar]
- 18.Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: Differential risks for preterm birth and small for gestational age. Obstet Gynecol. 2000;96:741–8. doi: 10.1016/s0029-7844(00)00982-0. [DOI] [PubMed] [Google Scholar]
- 19.Samuels P, Main EK, Mennuti MT, Gabbe SG. The origin of increased serum iron in pregnancy-induced hypertension. Am J Obstet Gynecol. 1987;157:721–5. doi: 10.1016/s0002-9378(87)80037-6. [DOI] [PubMed] [Google Scholar]
- 20.Ogbodo SO, Okeke AC, Obu HA, Shu EN, Chukwurah EF. Nutritional status of parasitemic children from malaria endemic rural communities in eastern Nigeria. Curr Pediatr Res. 2010;14:131–5. [Google Scholar]
- 21.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol. 1996;175:1356–9. doi: 10.1016/s0002-9378(96)70054-6. [DOI] [PubMed] [Google Scholar]
- 22.Scholl TO. High third-trimester ferritin concentration: Associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol. 1998;92:161–6. doi: 10.1016/s0029-7844(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood B. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70:1–7. [PubMed] [Google Scholar]
- 24.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 25.Kudo Y, Boyd CA. Characterization of L-tryptophan transporters in human placenta: A comparison of brush border and basal membrane vesicles. J Physiol. 2001;531:405–16. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yip L, McCluskey J, Sinclair R. Immunological aspects of pregnancy. Clin Dermatol. 2006;24:84–7. doi: 10.1016/j.clindermatol.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Riley EM, Schneider G, Sambou I, Greenwood BM. Suppression of cell-mediated immune responses to malaria antigens in pregnant Gambian women. Am J Trop Med Hyg. 1989;10:141–4. doi: 10.4269/ajtmh.1989.40.141. [DOI] [PubMed] [Google Scholar]
- 28.Rasheed FN, Bulme JN, Dunn DT, Menendez C, Jawla MF, Jepson A, et al. Suppressed peripheral blood and placental lymphoproliferative responses in first pregnancies: Relevance to malaria. Am J Trop Med Hyg. 1993;48:154–60. doi: 10.4269/ajtmh.1993.48.154. [DOI] [PubMed] [Google Scholar]
- 29.Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: Linking immunity and pathogenesis to prevention. Am J Trop Med Hyg. 2007;77(6 Suppl):14–22. [PubMed] [Google Scholar]


