Summary
Many invasive species have evolved behavioural and morphological characteristics that facilitate their dispersal into new areas, but it is unclear how selection on this level of the phenotype filters through to the underlying physiology. Cane toads have been dispersing westward across northern tropical Australia for more than 70 years. Previous studies of cane toads at the invasive front have identified several behavioural, morphological and locomotory characteristics that have evolved to facilitate dispersal of toads. We assessed a range of physiological characteristics associated with locomotory abilities in toads from the long-established, east coast of Australia, from the invasive front, and from a site in between these locations. We measured time to exhaustion and respiratory gases of toads exercising on a treadmill, time to recovery from exhaustion, blood properties (lactate, haematocrit, haemoglobin, red blood cell count, blood cell volume), and muscle properties associated with locomotion (activities of the enzymes citrate synthase and lactate dehydrogenase, and pH buffering capacity). None of the measured physiological parameters supported the hypothesis that toads from the invasive front possess physiological adaptations that facilitate dispersal compared to toads from areas colonised in the past. The strongest difference among the three groups of toads, time to exhaustion, showed exactly the opposite trend; toads from the long-established populations in the east coast had the longest time to exhaustion. Successful colonisers can employ many characteristics to facilitate their dispersal, so the extent to which behaviour, morphology and physiology co-evolve remains an interesting question. However, in the present case at least, behavioural adaptations do not appear to have altered the organism's underlying physiology.
Keywords: anura, Bufo marinus, cane toads, dispersal, endurance, invasive species, locomotion, Rhinella marina
Introduction
Range-shift is a common phenomenon in nature (Vermeij, 2005). Species shift their range either because they are invasive, or because their habitat shifts (e.g., through climate change). Importantly, the process of range expansion can exert strong evolutionary pressure on populations in the vanguard of the range advance. These vanguard populations are assorted by dispersal ability and exist at low conspecific densities, and these conditions select for individuals on the front with higher rates of dispersal and reproduction (Phillips, Brown & Shine, 2010). Evolved increases in these life-history traits on the invasion front leads to accelerating range advance (Travis and Dytham, 2002; Holt, Barfield & Gomulkiewicz, 2006).
Perhaps one of the clearest examples of these processes at work comes from the invasion of cane toads (Rhinella marina, formerly Bufo marinus; Pramuk et al., 2008) in northern Australia. The rate at which toads have spread across northern Australia has increased five-fold in the 75 years since they were introduced (Phillips et al., 2006). When toads were first introduced near Cairns in north Queensland, they expanded their range at ten kilometres per year, but they now invade new areas further west at 55 km year−1 (Phillips et al., 2007; Urban et al., 2008). Comparison of field dispersal of toads from frontal versus older, long-established populations reveals clear increases in dispersal associated with this accelerated range advance. Toads from frontal populations move more often, move farther when they do move, and follow straighter paths than do toads from older populations (Phillips et al., 2008; Alford et al., 2009). Also, toads at the invasive front have relatively longer legs than those from areas colonised longer ago (Phillips et al., 2006). Additionally, toads from frontal populations grow significantly faster than conspecifics from older populations, suggesting that reproductive rate also may be higher in frontal populations (Phillips, 2009).
Increases in dispersal and reproductive rate are likely associated with changes in many aspects of the phenotype: changes in those traits directly contributing to dispersal and reproduction, as well as those traits that trade-off against dispersal and reproduction (Brown et al., 2007). Thus, selection on increased dispersal and reproduction can have far-reaching phenotypic consequences. One intuitive prediction we might make, for example, is that evolution of increased dispersal rate might be apparent, not only at the actual level of selection (i.e., changed dispersal behaviour), but also at levels less proximate to selection (e.g., changed exercise physiology driven by demands of changed behaviour).
Given the clear selective pressures on invasion front populations, and evidence for behavioural and morphological adaptations related to dispersal in cane toads in Australia, a unique opportunity exists to test this idea. Is the rapid dispersal in invasion front toads associated with increased locomotory speed, endurance, or other physiological aspects of locomotion? Here we test this by measuring physiological parameters associated with locomotion in toads sampled from across their geographic range in northern Australia.
Materials and Methods
Physiological adjustments associated with an increased ability to perform sustained aerobic locomotion generally include enhanced blood oxygen delivery to muscles (high Hct and [Hb], and reduced erythrocyte size; see Lay & Baldwin, 1999), and high activities of muscle respiratory enzymes such as citrate synthase (Newsholme & Start, 1973). Locomotory muscles also tend to have higher proportions of LDH-H relative to LDH-M subunit isozymes, reflected as higher pyruvate inhibition ratios (lactate dehyrdogenase activity at low concentrations of pyruvate (0.33 mmol L−1) relative to the activity at a high concentration of pyruvate (10 mmol L−1). High H subunit LDH isozymes are inhibited at higher pyruvate concentrations and preferentially act in the direction of converting lactate to pyruvate for use as an aerobic fuel (Wilson, Cahn & Kaplan, 1963; Dawson, Goodfriend & Kaplan, 1964). The applicability of this indicator to toad muscles was confirmed when we obtained pyruvate inhibition ratios of 2.20 for the more aerobic toad heart ventricle compared to 1.62 for thigh muscle. In addition, more aerobic muscles often show lower maximum activities of lactate dehydrogenase and a lower pH buffering capacity, indicating reduced reliance on short term bursts of anaerobic metabolism (Castellini & Somero 1981; Blomberg & Baldwin, 1991). Thus, to examine locomotor endurance we tested, not only whole animals for their endurance, but also a range of physiological variables including standard metabolic rate, blood lactate levels, general haematology, and muscle enzyme activity.
Species and collection
Cane toads were introduced to north-eastern Australia in 1935 and they have subsequently dispersed along the east and north coasts of the continent (Kearney et al., 2008). The toads used in this study were a subset of a much larger group involved in a broader study (Phillips, 2009; Greenlees, Phillips & Shine 2010; Webb et al., 2008). In October 2006, we collected toads from three sites representing three lengths of time since colonisation: Cairns (colonised 1936), Borroloola (1988) and Timber Creek (2006) (Phillips, 2009). After collection all toads were housed outdoors in semi-natural conditions at Middle Point, near Darwin (Phillips, 2009) for three to four weeks. Six toads from each study site, ranging in mass from 63.9g to 148.0g (mean = 102.0, s.d. = 21.4), were then taken to a laboratory at Charles Darwin University, Darwin, Northern Territory, for measurements of endurance, respiratory gases, blood characteristics, and muscle enzymes. Another group of toads (5 from Cairns, 8 from Borroloola, and 8 from Timber Creek; mean mass 114.2 ± 39.3 g, range 63.8–178.9g) were brought into the laboratory for measurements of thermal tolerance and sprint rate as a function of temperature. Experiments began two days after bringing the animals into the laboratory.
Respiratory gas analyses
Oxygen consumption was measured with an Ametek Applied Electrochemistry O2 analyser (model S-3 A/II, Pittsburgh, Penn.). For measurements of standard metabolic rate (SMR), toads were individually placed inside acrylic metabolic chambers (9 cm × 9.5 cm diameter) that were placed in a controlled-temperature incubator set at 30°C. During measurements, room air was drawn through the metabolic chamber containing the toad, a carbon dioxide absorbent (Drägersorb, Lübeck, Germany), a drying column, and a mass flowmeter before a sample of the air was drawn into the gas analyser. Flow rates and oxygen concentration were recorded on a MacLab (model 8e, AD Instruments, Castle Hill, Australia). Metabolic rate was measured over 50 min intervals (with a 10 min baseline measurement before and after each period) for 24 h, and the lowest 50 min sampling period during the day or night was taken as the resting metabolic rate. The movement of toads inside the chambers was clearly evident from these nearly continuous traces, allowing us to select data from inactive periods for analysis (Schultz, Webb & Christian, 2008).
The respiratory gases of exercising toads were measured using a similar arrangement except that toads were placed on a treadmill with a vinyl mask tied over their heads to collect all the expired air (Christian et al., 1996), and there was no carbon dioxide absorbent in the air stream. Carbon dioxide production was measured with a Fuji (model ZFU) infrared CO2 analyser. A flow of 250 mL min−1 was drawn through the mask. Prior to exercise, a 0.2 mL heparinised blood sample was taken by cardiac puncture. Prodding of the hindquarters ensured that the animals kept pace with the treadmill, which was set at speeds ranging from 0.10 to 0.15 m s−1. The treadmill was located in a laboratory at 24°C. The highest metabolism over a 5 min period was used as a measure of maximal oxygen consumption. The time the animals hopped on the treadmill was recorded until the toad was deemed exhausted, as defined by a failure to respond to prodding for more than 1 min. After this point was reached, the treadmill was stopped, a second blood sample was taken by cardiac puncture, and the animal was covered with a cloth and allowed to sit on the treadmill as the respiratory gases were recorded for an additional 30 min. These data were analysed at 5 min intervals, but were not significantly different from SMR after 15 min, so only the first 15 min are reported here. After 30 min, a third blood sample was taken, and the toads were killed immediately with a lethal injection of buffered (pH 7.0, 500 mg L−1) tricane methanesulfonate (MS222).
Blood lactate
Aliquots of whole blood (0.1 mL) were deproteinised by the addition of 0.2 mL of 0.6 mol L−1 perchloric acid. Acidified samples were left on ice for 30 min, centrifuged at 13,000 g for 10 min, and decanted supernatants were stored at −20°C until assayed. Lactate was measured by the standard spectrophotometric method (Wells et al., 2007) using NAD and lactate dehydrogenase (test kit 826-u.v.; Sigma-Aldrich Co., St Louis, Mo, USA).
Haematology
The initial heparinised blood sample taken by cardiac puncture prior to exercise was analysed immediately after collection, using standard haematological methods (Dacie & Lewis, 1984). Haematocrit (Hct) was measured following 3 min centrifugation at 3000 g in microhaematocrit capillaries. Haemoglobin concentration ([Hb]) was measured spectrophotometrically at 540 nm following lysis and dilution of whole blood in modified Drabkin's reagent [200 mg K3Fe(CN)6 + 50 mg KCN L−1, pH 9.6]. An additional step to improve optical clarity was taken by centrifuging out cell debris from the lysed, nucleated erythrocytes (5 min at 13,000 g) (Wells et al., 2005). Red blood cell counts (RBCC) were made following dilution of whole blood in 1.2% NaCl, using an improved Neubauer chamber. Mean cell volume (MCV) was calculated from Hct and RBCC values.
Muscle enzymes
Freshly excised samples of gastrocnemius muscle (∼1 g) were finely minced on ice with scissors and homogenised in 10 ml of ice-cold 100 mmol L−1 sodium phosphate buffer, pH 7.5. Homogenates were centrifuged at 13,000 g for 3 min, and decanted supernatants were held on ice for immediate analysis.
Activity of citrate synthase and lactate dehydrogenase were measured with a recording spectrophotometer in which cuvette temperature was maintained at 25°C with a circulating water bath. Absorbance changes were followed at 340 nm for lactate dehydrogenase, and 412 nm for citrate synthase. Preliminary trials were made to determine optimal concentrations of reagent, and suitable controls lacking substrates were run to correct for non-specific activity. Assays were performed with 10 µl of suitably diluted muscle sample in a total volume of 1 ml. All determinations were made in duplicate on muscle samples from six toads from each collection site. The compositions of the reaction mixtures were as listed in Table 1.
Table 1. Reaction mixtures for toad muscle enzyme assays.
| Reaction | Mixture |
| Citrate synthase | 0.1 mmol L−1 acetyl CoA, 0.5 mmol L−1 oxaloacetate |
| 0.2 mmol L−1 5,5-dithiobis-(2nitrobenzoic acid) | |
| 75 mmol L−1 Tris-HCl buffer, pH 8.0 | |
| Lactate dehydrogenase (maximum activity) | 1 mmol L−1 puruvate |
| 0.1 mmol L−1 NAD | |
| 100 mmol L−1 sodium phosphate buffer, pH 7.5 | |
| Lactate dehydrogenase (pyruvate inhibition ratio) | 10 mmol L−1 or 0.33 mmol L−1 puruvate |
| 0.1 mmol L−1 NADH | |
| 100 mmol L−1 sodium phosphate buffer, pH 7.5 |
Muscle pH buffering capacity
The intracellular pH buffering capacity due to non-bicarbonate buffers present in muscle was determined by the method of Castellini & Somero (1981) as modified by Blomberg & Baldwin, (1991). Fresh gastrocnemius muscle (0.5 g) was diced with scissors and homogenised in 10 ml of 0.9% NaCl. Homogenates were adjusted to pH 6.0 with 1.0 mol L−1 HCl and titrated at 25°C against 20 µl aliquots of 0.2 mol L−1 NaOH while mixed continuously on a magnetic stirrer. pH values were recorded after each addition with an Activon digital pH meter equipped with an AEP412 combination pH electrode (Activon Scientific Products, Vic, Australia). Individual buffer curves were linear between pH 6 and pH 7. Buffering capacity, β slykes, is defined as the number of µmoles of base required to change the pH of 1 g of muscle by 1 pH unit over the range pH 6 to pH 7.
Other characteristics
Sprint speed was measured at five temperatures (15°C, 20°C, 25°C, 30°C, and 35°C) by racing toads along a two-metre long track that was marked out on the floor of an air-conditioned room maintained at 25°C. The track was 50 cm wide with flat side walls 40 cm high and there was a short area for acceleration and deceleration at either end. The core body temperature of each individual was manipulated by placing it in a water bath (Grant LT D6G) so that only the head remained exposed to the air, and measuring the core body temperature by intermittently inserting into the cloaca a thermocouple attached to a calibrated Fluke 51 K thermometer until the desired body temperature was achieved. Once the individual had reached the correct temperature, the time it took the individual to travel down the track was measured using a Micronta LCD Stopwatch. The toads were encouraged to move by the stamping of feet behind them, but were not physically induced to move.
We determined the critical thermal maximum and minimum (CTmax, CTmin) temperatures of toads from each population by manipulating the core body temperature of toads until they were unable to right themselves when placed on their backs. The core body temperature was controlled with a water bath as described above. The temperature of the water was changed at 1 oC increments and the individual was held at this temperature for a maximum of ten minutes. If at the end of this period the individual was able to right itself, then the temperature was again adjusted. When a toad was no longer able to right itself, the core body temperature was measured by inserting a thermocouple into the cloaca. All temperatures were measured using a calibrated Fluke 51 electronic thermometer.
Statistical analyses
Analysis of covariance was used to analyse standard and maximal metabolic rates with mass as the covariate. However, there were no significant effects due to mass (all P values for mass were 0.934 > P > 0.238). Hence, analysis of variance was used to compare the means of the three populations for all the physiological and biochemical metrics measured. A Tukey's HSD test (at the P = 0.05 level) was used to discriminate differences among populations. Because we analysed for population differences in several characteristics, we used the method of Benjamini and Hochberg (1995) to reduce the chances of false discovery inherent when doing multiple comparisons.
Results
The most striking difference among the populations was their time to exhaustion on the treadmill, with the toads from Cairns hopping for more than twice as long as the toads from the other two populations (Fig. 1), which were not statistically different from each other (F2,14 = 45.4, P<0.0001). There were no statistically significant differences among toad populations with respect to mass, SMR, maximal oxygen consumption, maximal carbon dioxide production, oxygen consumption or carbon dioxide production 5, 10 or 15 min after running on the treadmill, sprint speed or thermal tolerance (Table 2).
Fig. 1. Time to exhaustion (min) on a treadmill was significantly longer for toads from Cairns than those from Timber Creek or Borroloola (ANOVA: F2,14 = 45.4, P<0.0001), as indicated by the asterisk (Tukey's HSD post hoc test).
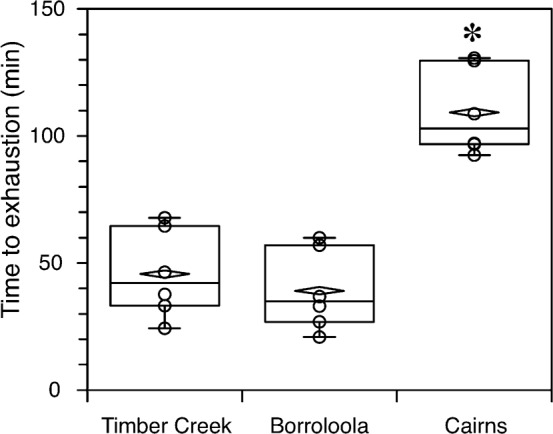
Six toads from each site were measured. Horizontal lines are the median, boxes are the 5th and 95th percentiles, whiskers are the range, and diamonds are the mean.
Table 2. Summary of metabolic, locomotory and thermal parameters for toads from each of 3 sites (see text for sample sizes).
Recovery periods 1, 2 and 3 refer to respiratory gases measured at 5, 10 and 15 min after the treadmill had stopped. Values are means ± 1 standard deviation, and P values are from ANOVAs.
| Cairns | Borroloola | Timber Creek | P value | |
| Mass (g) | 96.7 ± 18.7 | 99.5 ± 23.6 | 109.7 ± 23.3 | 0.590 |
| SMR (mL O2 h−1) | 4.98 ± 1.91 | 6.68 ± 2.40 | 8.09 ± 4.03 | 0.161 |
| Max O2 consumption (mL O2 h−1) | 35.9 ± 4.94 | 29.3 ± 8.63 | 48.2 ± 6.61 | 0.113 |
| O2 consumption recovery 1 (mL O2 h−1) | 14.5 ± 8.34 | 18.2 ± 6.41 | 20.0 ± 6.45 | 0.264 |
| O2 consumption recovery 2 (mL O2 h−1) | 9.23 ± 5.01 | 7.21 ± 8.07 | 12.8 ± 5.16 | 0.242 |
| O2 consumption recovery 3 (mL O2 h−1) | 6.75 ± 2.73 | 10.6 ± 4.47 | 9.12 ± 1.67 | 0.392 |
| Max CO2 production (mL CO2 h−1) | 44.0 ± 6.38 | 32.2 ± 6.39 | 41.5 ± 7.66 | 0.263 |
| CO2 production recovery 1 (mL CO2 h−1) | 18.8 ± 9.29 | 28.5 ± 7.12 | 26.2 ± 6.35 | 0.214 |
| CO2 production recovery 2 (mL CO2 h−1) | 17.9 ± 6.52 | 11.2 ± 7.67 | 18.5 ± 7.44 | 0.096 |
| CO2 production recovery 3 (mL CO2 h−1) | 9.02 ± 5.01 | 6.75 ± 6.53 | 11.5 ± 3.73 | 0.236 |
| Sprint speed (cm s−1) | ||||
| 15°C | 10.0 ± 1.3 | 9.4 ± 1.2 | 9.9 ± 0.9 | 0.601 |
| 20°C | 23.1 ± 3.1 | 21.7 ± 5.5 | 22.0 ± 3.4 | 0.719 |
| 25°C | 30.8 ± 6.6 | 31.0 ± 9.3 | 27.5 ± 7.5 | 0.575 |
| 30°C | 44.1 ± 10.8 | 40.3 ± 6.5 | 33.7 ± 13.2 | 0.180 |
| 35°C | 33.8 ± 7.3 | 29.6 ± 6.4 | 25.7 ± 3.6 | 0.080 |
| CTmin (°C) | 11.3 ± 0.7 | 10.9 ± 0.7 | 10.4 ± 0.8 | 0.273 |
| CTmax (°C) | 39.8 ± 1.0 | 40.0 ± 0.9 | 39.5 ± 0.5 | 0.552 |
A summary of the results obtained for blood lactates, haematology, muscle enzyme activities and muscle pH buffering capacity is presented in Table 3. Blood lactate levels prior to exercise were similar in toads from all three populations. Lactate levels immediately post exercise and 30 min after exercise were significantly higher in Borroloola toads, relative to toads from Cairns or Timber Creek (Table 3). Haematology revealed no statistically significant differences among the three populations with respect to Hct, RBCC, MCV or [Hb] (Table 3). From the gastrocnemius muscles, maximum activities of lactate dehydrogenase and pyruvate inhibition ratios did not differ significantly among the three populations, but maximum activities of citrate synthase were significantly higher in toads from Cairns (Table 3). pH buffering capacity was significantly lower in gastrocnemius muscle of toads from Borroloola compared with toads from Cairns and Timber Creek (Table 3).
Table 3. Summary of results for blood lactate, haematology, muscle enzyme activities and muscle pH buffering capacity for toads from three sites.
Values are means ± 1 standard deviation, and sample size was 6 for all values. Similar superscripted letters represent means that are not statistically different among sites, and P values are based on the results of ANOVA. The column on the far right shows P values after using the method of Bemjamini Hochberg to control the false discovery rate with multiple comparisons.
| Cairns | Borroloola | Timber Creek | P value | Benjamini Hochberg | |
| Lactate – pre-exercise (mmol L−1) | 5.08 ± 4.82 | 7.95 ± 3.92 | 2.73 ± 1.65 | 0.081 | 0.099 |
| Lactate – peak (mmol L−1) | 8.10 ± 5.58a,b | 15.68 ± 6.42a | 6.62 ± 2.48b | 0.017 | 0.046* |
| Lactate – post-recovery (mmol L−1) | 8.23 ± 4.57a.b | 12.81 ± 4.81a | 4.53 ± 1.83b | 0.009 | 0.049* |
| Haematocrit (%) | 0.27 ± 0.06 | 0.19 ± 0.04 | 0.25 ± 0.04 | 0.030 | 0.055 |
| Haemoglobin (g L−1) | 70.41 ± 10.65 | 40.96 ± 9.60 | 60.86 ± 8.3 | 0.008 | 0.088 |
| Cell count (106/mL) | 0.65 ± 0.25 | 0.41 ± 0.13 | 0.44 ± 0.06 | 0.051 | 0.080 |
| Mean cell volume (fL) | 431.8 ± 108.8 | 471.2 ± 73.7 | 553.5 ± 66.9 | 0.070 | 0.096 |
| Citrate synthase activity (µmol substrate min−1 g−1 muscle, 25°C) | 5.45 ± 1.03a | 3.37 ± 0.92b | 3.85 ± 1.22b | 0.010 | 0.037* |
| Lactate dehydrogenase (LDH) (µmol substrate min−1 g−1 muscle, 25°C) | 273.7 ± 34.1 | 186.5 ± 68.0 | 240.4 ± 89.9 | 0.114 | 0.120 |
| Pyruvate inhibition ratio (0.33/10) | 1.79 ± 0.08 | 1.81 ± 0.05 | 1.73 ± 0.09 | 0.186 | 0.186 |
| Buffering capacity (β) | 42.22 ± 5.81a,b | 34.35 ± 6.57b | 43.28 ± 2.93a | 0.022 | 0.048* |
Discussion
Although there were significant differences among sites in some of the physiological components that we studied (Tables 2 and 3), none showed clear trends associated with invasion history. Thus, we find no evidence that there are physiological correlates to the trends in dispersal rate showing that toads at the invasion front disperse more rapidly than their conspecifics from all the older, long-established populations (Phillips et al., 2006, 2008).
The physiological components that we studied were those that would logically be associated with locomotion. They can be interpreted with respect to their contribution to aerobic capacity, except for the initial lactate levels (which show that toads from all populations began the experiments in similar states), and cell volume and cell count (which can be interpreted as components of the broader characteristics of haemoglobin and haematocrit). Of the remaining variables from Table 3, high aerobic capacity is indicated by high values in some (haematocrit, haemoglobin, citrate synthase, LDH) and by low values in others (lactate measurements). By and large, our measurements of locomotor performance, as measured by endurance time (Fig. 1), correlate well with the other statistically significant variables we measured. After the correction for multiple comparisons (Table 3), there were 5 variables that were significantly different among sites: endurance time, peak lactate, lactate post-recovery, citrate synthase, and buffering capacity. All five of these variables indicated low aerobic capacity for the toads from Borroloola. The toads from Timber Creek had three indicators of high aerobic capacity and 2 indicators of low aerobic capacity (endurance time and citrate synthase). All five variables indicated high aerobic capacity in the toads from Cairns.
The samples sizes used in this study were small, thus increasing the likelihood of making a Type II (false negative) statistical error. Hence, larger studies of the physiological adaptations across their range in northern Australia would still be warranted to confirm these findings. Nevertheless, the result in endurance times (Fig. 1), for which the toads from the invasion front had significantly lower endurance than toads from Cairns, cannot be ignored. Similarly, the fact that toads from the invasion front only had three physiological variables indicating high aerobic capacity (peak lactate, lactate post-recovery, and buffering capacity), whereas toads from Cairns had five indicators of high aerobic capacity, does not support the hypothesis that toads at the invasion front have physiological adaptations to facilitate dispersal relative to other populations. Although toads at the invasive front have higher aerobic capacity compared to toads from Borroloola, there is no indication that their aerobic capacity is greater than that of toads from Cairns. Indeed, given that toads from Cairns have significantly higher endurance times and citrate synthase activity than toads from the invasion front, there would need to be several Type II errors (due to small sample size) before the aerobic capacity of the toads from the invasion front would be comparable to those from Cairns, and even more false negatives would be required to indicate support for the hypothesis that toads from the invasion front are physiologically superior in any way.
The most notable result from this study was that endurance, as measured by time to exhaustion on a treadmill, was significantly higher in the toads from Cairns, which were able to hop for a much longer period than toads from other locations (Fig. 1). This is in stark contrast to the findings of Llewelyn et al., (2010), who found that toads from Cairns had much lower endurance than toads sampled from the invasion front. The methods of measuring endurance in this study and in that of Llewelyn et al., (2010) are difficult to compare directly. However, in addition to direct measures of locomotion, we measured blood properties that we would expect to be associated with aerobic muscle work. Four of these were correlated with endurance in ways that support the endurance result, and the toads from Cairns had no indicators of low aerobic capacity compared to the toads from other populations. Thus, our endurance data are consistent with our hematological data, indicating that the results presented here are not simply an artifact of the method: our sampled toads from Cairns were in no way physiologically inferior to the toads sampled from the invasive front. The values for our sample of toads from Cairns were also similar to published measurements (Seebacher & Franklin, 2011) from another eastern Australian population of this species (except that our values for citrate synthase activity were lower), further indicating that our sample of toads from eastern Australia was not atypical.
There is disagreement in the literature with respect to the sprint speed of toads from different locations. Phillips et al., (2006) found that toads from the invasive front sprinted faster over 1 m than toads from Cairns. However, in our study and the study by Llewelyn et al., (2010), there were no statistical differences among populations from different locations when the toads were sprinted over longer distances (2 m and 10 m, respectively). Thus, locomotor tests in this instance do not appear to be particularly robust to small methodological changes.
Intuitively, having the ability to hop for long periods of time before stopping (i.e., higher endurance) would be expected to enhance the ability of toads to disperse to new areas. Estimates of time spent hopping for the highly dispersive toads on the invasion front, however, suggests that toads actually spend less than 4% of their nocturnal activity period moving (Kearney et al., 2008). Thus, it may be that locomotor endurance has little to do with dispersal rates in this species, and that behavioural variation (e.g., directionality of movement, small changes in percentage of time spent moving) may have the larger effect. Thus, the wildly varying results around locomotor performance and endurance in this species may be indicative of the general health of the sampled toads rather than their recent evolutionary history. Locomotor performance in this species may, thus, indicate the effect of local conditions at a fine spatiotemporal scale rather than broader patterns associated with the evolution of dispersal during range advance.
Dispersal abilities could be enhanced by changes in morphology, physiology, behaviour, or some combination of these factors. There are many examples of general relationships between morphology or physiology on the one hand and behaviour on the other (e.g. Feder et al., 1987, Pough et al., 1992), however, it is unlikely that there should always be a direct connection between the two. Natural selection will act most directly on whole organism performance, not underlying physiological capacities (Garland & Carter, 1994, Garland & Losos, 1994), so whole organism performance should correlate with behavioural ecology more closely than lower-level traits like physiological abilities. Behaviour, thus, can act as a buffer for selective pressures on physiology (Arnold, 1983, Garland & Carter, 1994, Garland & Kelly, 2006), leaving relatively weak selective pressure on the underlying physiology of performance. In reviewing the literature it appears that the evidence for a relationship between voluntary activity and endurance capacity is mixed. For example, while Garland (1999) found that, among species of lizards, endurance on a treadmill was positively correlated with distance moved in the field and Swallow, Rhodes & Garland, (2005) found increased maximum oxygen consumption in mice selected for high voluntary wheel running, others have found no significant correlation between endurance on a treadmill and voluntary wheel running behaviour in mice (Friedman, Garland & Dohm, 1992) or rats (Lambert et al., 1996).
We conclude that the behavioural enhancement of the dispersal of cane toads at the invasive front does not flow through cleanly to the underlying physiology. Although the evidence for behavioural trends among populations of toads across northern Australia is strong (Phillips et al., 2008), the evidence for physiological differences is not. In this instance, the selective forces resulting in behavioural adaptations related to dispersal have not had a corresponding effect on physiological parameters. The extent to which behaviour, physiology and morphology respond to selective forces and how selective responses at one level of organisation flow through to other levels remains an intriguing question.
Acknowledgments
Toads were collected and housed under permits from the Charles Darwin University Animal Ethics Committee and the Parks and Wildlife Commission of the Northern Territory. We thank the Australian Research Council (ARC grants DP0452700 and DP0879851) and Charles Darwin University for financial support. Greg Betts assisted with experiments, especially with the sprint speed and thermal tolerance measurements.
References
- Alford R. A., Brown G. P., Schwarzkopf L., Phillips B. L., Shine R. (2009). Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wildlife Research 36, 23–28 10.1071/WR08021 [DOI] [Google Scholar]
- Arnold S. J. (1983). Morphology, performance, and fitness. American Zoologist 23, 347–361 [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statisistical Society B 57, 289–300 10.2307/2346101 [DOI] [Google Scholar]
- Blomberg S., Baldwin J. (1991). Non-bicarbonate intracellular pH buffering of reptilian muscle. Journal of Comparative Physiology B 161, 101–107 10.1007/BF00258753 [DOI] [Google Scholar]
- Brown G. P., Shilton C., Phillips B. L., Shine R. (2007). Invasion stress and spinal arthritis in cane toads. Proceedings of the National Academy of Sciences 104, 17698–17700 10.1073/pnas.0705057104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini M. A., Somero G. N. (1981). Buffering capacity of vertebrate muscle: correlations with potentials for anaerobic function. Journal of Comparative Physiology B 143, 191–198 10.1007/BF00797698 [DOI] [Google Scholar]
- Christian K., Green B., Bedford G., Newgrain K. (1996). Seasonal metabolism of a small, arboreal monitor lizard, Varanus scalaris, in tropical Australia. Journal of Zoology 240, 383–396 10.1111/j.1469-7998.1996.tb05293.x [DOI] [Google Scholar]
- Dacie J. V., Lewis S. N. (1984). Practical Haematology. Fifth ed. Churchill Livingston, Edinburgh [Google Scholar]
- Dawson D. M., Goodfriend T. L., Kaplan N. O. (1964). Lactic dehydrogenases: functions of the two types. Science 143, 929–933 10.1126/science.143.3609.929 [DOI] [PubMed] [Google Scholar]
- Feder M. E., Bennett A. F., Burggren W. W., Huey R. B. (1987). New directions in ecological physiology. Cambridge University Press, New York [Google Scholar]
- Friedman W. A., Garland T., Jr, Dohm M. R. (1992). Individual variation in locomotor behavior and maximal oxygen consumption in mice. Physiology and Behavior 52, 97–104 10.1016/0031-9384(92)90438-8 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr (1999). Laboratory endurance capacity predicts variation in field locomotor behaviour among lizard species. Animal Behaviour 58, 77–83 10.1006/anbe.1999.1132 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Carter P. A. (1994). Evolutionary physiology. Annual Review of Physiology 56, 579–621 10.1146/annurev.ph.56.030194.003051 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Kelly S. A. (2006). Phenotyic plasticity and experimental evolution. Journal of Experimental Biology 209, 2344–2361 10.1242/jeb.02244 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Losos J. B. (1994). Ecological morphology of locomotor performance in squamate reptiles. Ecological Morphology: Integrative Organismal Biology (eds Wainwright P. C., Reilly S. M.), pp. 240–302 University of Chicago Press, Chicago [Google Scholar]
- Greenlees M. J., Phillips B. L., Shine R. (2010). Adjusting to a toxic invader: native Australian frogs learn not to prey on cane toads. Behavioral Ecology 21, 966–971 10.1093/beheco/arq095 [DOI] [Google Scholar]
- Holt R. D., Barfield M., Gomulkiewicz R. (2006). Theories of niche conservatism and evolution. Could exotic species be potential tests? Species invasions: insights into ecology, evolution, and biogeography (eds Sax D. F., Stachowicz J. J., Gaines S. D.), pp. 259–290 Sinauer Associates, Sunderland, MA [Google Scholar]
- Kearney M., Phillips B. L., Tracy C. R., Christian K. A., Betts G., Porter W. P. (2008). Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434 10.1111/j.0906-7590.2008.05457.x [DOI] [Google Scholar]
- Lambert M. I., Van Zyl C., Jaunky R., Lambert E. V., Noakes T. D. (1996). Tests of runing performance do not predict subsequent spontaneous running in rats. Physiology and Behavior 60 10.1016/0031-9384(96)00012-1 [DOI] [PubMed] [Google Scholar]
- Lay P. A., Baldwin J. (1999). What determines the size of teleost erythrocytes? Correlations with oxygen transport and nuclear volume. Fish Physiology and Biochemistry 20, 31–35 10.1023/A:1007785202280 [DOI] [Google Scholar]
- Llewelyn J., Phillips B. L., Alford R. A., Schwarzkopf L., Shine R. (2010). Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia 162, 343–348 10.1007/s00442-009-1471-1 [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Start C. (1973). Regulation in Metabolism. John Wiley and Sons, London [Google Scholar]
- Phillips B. L. (2009). The evolution of growth rates on an expanding range edge. Biology Letters 5, 802–804 10.1098/rsbl.2009.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. L., Brown G. P., Greenlees M., Webb J. K., Shine R. (2007). Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecology 32, 169–176 10.1111/j.1442-9993.2007.01664.x [DOI] [Google Scholar]
- Phillips B. L., Brown G. P., Shine R. (2010). Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. Journal of Evolutionary Biology 23, 2595–2601 10.1111/j.1420-9101.2010.02118.x [DOI] [PubMed] [Google Scholar]
- Phillips B. L., Brown G. P., Travis J. M. J., Shine R. (2008). Reid's paradox revisited: the evolution of dispersal in range-shifting populations. The American Naturalist 172, S34–S48 10.1086/588255 [DOI] [PubMed] [Google Scholar]
- Phillips B. L., Brown G. P., Webb J. K., Shine R. (2006). Invasion and the evolution of speed in toads. Nature 439, 803 10.1038/439803a [DOI] [PubMed] [Google Scholar]
- Pough F. H., Magnusson W. E., Ryan M. J., Wells K. D., Taigen T. L. (1992). Behavioral energetics. Environmental Physiology of the Amphibia (eds Feder M. E., Burggren W. W.), pp. 395–436 University of Chicago Press, Chicago [Google Scholar]
- Pramuk J. B., Robertson T., Sites J. W., Jr, Noonan B. P. (2008). Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae) Global Ecology and Biogeography 17, 72–83 [Google Scholar]
- Schultz T., Webb J. K., Christian K. A. (2008). The physiological cost of pregnancy in a tropical live-bearing snake. Copeia 2008, 637–642 10.1643/CP-06-182 [DOI] [Google Scholar]
- Seebacher F., Franklin C. E. (2011). Physiology of invasion: cane toads are constrained by thermal effects on physiological mechanisms that support locomotor performance. Journal of Experimental Biology 214, 1437–1444 10.1242/jeb.053124 [DOI] [PubMed] [Google Scholar]
- Swallow J. G., Rhodes J. S., Garland Jr T. (2005). Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integrative and Comparative Biology 45, 426–437 10.1093/icb/45.3.426 [DOI] [PubMed] [Google Scholar]
- Travis J. M. J., Dytham C. (2002). Dispersal evolution during invasions. Evolutionary Ecology Research 4, 1119–1129 [Google Scholar]
- Urban M. C., Phillips B. L., Skelly D. K., Shine R. (2008). A toad more travelled: the heterogeneous invasion dynamics of cane toads in Australia. The American Naturalist 171, E134–E148 10.1086/527494 [DOI] [PubMed] [Google Scholar]
- Vermeij G. (2005). Invasion as expectation: a historical fact of life. Species invasions: insights into ecology, evolution, and biogeography (eds Sax D. F., Stachowicz J. J., Gaines S. D.), pp. 315–339 Sinauer Associates, Sunderland, MA [Google Scholar]
- Webb J. K., Brown G. P., Child T., Greenlees M. J., Phillips B. L., Shine R. (2008). A native dasyurid (common planigale, Planigale maculata) rapidly learns to avoid a toxic invader. Austral Ecology 33, 821–829 10.1111/j.1442-9993.2008.01847.x [DOI] [Google Scholar]
- Wells R. M. G., Baldwin J. (2006). Plasma lactate and glucose flushes following burst swimming in silver trevally (Pseudocaranx dentex: Carangidae) supports the “releaser” hypothesis. Comparative Biochemistry and Physiology A 143, 347–352 10.1016/j.cbpa.2005.12.015 [DOI] [PubMed] [Google Scholar]
- Wells R. M. G., Baldwin J., Seymour R. S., Christian K., Brittain T. (2005). Red blood cell function and haematology in two tropical freshwater fishes from Australia. Comparative Biochemistry and Physiology A 141, 87–93 10.1016/j.cbpb.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Wells R. M. G., Baldwin J., Seymour R. S., Christian K. A., Farrell A. P. (2007). Air breathing minimizes post-exercise lactate load in the tropical Pacific tarpon, Megalops cyprinoides Broussonet 1782 but oxygen debt is repaid by aquatic breathing. Journal of Fish Biology 71, 1649–1661 10.1111/j.1095-8649.2007.01625.x [DOI] [Google Scholar]
- Wilson A. C., Cahn R. D., Kaplan N. O. (1963). Functions of two forms of lactate dehydrogenase in the breast muscle of birds. Nature 197, 331–334 10.1038/197331a0 [DOI] [PubMed] [Google Scholar]


