Summary
The aim of this research was to isolate and characterize bacteria from spores of arbuscular mycorrhizal fungi (AMF). We designated these bacteria ‘probable endobacteria’ (PE). Three bacterial strains were isolated from approximately 500 spores of Gigaspora margarita (Becker and Hall) using a hypodermic needle (diameter, 200 μm). The bacteria were identified by morphological methods and on the basis of ribosomal gene sequences as Bacillus sp. (KTCIGM01), Bacillus thuringiensis (KTCIGM02), and Paenibacillus rhizospherae (KTCIGM03). We evaluated the effect of these probable endobacteria on antagonistic activity to the soil-borne plant pathogens (SBPPs) Fusarium oxysporum f. sp. lactucae MAFF 744088, Rosellinia necatrix, Rhizoctonia solani MAFF 237426, and Pythium ultimum NBRC 100123. We also tested whether these probable endobacteria affected phosphorus solubilization, ethylene production, nitrogenase activity (NA), and stimulation of AMF hyphal growth. In addition, fresh samples of spores and hyphae were photographed using an in situ scanning electron microscope (SEM) (Quanta 250FEG; FEI Co., Japan). Bacterial aggregates (BAs), structures similar to biofilms, could be detected on the surface of hyphae and spores. We demonstrate that using extraction with an ultrathin needle, it is possible to isolate AMF-associated bacterial species that are likely derived from inside the fungal spores.
Keywords: arbuscular mycorrhizal fungi, bacteria, biocontrol, nutrient biodynamics
Introduction
Most plant families from all phyla are colonized by arbuscular mycorrhizal fungi (AMF). Benefits of these fungi to their host plants include improving nutrient supply, protection from infection by soil-borne plant pathogens (SBPPs), and protection from drought stress (Harrier and Watson, 2004; Ruiz-Lozano and Bonfante, 2001). Due to their widespread presence in soils, mycorrhizae play significant roles in the rhizosphere (Budi et al., 1999). During the process of plant colonization, AMF interact with bacteria, and the fungal spores and hyphae provide sites where certain populations of bacteria can live (Schuessler et al., 1994). Bacteria can also be found in the cytoplasm of AMF spores (Cruz, 2004; Lumini et al., 2007). The beneficial effects of mycorrhizae in the rhizosphere are the result of synergistic interactions among all rhizosphere microbes, which are crucial for plant growth (Linderman, 1992). Thus, the relationship between AMF and their associated bacteria may be of great importance for sustainable agriculture.
The bacterial community can change as a result of mycorrhizal establishment (Marschner et al., 2001), and AMF may act as a vehicle for spreading rhizobacteria that promote plant growth to neighboring rhizospheres (Boddey et al., 1991). This hypothesis would be supported if rhizobacteria that promote plant growth could be found adhering to spores and hyphal structures (Banciotto et al., 1996). The attachment of bacteria to the surface of spores and hyphae involves colonization of a solid substrate, and eventually, the bacteria assemble into complex clusters termed biofilms, which contain polysaccharides (Costerton et al., 1995).
Bacteria often associate with eukaryotic cells to establish endocellular symbioses (Douglas, 1994), but AMF spores are unique in that they host bacteria in their cytoplasm (Lumini et al., 2007). Intracellular structures similar to bacteria, called bacteria-like organisms, have been observed inside AMF spores with transmission electronic microscope (Bonfante et al., 1994; Cruz, 2004). Most AMF carry bacteria, and this relationship can be dated back to the time when AMF established symbioses with emergent land plants (Bonfante, 2003). These bacteria can colonize the surface of AMF spores and hyphae (Xavier and Germida, 2003), and they can also be located inside the spore walls (Walley and Germida, 1995). In addition, the bacteria affect spore germination, hyphal growth, and root colonization (Horii and Ishii, 2006; Horii et al., 2008).
Despite some progress, knowledge of mechanisms involved in the relationship between AMF and bacteria is still limited. This is mostly due to the difficulties involved in cultivating some of the species in vitro (Jargeat et al., 2004). Based on 16S rRNA gene sequences, the endobacteria in Gigaspora margarita were identified as belonging to the genus Burkholderia (Banciotto et al., 1996). These bacteria are widespread in Gigasporaceae and represent a stable cytoplasmic component. Other reports have demonstrated vertical transmission of bacteria from one generation to the next (Ruiz-Lozano and Bonfante, 2000). The bacterial species, identified based on genetic features as Candidatus Glomeribacter gigasporarum (Banciotto et al., 2003; Bonfante, 2003; Minerdi et al., 2002), has not yet been grown in vitro. Nevertheless, the production of a genomic library enabled identification of certain functions related to nutrient uptake, root colonization (Ruiz-Lozano and Bonfante, 2000), and nitrogenase activity (Minerdi et al., 2001). Normally, Burkholderia spp. is easily cultivated. However, the specific strains related to Gigaspora margarita spores may be difficult to grow in vitro outside the spores. At a different level, the generation of a complete genome sequence of AMF spores is challenging because the spores contain multiple nuclei, making it difficult to select an appropriate primer for each DNA in the spore (Budi et al., 1999; Minerdi et al., 2002).
Nitrogen fixation is one mechanism by which bacteria may affect mycorrhizal formation (Bertaux et al., 2003) via the release of ammonium (Rambelli, 1973). Several studies have reported the presence of acetylene-reducing bacteria, such as Bacillus spp. (Li et al., 1992), in ectomycorrhizae (Li and Hung, 1987). AMF also benefit from the production of bacterial metabolites that are used directly by the fungus. These include organic acids (Duponnois and Garbaye, 1990), volatile compounds (ethylene), and non-volatile compounds (Horii and Ishii, 2006). Previously, some bacteria were isolated from G. margarita spores by using osmotic solutions (Cruz et al., 2008). However, cultivable bacteria extracted using an ultrathin needle have not yet been reported. This research aimed to isolate and identify cultivable bacterial strains from G. margarita spores and analyze their performance with regard to nutrient biodynamics and biocontrol of SBPPs.
Materials and Methods
Extraction and identification of probable endobacteria from Gigaspora margarita spores
Approximately 500 spores of G. margarita (Central Glass Co. Ltd, Tokyo, Japan) were surface sterilized with 50% ethanol in ultrasound for 30 s, and then inserted in an antibiotic solution containing chloramines-T (7000 mg L−1), streptomycin (56 mg L−1), chloramphenicol (20 mg L−1), and a few drops of Tween 80. The spores were kept in the antibiotic solution in a refrigerator for 3 days. After sterilization, the spores were placed on petri dishes containing Nissui media (Nissui Co., Japan) for 2 more days to verify the efficiency of sterilization. The spores without bacterial growth around them were selected for extraction.
A 200-μm diameter hypodermic needle (Nano path 33; Terumo Co., Ltd., Japan) was aseptically inserted into a spore to extract the liquid contents. The contents were inserted into a glass tube with liquid medium containing polypeptone and bactoTM yeast extract (5 g L−1 and 1 g L−1, respectively). We collected spore contents in 500 tubes, which were placed on a shaker for 2 days at 25°C. Subsequently, 3 tubes containing bacteria were identified. The whole process was done aseptically to avoid contamination.
The morphology of each of the 3 bacteria was assessed by light microscopy (1500×) and a Gram test. In addition, molecular identification was carried out on DNA extracted using an Isoplant kit (Nippongene Co., Japan), using the polymerase chain reaction with the bacterial 27f/1492r primers and sequencing of the resulting fragments (Cruz et al., 2008; Horii et al., 2008). Existing DNA databases were searched for similar sequences using the BLAST program. Similar sequences from bacteria from certain lineages were aligned manually. The maximum similarity of sequences to identify the species was chosen by the criteria of highest (%) and lowest E-value.
Assessment of effects of probable endobacteria on nutrient biodynamics, SBPP suppression, and AMF hyphal growth stimulation
Phospohorus solubilization was determined in liquid and on solid Pikovskaya medium (Alam et al., 2002). To analyze ethylene production, a 5-ml sample of bacteria (108 cfu) was inserted in a flask. After shaking the flask at 65 rpm for a week, a 1-mL gas sample was removed. The ethylene concentration was determined using a gas chromatograph equipped with a flame ionization detector and an activated alumina (60–80 mesh) glass column (2×2 mm; Hitachi) at 130°C. Nitrogenase activity, an indicator of N-fixation, was determined by an acetylene reduction assay. Acetylene gas (10 ppm of internal air volume) was injected into the flask containing a 5-mL sample of bacteria (108 cfu) prior to incubation. Measurement of ethylene concentration followed the same procedure described above.
To assess disease suppression, 2 disks with probable endobacteria were placed on the peripheral edge of a petri dish containing potato dextrose agar and a 5-mm disk of the SBPPs F. oxysporum, R. necatrix (isolated from roots of Japanese apricot (Prunus mume) by Dr Norihiko Kobayashi – Kyoto Prefectural University), R. solani, or P. ultimum. Five days after incubation at 27°C, the clear area surrounding the bacterial disk was used to estimate the degree of antagonism based on the criteria published previously (Cruz et al., 2008; Horii et al., 2008).
The effect of PE on hyphal growth was investigated by placing disks of bacteria grown on Nissui media in petri dishes containing 10 mL of 1% IH base media (Ishii and Horii, 2007). Spores of Gigaspora margarita were sterilized using 50% ethanol in ultrasound for 30 s followed by incubation in 14% of the antibiotic solution described above. This was followed by washing the spores 7 times with sterilized distilled water. Four spores were individually transferred to the agar media at a distance of 2 cm from the bacterial disk. The petri dishes were incubated at 26°C in the dark, and after 2 weeks, the hyphal length was measured using a charge-coupled device camera attached to a TV (Cruz et al., 2000).
Statistical analysis
The statistical design was composed of 3 bacterial treatments and 1 control with 4 replicates each treatment. The results were evaluated based on standard error and t-test that was used to detect significant effects of the bacteria.
Imaging of PE on Gigaspora margarita using an in situ scanning electron microscope
For SEM observation, the spores were sterilized and placed in petri dishes as described above. After 1 week, germinated spores that had developed considerable hyphal growth were selected for bacterial inoculation. PE were grown in liquid media and centrifuged at 10,000 rpm for 1 min. After discarding the supernatant (liquid media), the bacteria were mixed with sterilized distilled water and placed on the hyphae, and the petri dishes containing both AMF and bacteria were incubated for 5 more days at 25°C. The whole process from the removal of liquid media until the incubation was done aseptically (flaming and ultraviolet) on a clean bench, using materials that had been sterilized by autoclaving (120°C for 30 min). Following this, the petri dishes containing spores, hyphae, and bacteria were imaged using an in situ SEM Quanta 250FEG (FEI Co., Japan), without prior fixation, dehydration, embedding, or staining.
Results
Characterization of bacterial strains
All isolated bacteria were gram positive, 0.5 μm wide, between 2 and 5 μm in length, and rod shaped. With regard to the 16S rRNA gene sequences, strain KTCIGM01 was 99% identical to Bacillus sp., whereas KTCIGM02 and KTCIGM03 were 100% identical to Bacillus thuringiensis and Paenibacillus rhizospherae, respectively (Table 1).
Table 1. Identification of the probable endobacteria (PE) from G. margarita spores by DNA methods and morphology.
| Methods | Code | KTCIGME01 | KTCIGME02 | KTCIGME03 |
| DNA | Specie | Bacillus sp. | B. thuringiensis | Paenibacillus rhizosphaerae |
| Acess. No. | FJ528077 | Z84587 | EU857426 | |
| Ident. (%) | 99 | 100 | 99 | |
| E-value | 1.00E-131 | 0 | 0 | |
| Morphol. | Gram test | + | + | + |
| Out shape | Rod | Rod | Rod | |
| Size (µm)* | 1/5 | 1/5 | 1/3 |
Rod shape: smaller size / larger size
Functions of the bacterial strains
All of parameters analyzed (antagonism to SBPP, hyphal growth, ethylene production, nitrogenase activity, P solubilization on agar media, and P solubilization on liquid media) were influenced by some of bacterial strains.
P. ultimum was strongly suppressed by KTCIGM01, whereas KTCIGM02 had an antagonistic effect on R. solani. R. necatrix was suppressed by both KTCIGM01 and KTCIGM02. None of the strains inhibited F. oxysporum to a great extent, while R. necatrix was most susceptible to bacterial suppression (Table 2).
Table 2. Antagonism between PE and soil-borne plant pathogens.
| Strain code | Growth inhibition* | |||
| Pythium ultimum | Fusarium oxysporum | Rhizoctonia solani | Roselinia necatrix | |
| KTCIGME01 | 1.8±0.3† | 1.0±0.1 | 0.9±0.2 | 2.0±0.4 |
| KTCIGME02 | 0.6±0.3 | 1.3±0.4 | 2.0±0.1 | 1.9±0.7 |
| KTCIGME03 | 0.2±0.1 | 0.7±0.3 | 0.8±0.2 | NE |
NE – No effect i.e. no inhibition on growth
Antagonism scale: 0-No; 1-Slightly; 2-Moderate; 3-Strong
Mean±standard error (SE), n = 4
All bacterial strains were able to form a halo around colonies on solid Pikovskaya medium, indicating their ability to solubilize P in this medium (Fig. 1a). Similar results were detected in liquid media. Strain KTCIGM03 was the strongest P solubilizer (Fig. 1b).
Fig. 1. P solubilization ability by the PE on agar (a) and liquid media (b).
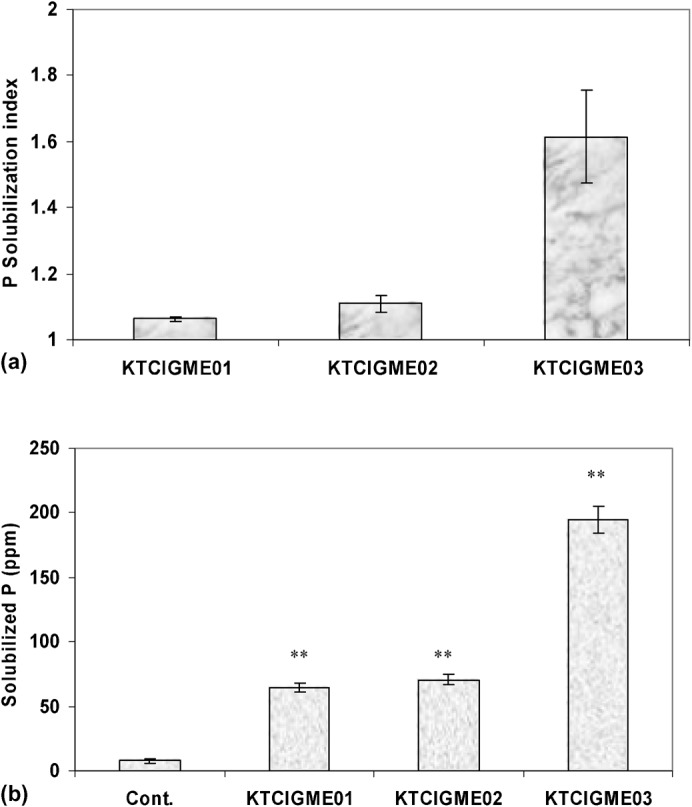
Vertical bars indicate SE (n = 4). **Significantly different at 99% level as compared with the control
KTCIGM01 and KTCIGM02 both produced ethylene. KTCIGM02 produced more ethylene than KTCIGM01, while KTCIGM03 produced no more than the control (no bacteria) (Table 3). Only KTCIGM02 showed significant nitrogenase activity and for the other strains the values were not significantly different from the control (Table 3). All bacterial strains affected hyphal growth. The effects of KTCIGM01 and KTCIGM03 were the strongest (Table 3).
Table 3. Effects of PE (Bacterial treatment) on hyphal growth of G. margarita, ethylene production and nitrogenase activity.
| Bacterial treat. | Hyphal growth (mm) | Ethylene production (C2H4 ppm) | Nitrogenase activity (C2H4 ppm) |
| Control | 21.4±1.8a | 0.35±0.1 | 0.21±0.1 |
| KTCIGME01 | 102.8±10.2*† | 0.96±0.1* | 0.63±0.3ns |
| KTCIGME02 | 64.3±5.4** | 3.17±0.2** | 1.46±0.5* |
| KTCIGME03 | 96.4±8.7** | 0.32±0.1ns | 0.48±0.2ns |
Mean±SE, n = 4
Significantly different at 95% (*) and 99% level (**) as compared with the control, using t-test
Non-significantly as compared with the control
Imaging of bacteria co-cultured with spores using in situ SEM
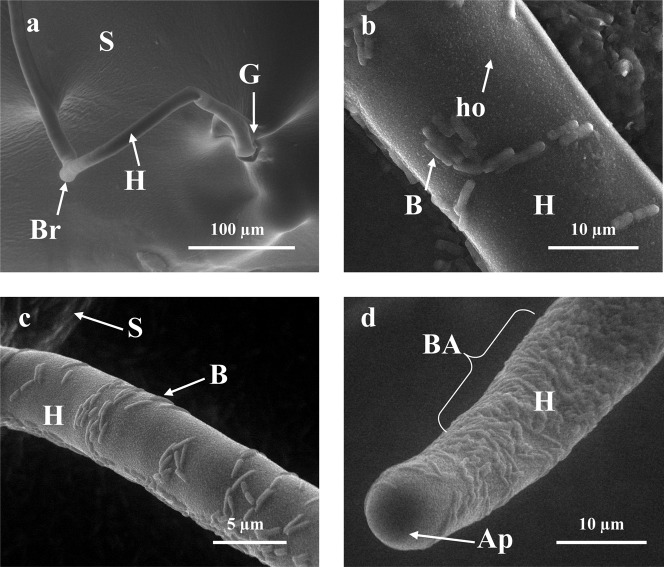
The in situ SEM images showed absence of bacterial growth around the AMF spores and hyphae in the control petri dish (Fig. 2a). SEM imaging also showed the point of invasion of bacteria into spores at the place of germination (Fig. 2b,c,d). On the surface of the hyphae, holes that might be used for gas and/or water exchange were also seen. The size of these holes seems to prevent the penetration of bacteria. Some bacterial aggregates, which were similar to biofilms, were observed. The aggregates were not detected on the apical parts (growing tips) of the hyphae (Fig. 2d).
Fig. 2. In situ SEM picture of G.margarita spore (S) and hyphae (II) colonized by PE (B), including their germination (G) and branching point (Br).
Some holes (ho) and bacterial aggregates (BA) were detected on the hvphal surface, but no bacterial colonization on the apical part of hyphae (Ap). Control (no bacterial) (a): KTCIGM01 (b); KTCIGM02 (c); KTCIGM03 (d).
Discussion
The use of an ultrathin needle to extract the contents of individual spores allowed the isolation of PE. From 500 spores, 3 PE strains were isolated, suggesting that most AMF-associated bacteria are located on the surface of spores and hyphae. In another experiment (data not shown), 1% of the genes sequenced from sterilized Glomus clarum spores belonged to Pseudomonas spp. Even though definitive evidence is lacking, the data suggest that the bacteria were probably derived from inside the spores. Our results demonstrated activity PE in vitro, which may not reflect their effects in soils, especially the effects of those bacteria living on the spore surface. Bacteria associated with spores have been detected previously using DNA methods (e.g., Burkholderia spp.) (Banciotto et al., 1996; Bonfante, 2003; Minerdi et al., 2002). However, use of this ultra thin needle extraction method enabled us to isolate cultivable strains, including Bacillus and Paenibacillus. It also enabled us to obtain a better understanding of the mechanisms underlying interactions between AMF and bacteria and to follow-up on previous work in which bacteria were isolated using osmotic pressure (Cruz et al., 2008).
The probable endobacteria suppressed SBPP, promoted hyphal growth, and stimulated nutrient biodynamics, as reflected by the phosphorus solubilization and nitrogenase activity measurements. We also observed variability in effectiveness among the strains. The bacterial effects can be chemical, by means of exudates, or physical due to their aggregation on the surfaces of spores and hyphae. Although the functional significance of bacteria for spores is still unclear, their presence may be required for the AMF-plant system. Other in vitro studies have shown that Paenibacillus associated with AMF could stimulate the growth of hyphae (Horii and Ishii, 2006; Horii et al., 2008), the formation of new spores (Hildebrandt et al., 2002), the N-fixation rate (Offre et al., 2007), the P solubilization rate (Horii et al., 2008; Larsen et al., 2006; Larsen et al., 2009), and pathogen suppression (Budi et al., 1999; Horii et al., 2008). An effect on N-fixation has also demonstrated with Bacillus strains (Sprent and Sprent, 1990).
In this experiment, the ethylene concentration was not correlated to hyphal growth. Other non-volatile compounds may be involved in the stimulation of hyphal growth (Horii and Ishii, 2006). Previously, cultivable bacteria were isolated from AMF spores and identified (Horii and Ishii, 2006; Horii et al., 2008). Volatile compounds and exudates released by these strains led to higher hyphal length of Gigaspora margarita, which suggests that the mechanisms involved in stimulating hyphal growth are related to ethylene and/or non-volatile compounds produced by these microorganisms.
The NA of these bacteria is interesting, as AMF may be involved in the N-fixation process through their associated microorganisms. This suggests that free-living bacteria associated with AMF can act as N fixers for plants and that mycorrhizal plants, such as those belonging to the family Gramineae, can fix atmospheric N by means of these bacteria. In fact, N-fixation by free-living bacteria in soil is a well-known phenomenon (Tortora et al., 2007). Concomitantly, the fungus can released exudates that enhance the NA of bacteria (Li and Hung, 1987; Rambelli, 1973), suggesting that fungi and bacteria interact in fixing nitrogen in the rhizosphere.
Bacteria interact physically with surfaces to form complex multicellular assemblies, often involving multiple species. These assemblies include biofilms and smaller aggregates (Danhorn and Fuqua, 2007). The SEM images showed the ability of bacteria to colonize AMF spores and hyphae. An interesting point was the appearance of bacterial aggregates, structures similar to biofilms, around hyphae and spores. By definition, a biofilm is a physical structure formed by aggregation of microorganisms, in which cells adhere to each other and/or to a surface. These aggregates could only be detected with in situ SEM, suggesting that the processing steps (fixation, dehydration, and embedding) required for traditional SEM (data not shown) removed these structures from hyphae and spores. In fact, bacteria can form a biofilm that protects plants (Seneviratne et al., 2008). Another interesting observation was that the bacteria could aggregate on hyphae without interrupting hyphal growth, as shown in Fig. 2d. The absence of bacterial aggregates on the apical part of hyphae indicate tight synchronization with AMF.
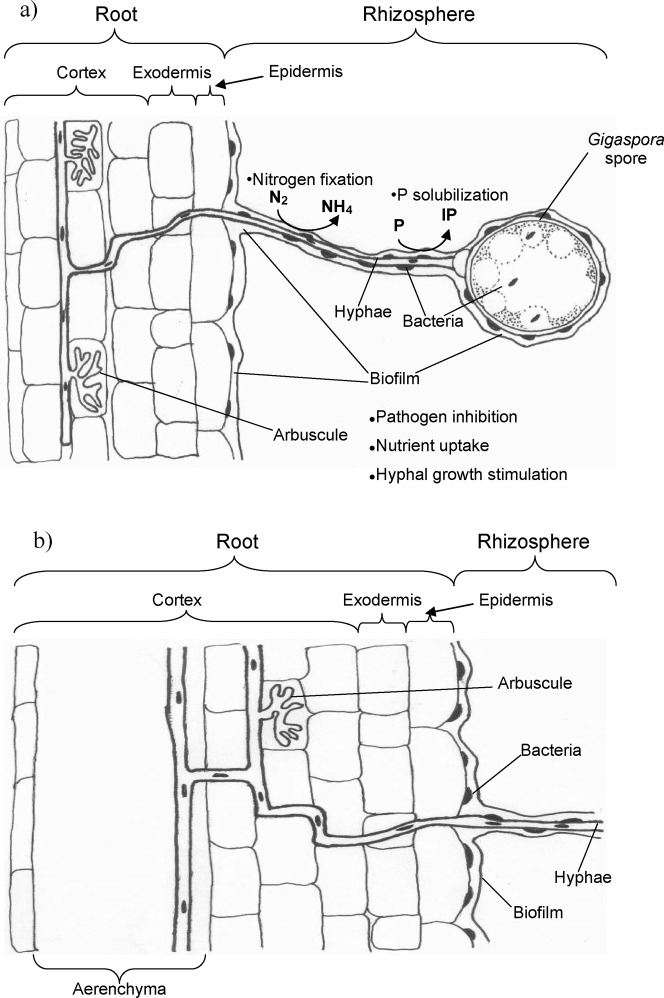
A theoretical model can be derived from these results, in which bacteria stimulate AMF hyphal growth to colonize plant roots under normal conditions (Fig. 3a) and under waterlogged conditions, when plants root contain aerenchyma (Fig. 3b). At the same time, bacteria can form aggregates (biofilms) on the surface of hyphae and spores; these aggregates suppress pathogens and affect nutrient biodynamics (Fig. 3b). Under waterlogged conditions, AMF hyphae were observed on the surface of roots, epidermal cells, and in the aerenchyma of roots, suggesting that oxygen transport takes place through AMF hyphae inside these aerenchyma (Matsumura et al., 2008). It is thought that hyphae help in transporting bacteria to the shoot through the aerenchyma, and not through the xylem due to the presence of lignin. A biofilm can facilitate hyphal penetration through the soil, and when hyphae colonize plant tissues, the bacteria can continue their functions. The bacteria located on hyphae can be released to the intercellular spaces of roots after hyphae are digested by endogenous enzymes. In the intercellular spaces, these bacteria can interact with other endophytic microorganisms. Bacteria have physical effects through biofilm formation, as well as chemical effects through the release of compounds in the exudates.
Fig. 3. Theoretical scheme of the association between AMF and their partner bacteria within the system plant-soil-microorganism system in roots under normal (a) and waterlogged conditions (b).
The bacteria can be located inside and/or surrounding spores and hyphae, and they are released into roots and cells after digestion of hyphae (see arrows). IP Insoluble phosphorus; P–solubilized phosphorus; Atmospheric nitrogen (N2); Ammonium (NH4).
We hypothesize that in the complex system of plant-AMF-bacteria, some functions thought to be carried out by AMF are actually dependent on their associated bacteria. However, further evidence is necessary to test this hypothesis. Clarification of the role of the bacteria in P solubilization, N-fixation, disease suppression, and hyphal growth stimulation will provide new opportunities to use AMF in sustainable agriculture. We believe that the stimulation of fungal growth by the bacteria,will be the feature that can be best exploited for choosing of bacteria and AMF for practical uses.
Acknowledgments
This research was supported by a Grant-in-aid for scientific research from the Ministry of education, sports and culture of Japan no. 80405240. This work was also supported, in part, by a research grant (2009–2011) of the Institute for Fermentation, Osaka, Japan. In addition, we thank Mr. Kodai Takemori, a master’s student at Kyoto Prefectural University, for conducting the experiment.
References
- Alam S., Khalil A., Ayub N., Rashid M. (2002). In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganisms (PSM) from maize rhizosphere. Int. J. Agric. & Biol. 4, 454–458 [Google Scholar]
- Banciotto V., Bandi C., Minerdi D., Sironi M., Ticky H. V., Bonfante P. (1996). An obligately endosymbiotic mycorrhizal fungus itself harbors obligately intracellular bacteria. Appl. Env. Microbiol. 62, 3005–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banciotto V., Lumini E., Bonfante P., Vandamme P. (2003). Candidatus Glomeribacter gigasporarum gen. nov: sp. nov., an endosymbiont of arbuscular mycorrhizal fungi. Int. J. Syst. Evol. Microbiol 53, 121–124 [DOI] [PubMed] [Google Scholar]
- Bertaux J., Schimid M., Chemidlin N. P. B., Chrin J. L., Hartmann A., Garbaye J., Frey-Klatt P. (2003). In situ identification of intracellular bacteria related to Paenibacillus ssp, in the mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Appl. Env. Microbiol. 68, 4243–4248 10.1128/AEM.69.7.4243-4248.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey R., Urquiaga S., Reis V., Döbereiner J. (1991). Biological nitrogen fixation associated with sugar cane. Plant Soil 137, 111–117 10.1007/BF02187441 [DOI] [Google Scholar]
- Bonfante P. (2003). Plants, mycorrhizal fungi and endobacteria: a dialog among cells and genomes. Biol. Bull. 204, 215–220 10.2307/1543562 [DOI] [PubMed] [Google Scholar]
- Bonfante P., Balestrini R., Mendgen K. (1994). Storage and secretion processes in the spore of Gigaspora margarita Becker and Hall as revelead by high-pressure freezing and freeze-substitution. New Phytol. 128, 93–101 10.1111/j.1469-8137.1994.tb03991.x [DOI] [PubMed] [Google Scholar]
- Budi S. W., van Tuinen D., Martinotti G., Gianinazzi S. (1999). Isolation from the sorghum bicolor mycorrhizosphere of a bacterium compatible with arbuscular mycorrhiza development and antagonistic towards soilborne fungal pathogens. Appl. Env. Microbiol. 65, 5148–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. (1995). Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745 10.1146/annurev.mi.49.100195.003431 [DOI] [PubMed] [Google Scholar]
- Cruz A., Ishii T., Kadoya K. (2000). Distribution of vesicular-arbuscular mycorrhizal hyphae in the rhizospheres of trifoliate orange (Poncirus trifoliata) and Bahia grass (Paspalum notatum) seedlings under an intercropping system. J. Jpn. Soc. Hort. Sci. 69, 237–242 10.2503/jjshs.69.237 [DOI] [Google Scholar]
- Cruz A. F. (2004). Element storage in spores of Gigaspora margarita Becker & Hall measured by electron energy loss spectroscopy (EELS). Acta. Bot. Bras. 18, 473–480 10.1590/S0102-33062004000300007 [DOI] [Google Scholar]
- Cruz A. F., Horii S., Ochiai S., Yasuda A., Ishii T. (2008). Isolation and analysis of bacteria associated with spores of Gigaspora margarita. J. Appl. Microbiol. 104, 1711–1717 10.1111/j.1365-2672.2007.03695.x [DOI] [PubMed] [Google Scholar]
- Danhorn T., Fuqua C. (2007). Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 61, 401–422 10.1146/annurev.micro.61.080706.093316 [DOI] [PubMed] [Google Scholar]
- Douglas A. (1994). Symbiotic Interactions Oxford: Oxford University Press [Google Scholar]
- Duponnois R., Garbaye J. (1990). Some mechanisms involved in growth stimulation of ectomycorrhizal fungi by bacteria. Can. J. Bot. 68, 2148–2152 10.1139/b90-280 [DOI] [Google Scholar]
- Harrier L. A., Watson C. A. (2004). The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest Manag. Sci. 60, 149–157 10.1002/ps.820 [DOI] [PubMed] [Google Scholar]
- Hildebrandt U., Janetta K., Bothe H. (2002). Towards growth of arbuscular mycorrhizal fungi independent of a plant host. Appl. Env. Microbiol. 68, 1919–1924 10.1128/AEM.68.4.1919-1924.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii S., Ishii T. (2006). Identification and function of Gigaspora margarita growth-promoting microorganisms. Symbiosis 41, 135–141 [Google Scholar]
- Horii S., Matsumura A., Cruz A., Ishii T. (2008). Effect of arbuscular mycorrhizal fungi and their helper microorganisms on root colonization and growth of trifoliate orange seedlings. Proc. Int. Soc. Citriculture 1, 567–571 [Google Scholar]
- Ishii T., Horii S. (2007). Establishment of axenic cultural techniques of arbuscular mycorrhizal fungi. Hort. Res. (Jpn) (Suppl. 2) 6, 136 [Google Scholar]
- Jargeat P., Cosseau C., Ola'h B., Jauneau A., Bonfante P. (2004). Isolation, free-living capacities, and genome structure of Candidatus glomeribacter gigasporarum, the endocellular bacterium of the mycorrhizal fungus Gigaspora margarita. J. Bacteriol. 186, 6876–6884 10.1128/JB.186.20.6876-6884.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J., Cornejo P., Barea J. M. (2006). Plant growth promoting bacteria from the genus Paenibacillus suppress the arbuscular mycorrhizal fungus Glomus intraradices. Intern. Cong. Mycorrhizae (ICOM 5) p. 203 [Google Scholar]
- Larsen J., Cornejo P., Barea J. M. (2009). Interactions between the arbuscular mycorrhizal fungus Glomus intraradices and the plant growth promoting rhizobacteria Paenibacillus polymyxa and P. macerans in the mycorrhizosphere of Cucumis sativus. Soil Biol. Biochem. 41, 286–292 10.1016/j.soilbio.2008.10.029 [DOI] [Google Scholar]
- Li C., Hung L. (1987). Nitrogen-fixing (acetylene-reducing) bacteria associated with ectomycorrhizae of Douglas-fir. Plant Soil 98, 425–428 10.1007/BF02378363 [DOI] [Google Scholar]
- Li C. Y., Massicote H. B., Moore L. V. H. (1992). Nitrogen-fixing Bacillus sp. associated with Douglas-fir tuberculate ectomycorrhizae. Plant Soil 140, 35–40 10.1007/BF00012804 [DOI] [Google Scholar]
- Linderman R. G. (1992). Vesicular-arbuscular mycorrhizae and soil microbial interactions. Mycorrhizae In Sustainable Agriculture, Vol. 54 ed. (Bethlenfalvay G. J., Linderman R. G.), pp. 45–70 Madison: ASA [Google Scholar]
- Lumini E., Bianciotto V., Jargeat P., Novero M., Salvioli A., Faccio A., Becard G., Bonfante P. (2007). Presymbiotic growth and sporal morphology are affected in the arbuscular mycorrhizal fungus Gigaspora margarita cured of its endobacteria. Cell. Microbiol. 9, 1716–1729 10.1111/j.1462-5822.2007.00907.x [DOI] [PubMed] [Google Scholar]
- Marschner P., Crowley D., Lieberei R. (2001). Arbuscular mycorrhizal infection changes the bacterial 16 S rDNA community composition in the rhizosphere of maize. Mycorrhiza 11, 297–302 10.1007/s00572-001-0136-7 [DOI] [PubMed] [Google Scholar]
- Matsumura A., Horii S., Ishii T. (2008). Observation of arbuscular mycorrhizal network system between trifoliate orange and some grasses under waterlogged conditions. Acta Hortic. 773, 69–75 [Google Scholar]
- Minerdi D., Fani R., Gallo R., Boarino A., Bonfante P. (2001). Nitrogen fixation genes in an endosymbiotic Burkholderia strain. Appl. Environ. Microbiol. 67, 725–732 10.1128/AEM.67.2.725-732.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minerdi D., Banciotto V., Bonfante P. (2002). Endosymbiotic bacteria in mycorrhizal fungi: from their morphology to genomic sequences. Plant Soil 244, 211–219 10.1023/A:1020211821753 [DOI] [Google Scholar]
- Offre P., Pivato B., Siblot S., Gamalero E., Corberand T., Lemanceau P., Mougel C. (2007). Identification of bacterial groups preferentially associated with mycorrhizal roots of Medicago truncatula. Appl. Environ. Microbiol. 73, 913–921 10.1128/AEM.02042-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambelli A. (1973). The rhizosphre of mycorrhizae. Ectomycorrhizae, Their Ecology And Physiology, ed. (Marks G., Kozlowski T.), pp. 299–349 New York: Academic Press [Google Scholar]
- Ruiz-Lozano J. M., Bonfante P. (2000). A Burkholderia strain living inside the arbuscular mycorrhizal fungus Gigaspora margarita possesses the vacB gene, which is involved in host cell colonization by bacteria. Microbiol Ecol. 39, 137–144 10.1007/s002480000008 [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano J. M., Bonfante P. (2001). Intracellular Burkholderia strain has no negative effect on the symbiotic efficiency of the arbuscular mycorrhizal fungus Gigaspora margarita. Plant Growth Regul. 34, 347–352 10.1023/A:1013336413988 [DOI] [Google Scholar]
- Schuessler A., Mollenhauer D., Schnepf E., Kluge M. (1994). Geosiphon pyriforme, and endosymbiotic association of fungus and cyanobacteria: The spore structure resembles that of arbuscular mycorrhizal (AM) fungi. Bot. Acta 107, 36–45 [Google Scholar]
- Seneviratne G., Zavahir J., Bandara W., Weerasekara M. (2008). Fungal-bacterial biofilms: their development for novel biotechnological applications. World J. of Microbiol. and Biotechnol. 24, 739–743 10.1007/s11274-007-9539-8 [DOI] [Google Scholar]
- Sprent J., Sprent P. (1990). Nitrogen-fixing Organisms: Pure And Applied Aspects London: Chapman & Hall [Google Scholar]
- Tortora G. J., Fuke B. R., Case C. L. (2007). Microbiology: An Introduction San Francisco: Pearson/Benjamin Cummings [Google Scholar]
- Walley F. L., Germida J. J. (1995). Failure to decontaminate Glomus clarum NT4 spores is due to spore wall-associated bacteria. Mycorrhiza 6, 43–49 10.1007/s005720050104 [DOI] [Google Scholar]
- Xavier L. J. C., Germida J. J. (2003). Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol. Biochem. 35, 471–478 10.1016/S0038-0717(03)00003-8 [DOI] [Google Scholar]