Summary
The ability of cultured pluripotent cells to contribute to the germline of chimaeric animals is essential to their utility for genetic manipulation. In the three years since rat embryonic stem (ES) cells were first reported the anticipated proliferation of genetically modified rat models from this new resource has not been realised. Culture instability, karyotypic anomalies, and strain variation are postulated to contribute to poor germline colonisation capacity. The resolution of these issues is essential to bring pluripotent cell-based genetic manipulation technology in the rat to the level of efficiency achieved in the mouse. Recent reports have described various alternative methods to maintain rat ES cells that include provision of additional small molecules and selective passaging methods. In contrast, we report that euploid, germline competent rat ES and embryonic germ (EG) cell lines can be maintained by simple adherent culture methods in defined medium supplemented with the original two inhibitors (2i) of the mitogen-activated protein kinase (ERK1/2) cascade and of glycogen synthase kinase 3, in combination with the cytokine leukaemia inhibitory factor (LIF). We demonstrate genetic modification, clonal expansion and transmission through the germline of rat ES and EG cell lines. We also describe a marked preference for full-term chimaera contribution when SD strain blastocysts are used as recipients for either DA or SD pluripotent stem cells.
Keywords: rat, embryonic stem cell, embryonic germ cell, 2i, chimaera, germline transmission
Introduction
Mouse ES cells have become an unparalleled tool for the manipulation of the mouse genome (Rossant and Nagy, 1995; Capecchi, 2005; Skarnes et al., 2011). However, past attempts to derive ES cells from rat blastocysts using conventional serum-based culture conditions yielded cell lines with mixed extra-embryonic identities that did not demonstrate robust chimaeric contributions and were not capable of germline colonisation (Ouhibi et al., 1995; Stranzinger, 1996; Vassilieva et al., 2000; Buehr et al., 2003). As the rat is the model organism of choice for many areas of mammalian biology, including physiology and neurobiology, it remains desirable to employ ES cell-based genetic manipulation technologies. Methods for direct genetic modification of germ cells and zygotes have been utilised to generate genetically modified rats without using ES cells (Smits et al., 2006; Geurts et al., 2009; Izsvák et al., 2010). However, these approaches require extensive screening of progeny to identify suitable founders and they do not allow for same breadth of sophisticated reporter, knock-in and conditional alleles that can be generated using ES cell-based technologies.
In 2008, derivation of genuine embryonic stem (ES) cells from rat blastocysts was first achieved using two small molecule inhibitors (2i) of the mitogen-activated protein kinase (ERK1/2) cascade and of glycogen synthase kinase 3 (Buehr et al., 2008; Li et al., 2008). These ES cell lines exhibit all the properties of naive pluripotent cells, including responsiveness to LIF, capacity for clonal expansion and, most notably, the ability to contribute functionally to the germline of chimaeras. We have also been able to derive embryonic germ (EG) cell lines from rat primordial germ cells that satisfied all but the last of these criteria (Leitch et al., 2010). Subsequently, other studies have employed similar systems to derive germline competent rat ES and EG cells (Kawamata and Ochiya, 2010; Hirabayashi et al., 2010; Northrup et al., 2011), and to propagate rat induced pluripotent stem (iPS) cells (Hamanaka et al., 2011)
Homologous recombination in rat ES cells has been employed to generate germline-modified rats (Tong et al., 2010; 2011). However, the generation of genetically modified rats using ES cell-based technologies has not become routine. Our group and others have noted that rat pluripotent cells are more difficult to maintain in culture than their mouse counterparts and that karyotypic anomalies tend to develop at higher passage number. Additionally, there is evidence for an effect of strain variability on chimaera penetrance and germline contribution efficiency (Li et al., 2008), but this has yet to be thoroughly explored. These issues must be resolved to achieve the efficiency of ES cell-based genetic modification in the rat that has been achieved in the mouse.
Several techniques have been suggested to stabilise rat ES cells and overcome the problem of karyotypic instability. Tong and colleagues used morphological selection of rounded, floating colonies at each passage (Tong et al., 2010; 2011), while Meek et al. employed pulled pipettes and enzyme-free dissociation buffer (Meek et al., 2010). Kawamata et al. report that the addition of Rho-kinase inhibitor is required to maintain rat ES cell lines (Kawamata and Ochiya, 2010), while Li et al. suggest that provision of an inhibitor of the ALK5 receptor stabilises putative rat iPS cells (Li et al., 2009). However, the former study also supplemented their cultures with 20% serum, while validated germline-competent rat iPS cells have subsequently been derived without ALK5 inhibitor (Hamanaka et al., 2011).
Here, we investigate whether standard culture methods can be employed to maintain stable cultures of rat ES and EG cells and allow genetic manipulation and clonal expansion without loss of germline competence. We also compare the utility of the DA and SD strains as donors and hosts for blastocyst injection.
Results and Discussion
Routine culture of rat pluripotent cells
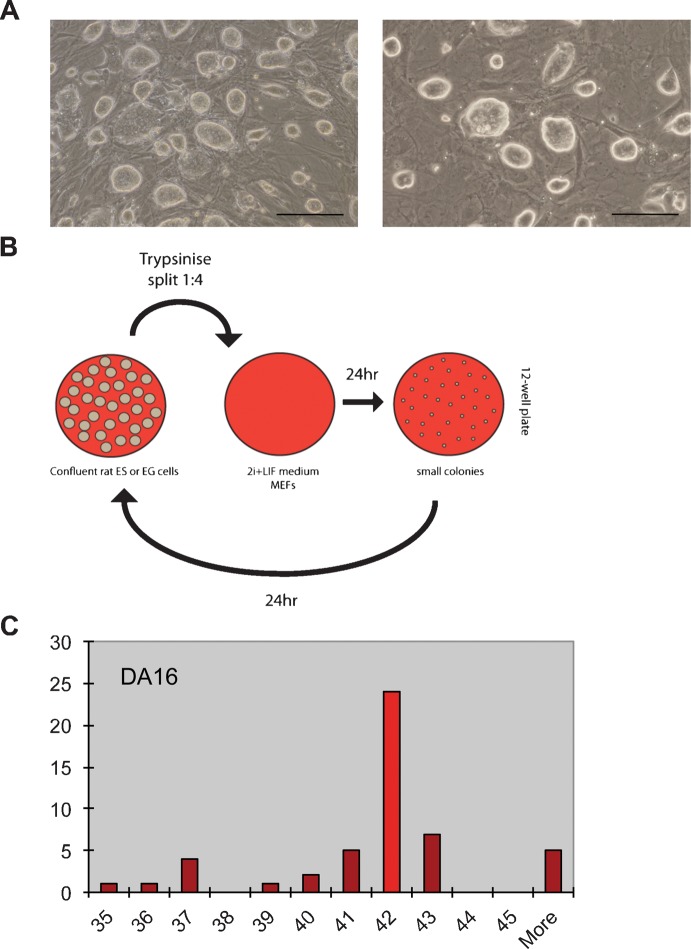
Rat ES and EG cells were derived as previously described (Buehr et al., 2008; Leitch et al., 2010) and maintained in 2i+LIF on mitotically inactivated, high-density mouse embryonic fibroblasts (MEFs) or human foetal foreskin fibroblasts (HS27). In these conditions, cultures maintained compact, domed colony morphology (Fig. 1A). Cultures split 1:10, a routine passaging ratio for mouse ES cells, form undifferentiated colonies that are expandable, but these cultures were prone to instability upon repeated passaging. Such instability manifested as a slowing in proliferation rate and could be accompanied by increased cell fragmentation and/or spontaneous differentiation. We therefore assessed passaging rat ES cell lines at high density. Cultures were maintained between 40–80% confluence in 12 well plates dissociated to single cells with trypsin, and split at a ratio of 1:2 to 1:4 every other day (Fig. 1B). Stable cultures could be maintained over multiple passages in these conditions. A female, DA, rat ES line, DA16, was expanded with this protocol and yielded germline chimaerism at passage 15 (Table 1, supplementary material Fig. S1). This was reflected in a euploid karyotype when assessed by metaphase analysis at passage 13 (Fig. 1C).
Fig. 1. Rat pluripotent stem cell lines can be expanded using 2i+LIF as adherent cultures on MEFs and retain a stable karyotype.
(A) Phase contrast images of DA16 rat ES cells and WBY2 EG cells. Scale bars = 200 μm. (B) Schematic diagram of passaging technique. (C) Histogram showing chromosome number per cell in 50 metaphase spreads from line rat ES cell line DA16 at passage 13.
Table 1. Summary of blastocyst injections of rat ES and EG cell lines.
Blast =blastocysts. GLT=germline transmission. Chimaeras of the same sex as the parental ES cell lines were mated to test for GLT.
| Cell line | Sex | Strain | Modification | Passage number | Blast strain | Blast number | Pups born | Chimaeras | GLT | ||
| M | F | ||||||||||
| 1 | DA16 | F | DA | none | 15 | SD | 64 | 19 | 11 | 1 | 1/1 |
| 2 | 16g2 | F | DA | GFP | 22+12 | SD | 54 | 23 | 8 | 9 | 2/9 |
| 3 | g2.5 | F | SD | GFP | 6+14 | DA | 55 | 1* | – | – | – |
| 4 | g2.5 | F | SD | GFP | 6+14 | SD | 48 | 10 | 1 | 4 | 3/4 |
| 5 | DAK31 | M | DA | none | 8 | SD | 24 | 10 | 5 | 1 | 2/5 |
| 6 | DAK38 | M | DA | none | 7 | SD | 54 | 23 | 11 | 6 | 3/11 |
| 7 | DAK27 | M | DA | none | 12 | SD | 43 | 9 | 5 | 2 | 0/3‡ |
Found dead at P2.
Remaining male chimaeras are currently being tested for germline transmission.
Transfection, selection and clonal propagation using plasmid expression vectors and the piggyBac transposase system
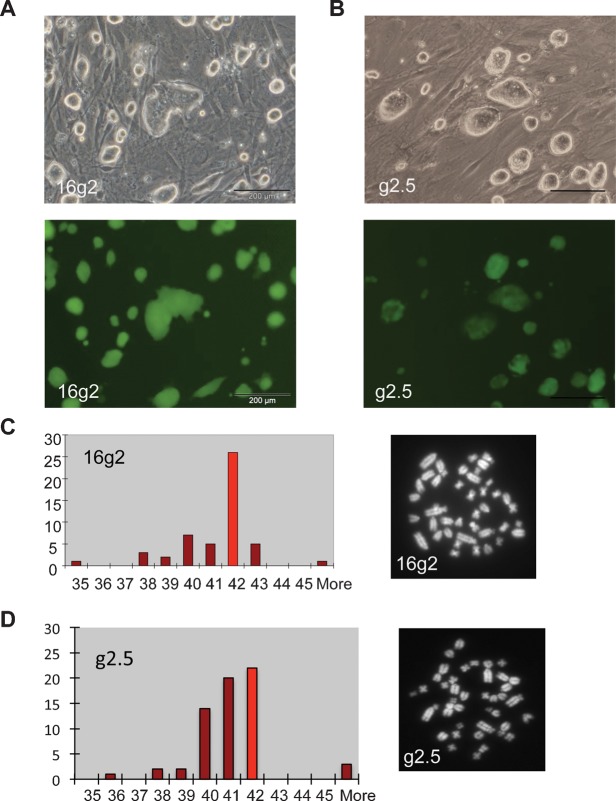
We then assessed stable transfection of rat pluripotent stem cells. Rat EG cells, established as previously described (Leitch et al., 2010), were plated at passage 6 at high density on DR4 drug resistant MEFs (Tucker et al., 1997). They were lipofected for 5 hours with a plasmid containing GFP-IRES-puroR driven by the CAG promoter (Niwa et al., 1991). Puromycin selection was applied after 24 hours and retained until the emergence of colonies. Eight drug resistant colonies appeared and were GFP positive. These were picked and expanded as separate lines. Six retained stable GFP expression in the absence of selection (Fig. 2B). Metaphase analysis revealed that five of these lines were pseudotetraploid, however, and only one had a modal chromosome number of 42 (Fig. 2D).
Fig. 2. Transfection and clonal selection of rat pluripotent stem cells to generate stable transgenic lines.
Phase contrast and fluorescence images of (A) 16g2 clonal rat ES cells and (B) g2.5 clonal rat EG cells. Scale bars = 200 μm. Histogram showing chromosome number per cell in 50 metaphase spreads from (C) rat ES cell line 16g2 and (D) rat EG cell line g2.5 with accompanying metaphase spreads showing 42 chromosomes.
Although a euploid line was recovered using this basic protocol, the efficiency is poor compared with mouse ES or EG cells. We therefore delayed selection by using the piggyBac transposase system (Ding et al., 2005; Wang et al., 2008) to generate a high frequency of stable transfectants in bulk culture. DA16 rat ES cells were plated at high density at passage 17 and lipofected overnight with a piggyBac-CAG-GFP-PGK-hygroR vector and a piggyBac transposase plasmid. Transfection efficiency, as assessed by fluorescence microscopy after 24 hours, was approximately 40% with no evidence of cellular toxicity. After six days of culture, hygromycin was applied for eight days to select for stable integration. A robust bulk culture with ES cell morphology and varying levels of GFP expression was obtained. This population of DA16-GFP cells was then sorted by flow cytometry and single cells were deposited into individual wells of a 96 well plate containing feeders pre-equilibrated with 2i+LIF. After six days of expansion, morphologically undifferentiated, uniformly GFP-positive colonies had formed in 26/84 wells (31%). When 10 cells were seeded per well, an average of 3.3 colonies formed per well. Fourteen colonies were picked, dissociated using trypsin, plated into fresh wells and expanded. Twelve lines were subsequently established, all of which exhibited typical ES cell morphology and uniform GFP expression (Fig. 2A). Six lines were assessed for euploid karyotype by metaphase spread analysis, six passages after cloning. Of these, one was found to have a normal karyotype, three had modal chromosome counts of 41, and two had high chromosome counts indicative of pseudotetraploidy (Fig. 2C, supplementary material Fig. S2).
Chimaeric contribution of rat ES and EG cells
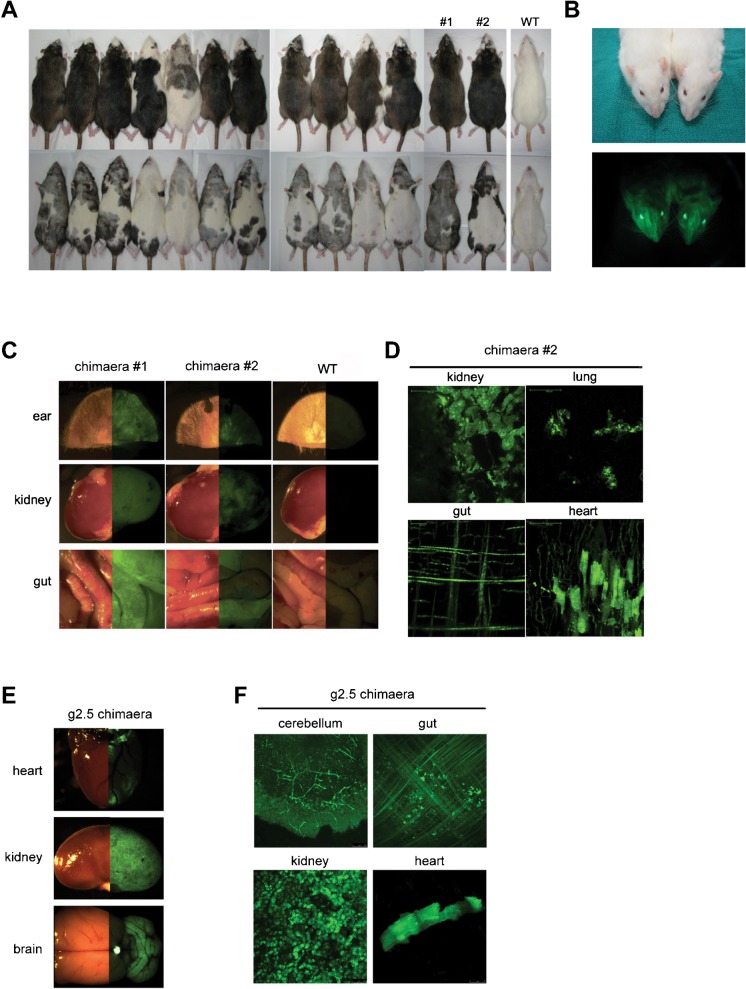
Having established euploid, clonal, transgenic ES and EG cells, we assayed their ability to colonise the developing embryo and their pluripotency by blastocyst injection. The transgenic rat EG cell line g2.5 was expanded for a further 14 passages prior to injection into 48 SD blastocysts. As g2.5 is of SD origin, chimaerism could not be assessed by coat colour. However, GFP fluorescence could be readily observed (Fig. 3B) and of the 10 pups born, 5 were overtly chimaeric. In agreement with our previous findings (Leitch et al., 2010), these rat EG cells exhibited widespread contribution to all adult organs examined (Fig. 3E and 3F).
Fig. 3. Genetically manipulated rat pluripotent stem cells contribute widely to chimaeras.
(A) Chimaeras from injection of 16g2 DA (agouti) rat ES cells into SD (albino) blastocysts. (B) Chimaeras from injection of g2.5 SD rat EG cells into SD blastocysts. Bright-field and fluorescence images of whole organs show contribution of (C) rat ES cells and (E) rat EG cells to a wide range of tissues. 3D projection of serial confocal sections through unfixed tissue squashes demonstrate the contribution of (D) rat ES cells and (F) rat EG cells to both tissue parenchyma and stromal cells. Chimaeras numbers 1 and 2 in (C) and (D) represent numbers 1 and 2 from (A).
The transgenic rat ES cell line 16g2 was expanded for 12 passages after sub-cloning prior to injection into 54 SD blastocysts. Of the 23 pups born, 17 were coat colour contribution chimaeras (14 shown in Fig. 3A). Notably, the majority of chimaeras formed were >70% agouti. To assess whether this coat colour contribution was indicative of high overall contribution of ES-derived cells to multiple germ layers, we examined the contribution of GFP-positive cells to organs and tissues of two chimaeras. We found that indeed a high proportion of GFP-positive cells were present in all organs examined (Fig. 3C and 3D). All ES cell and EG cell chimaeras were healthy and no abnormalities were observed in animals sacrificed.
Germline transmission of rat EG and ES cells
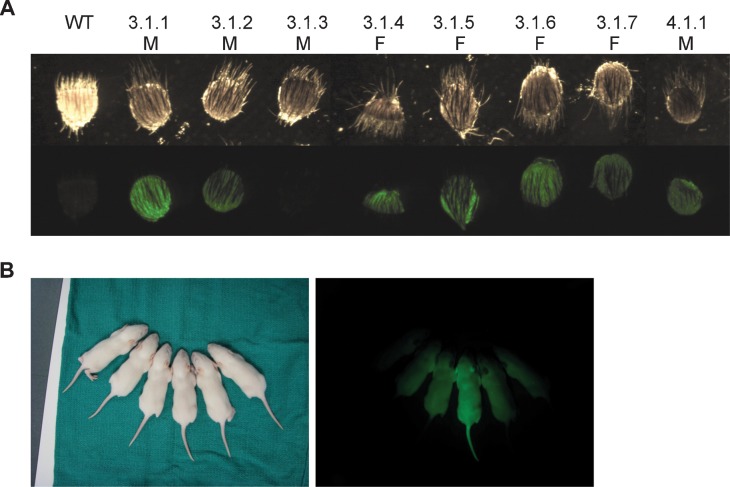
Four female chimaeras were generated by injection of g2.5 rat EG cells. Three of these gave germline transmission on the first litter (Table 1). The first chimaera produced seven offspring, one of which was GFP-positive (Fig. 4B). The second and third chimaeras produced 1/7 and 5/12 GFP-positive pups respectively. The 9 female chimaeras generated from 16g2 ES cell blastocyst injections were tested for germline transmission by mating with an albino male. Two bore agouti pups in the first litter (7/14 and 1/15 pups; Table 1), six bore all white litters, and one gave birth but ate her litter before coat colour could be assessed. Seven of the eight agouti offspring were GFP-positive as indicated by bright field and fluorescent images of ear punches (Fig. 4A).
Fig. 4. Germ line transmission from high passage number, genetically manipulated rat pluripotent stem cells.
(A) Ear punches from wildtype littermate and agouti/fluorescent pups indicating germline transmission of 16g2. (B) Fluorescent pups indicating germline transmission of g2.5.
Host/strain combination affects chimaerism
Significant variability in donor/host strain compatibility for the production of chimaeras has previously been noted in mice (Schwartzberg et al., 1989, Nagy et al., 2003), but remains largely uncharacterised in rats. Having established that 16g2 can efficiently colonise SD host blastocysts the suitability of the DA donor and SD host combination was further investigated. Three male DA lines (DAK27, DAK31, and DAK38) were derived in 2i+LIF on feeders and propagated as described (Fig. 1B). Two lines were assayed for karyotypic normality by metaphase spread at passage 10 and found to be euploid (Fig. S3). Each of the three lines was injected into SD host blastocysts between passage 7 and 12; all gave rise to chimaeras at a high rate, ranging from 60–78% of pups born (Table 1, Fig. S4). We have previously reported an efficiency of 5–13% when injecting DA cell lines into Fischer (F334) blastocysts (Buehr et al., 2008). The present results suggest that SD is a more suitable albino host blastocyst strain for injection of DA ES cells than F344. Male chimaeras produced from DAK31 and DAK38 ES cells have been tested for germline transmission; 2 of 5 DAK31 chimaeras and 3 of 11 DAK38 chimaeras have produced agouti pups in the first litter. Therefore, different DA rat ES cell lines generate high contribution chimaeras in SD recipient blastocysts and give germline transmission.
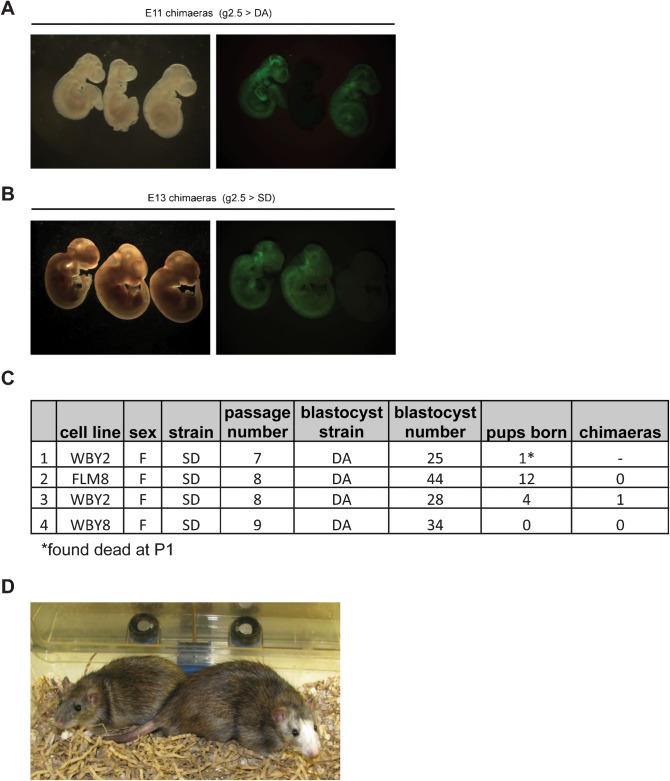
As rat EG cell line g2.5 produced high contribution chimaeras and efficient germline transmission following injection into SD blastocysts (Table 1) we used this line to test the suitability of DA host blastocysts. In three rounds of injection, g2.5 was injected into a total of 54 DA blastocysts that were transferred to 5 pseudopregnant females. One female was sacrificed at mid-gestation to assess foetal chimaerism. Six embryos were recovered and chimaeric contribution assessed by fluorescence microscopy. Five out of six embryos displayed fluorescence throughout the whole embryo, comparable to that observed when the same line was injected into SD host blastocysts (Fig. 5A and 5B). The remaining females were left to term but only 1 pup was born. This was found dead at P2 (Table 1). Injection of the unmodified parental line and two additional, unmodified SD rat EG cell lines into a total of 131 DA blastocysts yielded only 16 live pups of which only 1 showed very low chimaera contribution (Fig. 5C and 5D). This poor birth rate and low rate of chimaerism indicates that the DA strain is a suboptimal recipient for development to term of chimaeras with SD pluripotent stem cells.
Fig. 5. The DA strain is a suboptimal recipient for SD rat pluripotent cells.
(A) Bright-field and fluorescence images of two E11.5 chimaeric embryos and a litter mate control obtained from g2.5 injections into DA host blastocysts. (B) Bright-field and fluorescence images of two E13.5 chimaeric embryos and a litter mate control obtained from g2.5 injections into SD host blastocysts. (C) Summary of injections of unmodified SD rat EG cells into DA host blastocysts. (D) Low contribution adult chimaera from injection of WBY2 SD rat EG into DA blastocyst.
Overall, these findings validate conditions for stable culture of rat ES and EG cells in 2i+LIF on feeders. By routinely propagating lines at high density and passaging with trypsin, we can maintain cell lines with normal karyotype for multiple passages. These lines are readily amenable to transfection by lipofection. They can stably integrate DNA introduced using both standard expression vectors and the piggyBac transposase system and can withstand selection in hygromycin or puromycin. Clonal lines can be expanded from single cells from genetically modified bulk cultures. Only a subset of clones maintains stable euploid karyotype, however, and these must be identified by chromosome analysis. It is noteworthy that karyotype abnormalities appear in many of the clones after expansion from single cells. This is consistent with the loss of stability we observe when lines are serially passaged at low density. However, high density cultures appear robust. It is possible that rat pluripotent stem cells release paracrine growth factors. The use of conditioned medium may help to improve the stability of clones and should be investigated in future studies. We also report that multiple DA cell lines injected into SD host blastocysts form very high contribution chimaeras. In contrast SD cell lines have a limited ability to generate viable chimaeras when injected into DA blastocysts although they readily produce chimaeras and pass through the germline when injected into syngenic blastocysts. These data indicate that donor/host strain compatibility affects chimaera formation and should be considered in future work.
In conclusion, rat pluripotent stem cells are less stable than their mouse counterparts and future work should be directed at understanding the species differences and further optimising culture conditions for rat pluripotent cells. However, ES and EG cell lines can remain germline competent after genetic modification, expansion from single cells, freeze-thaw cycles and up to 30 passages in culture. This demonstrates that careful application of standard culture methods enables reliable rat transgenesis despite the fastidiousness of rat pluripotent stem cells.
Materials and Methods
Animals
The outbred albino rat strain Sprague Dawley (SD) and the inbred pigmented rat strain Dark Agouti (DA) were used in this study. Animal studies were authorised by a UK Home Office Project Licence and carried out in a Home Office designated facility
Transfection
Plasmids were introduced using Lipofectamine 2000 (Invitrogen).
Cell culture
Rat ES and EG cells were maintained as described in the text. For 2i+LIF medium, N2B27 basal medium (Stem Cells Inc.) was supplemented with 1 µM PD0325901, 3 µM CHIR99021 (Signalling Technologies, University of Dundee) and either human recombinant LIF (10,000 U/mL, prepared in house) or mouse LIF (10 ng/mL, prepared in house). Mitotically inactivated MEFs (prepared in house from E12–13 mouse embryos) and Hs27 human fibroblasts (ATCC) were used as feeders for routine culture. DR4 MEFs derived from DR4 transgenic mouse embryos that are resistant to neomycin, hygromycin, puromycin and 6-thioguanine were used during drug selection (Tucker et al., 1997).
Blastocyst injection
Rat ES and EG cells were prepared for injection by harvesting cultures with trypsin and 1 hour pre-plating on uncoated tissue culture plates to eliminate feeders. Cells were resuspended in N2B27 with 1M HEPES buffer (1:50) and kept on ice prior to injection. Blastocysts were harvested from time-mated rats at 4.5 days post coitum (dpc) in M2 medium. 15–20 pluripotent rat cells were injected into the blastocoel cavity. Injected blastocysts were transferred into 3.5 dpc pseudopregnant SD recipients.
Supplementary Material
Acknowledgments
Mia Buehr derived the cell line DA16. Mark Hyvonen provided recombinant mouse LIF. Joerg Betschinger provided the piggyBac-CAG-GFP-IRES-hygro construct. Sam Jameson and the staff of the biofacilities unit provided excellent animal husbandry. This work received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement No. HEALTH-F4-2010-241504 (EURATRANS) and from the Biotechnology and Biological Sciences Research Council of the United Kingdom. KB is a Gates Cambridge Scholar. HGL is supported by a Merk, Sharp and Dohme Award from the University of Cambridge School of Clinical Medicine MD/PhD programme and the James Baird Fund. AS is Medical Research Council Professor.
References
- Buehr M., Meek S., Blair K., Yang J., Ure J., Silva J., McLay R., Hall J., Ying Q.-L., Smith A. (2008). Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 10.1016/j.cell.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Buehr M., Nichols J., Stenhouse F., Mountford P., Greenhalgh C. J., Kantachuvesiri S., Brooker G., Mullins J., Smith A. G. (2003). Rapid Loss of Oct-4 and Pluripotency in Cultured Rodent Blastocysts and Derivative Cell Lines. Biol. Repro. 68, 222–229 10.1095/biolreprod.102.006197 [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. (2005). Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat. Rev. Genet. 6, 507–512 10.1038/nrg1619 [DOI] [PubMed] [Google Scholar]
- Ding S., Wu X., Li G., Han M., Zhuang Y., Xu T. (2005). Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483 10.1016/j.cell.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Geurts A. M., Cost G. J., Freyvert Y., Zeitler B., Miller J. C., Choi V. M., Jenkins S. S., Wood A., Cui X., Meng X. et al. (2009). Knockout Rats via Embryo Microinjection of Zinc-Finger Nucleases. Science 325, 433 10.1126/science.1172447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka S., Yamaguchi T., Kobayashi T., Kato-Itoh M., Yamazaki S., Sato H., Umino A., Wakiyama Y., Arai M., Sanbo M. et al. (2011). Generation of germline-competent rat induced pluripotent stem cells. PLoS One 6, e22008 10.1371/journal.pone.0022008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi M., Kato M., Kobayashi T., Sanbo M., Yagi T., Hochi S., Nakauchi H. (2010). Establishment of rat embryonic stem cell lines that can participate in germline chimaerae at high efficiency. Mol. Reprod. Dev. 77, 94 10.1002/mrd.21181 [DOI] [PubMed] [Google Scholar]
- Izsvák Z., Fröhlich J., Grabundzija I., Shirley J. R., Powell H. M., Chapman K. M., Ivics Z., Hamra F. K. (2010). Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat. Methods 7, 443–445 10.1038/nmeth.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata M., Ochiya T. (2010). Generation of genetically modified rats from embryonic stem cells. Proc. Natl. Acad. Sci. USA 107, 14223–14228 10.1073/pnas.1009582107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H. G., Blair K., Mansfield W., Ayetey H., Humphreys P., Nichols J., Surani M. A., Smith A. (2010). Embryonic germ cells from mice and rats exhibit properties consistent with a generic pluripotent ground state. Development 137, 2279–2287 10.1242/dev.050427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Tong C., Mehrian-Shai R., Jia L., Wu N., Yan Y., Maxson R. E., Schulze E. N., Song H., Hsieh C.-L. et al. (2008). Germline Competent Embryonic Stem Cells Derived from Rat Blastocysts. Cell 135, 1299–1310 10.1016/j.cell.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wei W., Zhu S., Zhu J., Shi Y., Lin T., Hao E., Hayek A., Deng H., Ding S. (2009). Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4, 16–19 10.1016/j.stem.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Meek S., Buehr M., Sutherland L., Thomson A., Mullins J. J., Smith A. J., Burdon T. (2010). Efficient gene targeting by homologous recombination in rat embryonic stem cells. PLoS One 5, e14225 10.1371/journal.pone.0014225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Gertsenstein M., Vintersten K., Behringer R. (2003). Manipulating the Mouse Embryo Third edition. (New York: Cold Spring Harbor Laboratory Press; ). [Google Scholar]
- Niwa H., Yamamura K., Miyazaki J. (1991). Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 10.1016/0378-1119(91)90434-D [DOI] [PubMed] [Google Scholar]
- Northrup E., Eisenblätter R., Glage S., Rudolph C., Dorsch M., Schlegelberger B., Hedrich H.-J., Zschemisch N.-H. (2011). Loss of Dnd1 facilitates the cultivation of genital ridge-derived rat embryonic germ cells. Exp. Cell Res. 317, 1885–1894 10.1016/j.yexcr.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Ouhibi N., Sullivan N. F., English J., Colledge W. H., Evans M. J., Clarke N. J. (1995). Initial culture behaviour of rat blastocysts on selected feeder cell lines. Mol. Reprod. Dev. 40, 311–324 10.1002/mrd.1080400307 [DOI] [PubMed] [Google Scholar]
- Rossant J., Nagy A. (1995). Genome engineering: the new mouse genetics. Nat. Med. 1, 592–594 10.1038/nm0695-592 [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Goff S. P., Robertson E. J. (1989). Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science 246, 799–803 10.1126/science.2554496 [DOI] [PubMed] [Google Scholar]
- Skarnes W. C., Rosen B., West A. P., Koutsourakis M., Bushell W., Iyer V., Mujica A. O., Thomas M., Harrow J., Cox T. et al. (2011). A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 10.1038/nature10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits B. M. G., Mudde J. B., van de Belt J., Verheul M., Olivier J., Homberg J., Guryev V., Cools A. R., Ellenbroek B. A., Plasterk R. H. A. et al. (2006). Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet. Genomics 16, 159–169 [DOI] [PubMed] [Google Scholar]
- Stranzinger G. F. (1996). Embryonic stem-cell-like cell lines of the species rat and Bovinae. Int. J. Exp. Pathol. 77, 263–267 [PMC free article] [PubMed] [Google Scholar]
- Tong C., Huang G., Ashton C., Li P., Ying Q.-L. (2011). Generating gene knockout rats by homologous recombination in embryonic stem cells. Nat. Protoc. 6, 827–844 10.1038/nprot.2011.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C., Li P., Wu N. L., Yan Y., Ying Q.-L. (2010). Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 467, 211–213 10.1038/nature09368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K. L., Wang Y., Dausman J., Jaenisch R. (1997). A transgenic mouse strain expressing four drug-selectable marker genes. Nucleic Acids Res. 25, 3745–3746 10.1093/nar/25.18.3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilieva S., Guan K., Pich U., Wobus A. M. (2000). Establishment of SSEA-1- and Oct-4-expressing rat embryonic stem-like cell lines and effects of cytokines of the IL-6 family on clonal growth. Exp. Cell Res. 258, 361–373 10.1006/excr.2000.4940 [DOI] [PubMed] [Google Scholar]
- Wang W., Lin C., Lu D., Ning Z., Cox T., Melvin D., Wang X., Bradley A., Liu P. (2008). Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 105, 9290–9295 10.1073/pnas.0801017105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.